Abstract
Background
Trends in state-level prevalence of pre-pregnancy diabetes mellitus (PDM; i.e., type 1 or type 2 diabetes diagnosed before pregnancy) among delivery hospitalizations are needed to inform healthcare delivery planning and prevention programs.
Purpose
To examine PDM trends overall, by age group, race/ethnicity, primary payer, and with comorbidities such as pre-eclampsia and pre-pregnancy hypertension, and to report changes in prevalence over 11 years.
Methods
In 2014, State Inpatient Databases from the Agency for Healthcare Research and Quality were analyzed to identify deliveries with PDM and comorbidities using diagnosis-related group codes and ICD-9-CM codes. General linear regression with a log-link and binomial distribution was used to assess the annual change.
Results
Between 2000 and 2010, PDM deliveries increased significantly in all age groups, all race/ethnicity groups, and in all states examined (p<0.01). The age-standardized prevalence of PDM increased from 0.65 per 100 deliveries in 2000 to 0.89 per 100 deliveries in 2010, with a relative change of 37% (p<0.01). Although PDM rates were highest in the South, some of the largest relative increases occurred in five Western states (≥9%). Non-Hispanic blacks had the highest PDM rates and the highest absolute increase (0.26 per 100 deliveries). From 2000 to 2010, the proportion of PDM deliveries with pre-pregnancy hypertension increased significantly (p<0.01) from 7.4% to 14.1%.
Conclusions
PDM deliveries are increasing overall and particularly among those with PDM who have hypertension. Effective diabetes prevention and control strategies for women of childbearing age may help protect their health and that of their newborns.
Introduction
From 1988–1994 to 2005–2010, the prevalence of diabetes in the U.S. increased significantly among young adults (aged 20–34 years).1 In addition, projections of diabetes prevalence among those aged <20 years indicate that, if the incidence continues to increase as it has over recent years, the number with type 1 diabetes will triple and the number with type 2 diabetes will quadruple by 2050.2 An increasing burden of disease among younger age groups places more women at risk of diabetes prior to pregnancy. Women diagnosed with pre-pregnancy type 1 or type 2 diabetes mellitus (PDM) could experience numerous adverse health outcomes if diabetes is poorly controlled during pregnancy.3 Yet, only 40%–60% of women with PDM achieve glycemic control prior to and early in pregnancy.4–6 Additionally, a large growth in the prevalence of diabetes and hypertension comorbidity among adults aged 20–44 years has been reported in the 2000s.7 To our knowledge, no recent state-level estimates of the prevalence of PDM in pregnancy are available. Because there are many complications associated with PDM for both the mother and child, it is crucial to monitor the prevalence of PDM to inform healthcare delivery planning and the identification and implementation of prevention programs. For U.S. states with available data, PDM trends from 2000 to 2010 by race/ethnicity, type of insurance, and associated comorbidities, such as pre-eclampsia and pre-pregnancy hypertension were assessed.
Methods
Data Source
Data from the Agency for Healthcare Research and Quality (AHRQ) State Inpatient Databases (SID) was used to identify maternal hospital discharges involving PDM. SID contains information on hospital inpatient stays for states that volunteer to participate in the Healthcare Cost and Utilization Project (HCUP). Annual data collection of SID includes all community hospitals from the participating states: Arizona, California, Colorado, Florida, Hawaii, Iowa, Kentucky, Maryland, Massachusetts, Michigan, New Jersey, New York, North Carolina, Oregon, South Carolina, Utah, Washington, West Virginia, and Wisconsin. Community hospitals are defined as short-term, non-federal, general and other hospitals, public and privately owned excluding hospital units of other institutions (e.g., prisons), and long-term rehabilitation centers.8 Community hospitals (and HCUP data) include obstetrics and gynecology, ear, nose, and throat, orthopedic, cancer, pediatric, public, and academic medical hospitals. Some states exclude hospitals with a main focus on long-term care, psychiatric, and alcoholism and chemical dependency treatment, although discharges from these types of units that are part of community hospitals are included. Although not all states include such hospitals, the numbers of deliveries from these types of hospitals are minimal and therefore any differences in the population of deliveries would be minimal. Further, the study population is largely a census of community hospitals, not a sample. Together, SID encompasses 97% of all U.S. community hospital discharges.8 AHRQ receives discharge data from the states and provide them in a uniform format so that multistate comparisons may be made.
Measures and Definitions
Hospital delivery discharges were identified using Diagnosis-Related Group (DRG) codes. DRGs comprise a patient classification system that categorizes hospital stays into groups that are clinically homogeneous with respect to resource use, including diagnosis and type of treatment/procedure. Each hospital stay has one DRG assigned to it. Delivery stays were identified by discharges having a DRG code of 767–768 and 774–775 (vaginal delivery) or 765–7766 (C-section) from October 2007 through December 2010 or a DRG code 372–375 (vaginal delivery) or 370–371 (C-section) from January 2000 through September 2007 listed anywhere on the discharge record.
Hospital delivery discharges with PDM were identified by the presence of an ICD-9-CM code of 648.0x (i.e., diabetes mellitus, pre-pregnancy), 250.xx (i.e., diabetes mellitus), or 249.xx (i.e., secondary diabetes) listed anywhere on the discharge record. Gestational diabetes is not included in any of these ICD codes.
Examined variables included maternal age at time of discharge, race/ethnicity, and primary insurance payer (e.g., private, Medicare/Medicaid, other government insurance including Worker's Compensation, Civilian Health and Medical Program of the Uniformed Services, Civilian Health and Medical Program of the Department of Veterans Affairs, Title V, other government programs, or uninsured). Medicare and Medicaid were combined, although deliveries expected to be funded by Medicare were only about 1% (e.g., nonretirees) of the total. PDM deliveries complicated by hyper-tension were also assessed. Pre-eclampsia was defined by ICD-9-CM codes 642.3x–642.6x listed anywhere on the discharge record, and pre-pregnancy hypertension by ICD-9-CM codes 642.0x, 642.2x, 642.7x, 401.0x, 401.1x, 401.9x, 437.2x, or 402.xx–405.xx9 listed anywhere on the discharge record. Age was categorized as: 15–19, 20–24, 25–29, 30–34, 35–39, and 40–44 years. Cesarean section was identified by DRG codes 370 and 371 from January 2000 through September 2007 and 765 and 766 from October 2007 through December 2010. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic, and Asian/Pacific Islander in the 12 states reporting race/ethnicity. Seven states (Iowa, Kentucky, North Carolina, Oregon, Utah, Washington, and West Virginia) reported no information on either race or ethnicity for ≥3 years during the study period. Race/ethnicity was the only variable in this analysis that was not consistently reported.
Statistical Methods
In 2014, age-standardized rates of PDM deliveries were estimated overall, by state, and by selected characteristics. The proportion of deliveries obtained from the 2000 HCUP National Inpatient Sample for the age groups 15–19, 20–24, 25–29, 30–34, 35–39, and 40–44 years was used to standardize the distribution of deliveries by age across years. SAS software, version 9.3 and SUDAAN, version 11.0.0 were used for data management and analyses to produce estimates. Data were missing for California in 2002 and Hawaii in 2005. For these two states, all variables were imputed for the missing years using PROC MI in SAS. Imputation was based on data from other years and the number of deliveries was obtained by DRG codes from HCUPnet.10 Less than 1% of data were missing for other variables for the remaining states and years, but for consistency they were also imputed. However, among the 12 states reporting race and ethnicity, race and ethnicity were missing, on average, 26.2% per year. The imputed overall age-standardized rates were compared with complete-case overall standardized PDM rates. Differences between the two methods were not more than 0.01 percentage point in any year. General linear regression with a log-link and binomial distribution was used to assess the annual change in PDM prevalence over 11 years and to test for a statistically significant change in prevalence over time. This study was reviewed by the Human Subjects Coordinator at CDC and as an analysis of secondary data without identifiers, was determined not to require IRB review.
Results
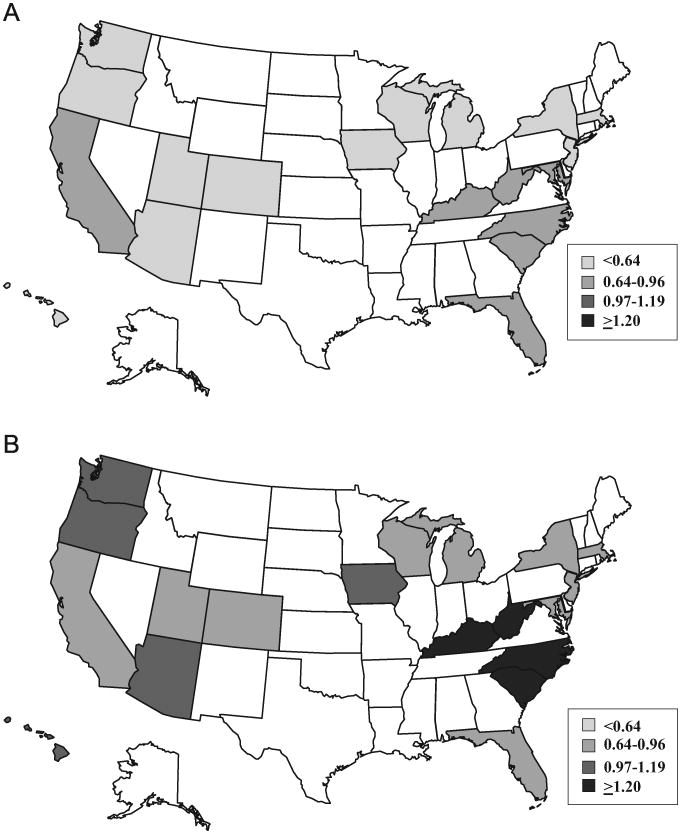
Overall, the number of PDM deliveries increased from 13,217 in 2000 to 18,168 in 2010. The age-standardized prevalence of PDM increased from 0.65 per 100 deliveries in 2000 to 0.89 per 100 deliveries in 2010, for a relative increase of 37% (Table 1). The greatest absolute increase occurred in Hawaii (from 0.66 to 1.15 per 100 deliveries) and the lowest increase, both absolute and relative, occurred in New Jersey (from 0.61 to 0.66 per 100 deliveries). Five of the six states with the highest relative increase in PDM deliveries (≥69%) were in the Western region (Hawaii, Oregon, Arizona, Utah, and Washington), but the region with the overall highest relative increase in this study was the Midwest (61%) (Figure 1A and 1B). Throughout most years, the four states with the highest rates were in the South (South Carolina, North Carolina, Kentucky, and West Virginia).
Table 1. Pre-pregnancy Diabetes Prevalence Among Deliveries in 19 States, HCUP SID 2000–2010.
| Rate Per 100 births | Absolute changea | Relative change (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |||
| Total 19 states | 0.65 | 0.67 | 0.71 | 0.73 | 0.76 | 0.80 | 0.81 | 0.80 | 0.83 | 0.88 | 0.89 | 0.24 | 37 |
| STATES BY U.S. CENSUS REGION | |||||||||||||
| North | 0.61 | 0.61 | 0.62 | 0.65 | 0.69 | 0.69 | 0.72 | 0.70 | 0.74 | 0.74 | 0.74 | 0.13 | 21 |
| Marylanda | 0.77 | 0.84 | 0.83 | 0.82 | 0.84 | 0.87 | 0.93 | 0.94 | 0.92 | 0.93 | 0.90 | 0.23 | 17 |
| Massachusettsa | 0.54 | 0.50 | 0.53 | 0.53 | 0.61 | 0.61 | 0.59 | 0.58 | 0.65 | 0.62 | 0.67 | 0.13 | 24 |
| New Jerseya | 0.61 | 0.59 | 0.59 | 0.61 | 0.62 | 0.61 | 0.60 | 0.64 | 0.64 | 0.63 | 0.66 | 0.05 | 8 |
| New Yorka | 0.60 | 0.59 | 0.60 | 0.64 | 0.70 | 0.68 | 0.72 | 0.70 | 0.74 | 0.73 | 0.72 | 0.12 | 20 |
| South | 0.79 | 0.80 | 0.82 | 0.88 | 0.89 | 0.95 | 0.94 | 0.97 | 1.00 | 1.04 | 1.08 | 0.29 | 37 |
| Floridaa | 0.71 | 0.73 | 0.74 | 0.77 | 0.75 | 0.84 | 0.81 | 0.84 | 0.88 | 0.90 | 0.90 | 0.19 | 27 |
| Kentucky | 0.79 | 0.89 | 0.76 | 0.92 | 0.92 | 1.06 | 1.02 | 1.06 | 1.06 | 1.12 | 1.20 | 0.41 | 52 |
| North Carolina | 0.86 | 0.85 | 0.99 | 0.98 | 1.06 | 1.06 | 1.09 | 1.09 | 1.14 | 1.20 | 1.22 | 0.36 | 42 |
| South Carolinaa | 0.94 | 0.91 | 0.95 | 1.03 | 1.10 | 1.11 | 1.14 | 1.13 | 1.16 | 1.26 | 1.33 | 0.39 | 41 |
| West Virginia | 0.91 | 1.02 | 0.85 | 1.04 | 1.13 | 1.23 | 1.09 | 1.17 | 1.30 | 1.28 | 1.28 | 0.37 | 41 |
| Midwest | 0.57 | 0.61 | 0.66 | 0.70 | 0.76 | 0.76 | 0.83 | 0.79 | 0.85 | 0.90 | 0.92 | 0.35 | 61 |
| Iowa | 0.55 | 0.49 | 0.61 | 0.60 | 0.68 | 0.71 | 0.72 | 0.77 | 0.76 | 0.88 | 0.98 | 0.43 | 78 |
| Michigana | 0.59 | 0.62 | 0.65 | 0.74 | 0.81 | 0.80 | 0.85 | 0.81 | 0.88 | 0.93 | 0.92 | 0.33 | 56 |
| Wisconsina | 0.54 | 0.67 | 0.70 | 0.66 | 0.71 | 0.72 | 0.86 | 0.77 | 0.83 | 0.85 | 0.89 | 0.35 | 65 |
| West | 0.64 | 0.65 | 0.71 | 0.72 | 0.75 | 0.79 | 0.79 | 0.78 | 0.81 | 0.89 | 0.88 | 0.24 | 38 |
| Arizonaa | 0.59 | 0.62 | 0.76 | 0.73 | 0.77 | 0.94 | 0.91 | 0.91 | 1.07 | 1.04 | 1.02 | 0.43 | 73 |
| Californiaa | 0.68 | 0.70 | 0.72 | 0.74 | 0.76 | 0.79 | 0.79 | 0.77 | 0.77 | 0.86 | 0.84 | 0.16 | 24 |
| Coloradoa | 0.56 | 0.51 | 0.56 | 0.52 | 0.62 | 0.57 | 0.60 | 0.60 | 0.64 | 0.73 | 0.67 | 0.11 | 20 |
| Hawaiia | 0.66 | 0.72 | 0.74 | 0.61 | 0.79 | 0.82 | 0.92 | 0.97 | 1.04 | 1.09 | 1.15 | 0.49 | 74 |
| Oregon | 0.57 | 0.59 | 0.76 | 0.84 | 0.82 | 0.78 | 0.92 | 0.92 | 0.93 | 0.99 | 1.09 | 0.52 | 91 |
| Utah | 0.41 | 0.49 | 0.53 | 0.62 | 0.57 | 0.61 | 0.65 | 0.73 | 0.66 | 0.82 | 0.79 | 0.38 | 93 |
| Washington | 0.58 | 0.58 | 0.63 | 0.69 | 0.74 | 0.82 | 0.79 | 0.77 | 0.84 | 0.90 | 0.98 | 0.40 | 69 |
Note: Annual change is significant (p<0.01) for all states. All estimates are age-standardized to the 2000 population of deliveries. For California in 2002 and Hawaii, in 2005 the number of deliveries was obtained from HCUPnet; all other variables for those 2 states for those 2 years were imputed from the other years' data from those states.
Indicates state had race/ethnicity data.
HCUP SID, Healthcare Cost & Utilization Project State Inpatient Databases.
Figure 1.
(A) Prevalence of pre-pregnancy diabetes per 100 deliveries among participating states, Healthcare Cost & Utilization Project State Inpatient Databases 2000. (B) Prevalence of pre-pregnancy diabetes per 100 deliveries among participating states, Healthcare Cost & Utilization Project State Inpatient Databases 2010.
PDM delivery rates in all 19 states increased significantly (p<0.01) across all age groups, but the greatest increases were among women aged 35–39 years, followed by those aged 15–19 years (relative changes of 47% and 41%, respectively) (Table 2). Crude and age-standardized PDM delivery rates per 100 births also increased significantly (p<0.01) among all racial/ethnic groups. Asians and Hispanics had higher crude relative increases (41% for Asian/Pacific Islanders, 39% for Hispanics, 33% for non-Hispanic blacks, and 31% for non-Hispanic whites). The age-standardized relative increases were similar across races and ethnicities (range, 24%–29%). However, non-Hispanic blacks, followed by Hispanics, had the highest absolute change in age-standardized rates (0.26 and 0.20 per 100 deliveries, respectively), and throughout the time period, had the highest rates of PDM. The relative increase in PDM among the 12 states that had race data during the entire period was 28%, whereas the relative increase was 84% among the seven states that did not report race or ethnicity consistently throughout the study.
Table 2. Prevalence of Deliveries with Pre-pregnancy Diabetes by Selected Characteristics, 19 States, HCUP SID 2000–2010.
| Rate Per 100 births | Absolute changea | Relative change (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |||
| Age group (years) | |||||||||||||
| 15–19 | 0.29 | 0.29 | 0.42 | 0.31 | 0.34 | 0.37 | 0.38 | 0.37 | 0.42 | 0.42 | 0.41 | 0.12 | 41 |
| 20–24 | 0.44 | 0.45 | 0.54 | 0.50 | 0.53 | 0.55 | 0.55 | 0.56 | 0.58 | 0.62 | 0.56 | 0.12 | 27 |
| 25–29 | 0.63 | 0.64 | 0.70 | 0.69 | 0.74 | 0.74 | 0.75 | 0.76 | 0.77 | 0.83 | 0.83 | 0.20 | 32 |
| 30–34 | 0.78 | 0.81 | 0.81 | 0.89 | 0.89 | 0.96 | 0.99 | 0.96 | 0.99 | 1.06 | 1.06 | 0.28 | 36 |
| 35–39 | 1.07 | 1.11 | 1.02 | 1.16 | 1.25 | 1.30 | 1.33 | 1.30 | 1.40 | 1.45 | 1.57 | 0.50 | 47 |
| 40–44 | 1.55 | 1.43 | 1.37 | 1.67 | 1.73 | 1.75 | 1.84 | 1.86 | 1.85 | 1.90 | 2.14 | 0.59 | 38 |
| RACE/ETHNICITYa | |||||||||||||
| Non-Hispanic white | |||||||||||||
| Crude | 0.59 | 0.60 | 0.64 | 0.66 | 0.69 | 0.71 | 0.73 | 0.71 | 0.72 | 0.78 | 0.77 | 0.18 | 31 |
| Age-standardized | 0.56 | 0.57 | 0.64 | 0.62 | 0.64 | 0.67 | 0.68 | 0.67 | 0.68 | 0.73 | 0.72 | 0.16 | 29 |
| Hispanic | |||||||||||||
| Crude | 0.72 | 0.69 | 0.75 | 0.76 | 0.78 | 0.87 | 0.86 | 0.85 | 0.92 | 0.95 | 1.00 | 0.28 | 39 |
| Age-standardized | 0.74 | 0.71 | 0.72 | 0.77 | 0.78 | 0.87 | 0.86 | 0.89 | 0.91 | 0.92 | 0.94 | 0.20 | 27 |
| Non-Hispanic black | |||||||||||||
| Crude | 0.91 | 0.99 | 1.00 | 1.07 | 1.11 | 1.12 | 1.13 | 1.08 | 1.18 | 1.21 | 1.21 | 0.30 | 33 |
| Age-standardized | 1.01 | 1.07 | 1.02 | 1.14 | 1.20 | 1.20 | 1.21 | 1.09 | 1.26 | 1.29 | 1.27 | 0.26 | 26 |
| Asian/Pacific Islander | |||||||||||||
| Crude | 0.61 | 0.61 | 0.67 | 0.60 | 0.74 | 0.73 | 0.74 | 0.79 | 0.83 | 0.85 | 0.86 | 0.25 | 41 |
| Age-standardized | 0.59 | 0.56 | 0.61 | 0.54 | 0.67 | 0.64 | 0.65 | 0.71 | 0.72 | 0.71 | 0.73 | 0.14 | 24 |
Note: Annual change is significant (p<0.01) for all states. All estimates are age-standardized to the 2000 population of deliveries. For California in 2002 and Hawaii, in 2005 the number of deliveries was obtained from HCUPnet; all other variables for those 2 states for those 2 years were imputed from the other years' data from those states.
States with race/ethnicity data include AZ, CA, CO, FL, HI, MA, MD, MI, NJ, NY, SC, WI.
HCUP SID, Healthcare Cost & Utilization Project State Inpatient Databases.
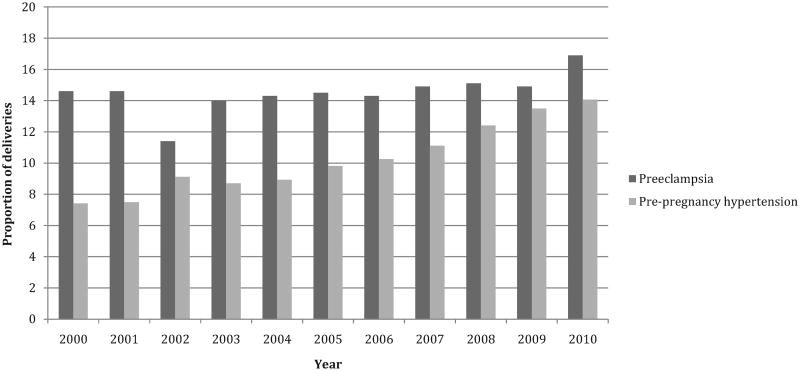
From 2000 to 2010, the proportion of PDM deliveries with pre-pregnancy hypertension increased from 7.4% to 14.1% (relative increase, 91%) (Figure 2). The proportion of PDM deliveries with preeclampsia increased from 14.6% to 16.9% (relative increase, 16%) and the annual change was significant (p=0.05). The proportion of PDM deliveries with private insurance as the expected primary payer decreased significantly from 55.5% to 40% (relative change, −28%; annual change, p<0.001), whereas the proportion expected to be funded by Medicaid/Medicare increased from 39.9% to 54.9% (relative increase, 38%; annual change, p<0.001). The proportion of PDM cesarean section deliveries increased from 50.9% in 2000 to 62.1% in 2010 (relative increase, 22%; annual change, p<0.001).
Figure 2.
Proportion of PDM deliveries with hypertension, 19 states 2000–2010.
PDM, pre-pregnancy type 1 or type 2 diabetes mellitus.
Discussion
This study indicates that PDM deliveries in 19 states are increasing across all demographic groups (e.g., age, race/ethnicity) and geographies (e.g., states, regions), thus, the increase in PDM is widespread in the 19 examined states. This is consistent with national trends of diabetes prevalence,1,11,12 including women aged ≥18 years.13 Numerous studies have found that elevated blood glucose levels in early pregnancy are associated with a significantly increased risk of pregnancy-related hypertension, birth defects, stillbirth, and preterm birth in infants of women with frank diabetes.14,15 However, the majority of women with PDM do not plan their pregnancies in advance and become pregnant with poor blood glucose control.16
The increase in PDM deliveries followed the emerging increase of diabetes in adolescents,17 which paralleled the increase in overweight prevalence among youth from 1963 to 1994.18 Not only are PDM deliveries becoming more common, but the proportion complicated by hypertension, whether pre-eclampsia or pre-pregnancy hypertension, has increased significantly. The 91% increase in pre-pregnancy hypertension among PDM deliveries is consistent with national data documenting a large growth in the prevalence of diabetes and hypertension comorbidity among adults aged 20–44 years in the 2000s.19 Further, the increase of hyper-tension in PDM deliveries poses a threat to their children because uncontrolled diabetes, untreated hypertension, and hypertension treatment are associated with congenital heart defects and other adverse health outcomes.3,19
It has long been established that diabetes disproportionately affects those with lower SES, particularly among women.20 Throughout the study period, the proportion of PDM deliveries expected to be funded by the Centers for Medicare and Medicaid Services increased significantly. Starting in 1991, all state Medicaid programs were required to cover pregnant women with incomes <133% of the federal poverty level.21 Notably, Medicaid funded over half of the PDM deliveries by 2010 in the 19 assessed states.
The increase in PDM rates among racial and ethnic minorities may be in part due to low SES and other unmeasured factors that increase PDM risk. The highest rate of PDM throughout the study period and the highest degree of absolute change in rate were observed among non-Hispanic blacks. This high prevalence is also consistent with higher rates of diabetes and obesity (a known risk factor for type 2 diabetes) among black women compared to Hispanic and white women.22,23 However, the highest crude rates of relative increase in PDM by race/ethnicity were among Hispanics and Asians; the highest age-standardized rate of relative increase was among non-Hispanic white women. From 1999 to 2008, the prevalence of obesity increased more among Mexican-American women aged 20–39 years (relative increase of 29%) than among non-Hispanic blacks (relative increase of 2%), possibly explaining the greater increase among Hispanics. Non-Hispanic white women of the same age experienced a similar increase in obesity as Hispanic women (relative increase of 28%).22 Although studies have shown that the prevalence of obesity among Asian women is substantially lower than the national average, Asians are at risk for diabetes at lower levels of body mass.24
States with the highest rates of PDM by 2010 were mostly in the South, whereas states in the North had the lowest rates as well as some of the lowest rates of increase. In general, diabetes prevalence is highest in the Southeastern U.S.25 Regional differences in PDM trends may be related to demographic variation in race/ethnicity and SES. Southern states with some of the highest PDM rates have a greater proportion of African Americans than other regions, while western states with the highest relative rates of increase in PDM have the highest proportion of Hispanics. Hawaii has the largest proportion of Asians in this study, which may explain in part of the high rate and high increase in that state.
One limitation of this study is that data are not available for all 50 states, thus the results may not be representative of the entire nation or all regions. However, no data source that covers all states is readily available. A second limitation is the inconsistency in availability of race/ethnicity data across states. Also, no data on pre-pregnancy weight or BMI were available. Because the effect of obesity varies across age and race/ethnicity with respect to PDM,26 it was not possible to further examine the variability across race/ethnicity between crude and age-standardized rates. Another limitation is that the PDM rates for race and ethnicity do not represent the 19 states in the entire study population. Also, close to 26% of discharges were missing data on race and ethnicity and thus were imputed. The seven states that did not report race and ethnicity consistently during the study experienced much higher relative rates of increase than the states that did report race; the reason may be due, in part, to their having the lowest rates to begin with and therefore having the greatest increase when using a multiplicative measure like relative change. Moreover, racial and ethnic differences noted may well be due to other unmeasured factors. Although ICD-9 codes are not as reliable as a clinical diagnosis of diabetes, validation studies done with hospital discharges found sensitivity of 78.0%–95.3%, specificity of 99.4%–100.0%, and positive predictive value of 94.0%–97.6%.27 In addition, the ICD codes for type of diabetes (e.g., type 1 or 2) are not reliable, thus, it was not possible to study the conditions that underlie these trends. In addition, no data are available for the proportion of type 2 PDM in the U.S. However, in the 1990s, the incidence of type 2 diabetes increased among youth aged 10–19 years who were obese and had a family history of type 2 diabetes.17 Finally, using ICD codes may under-report complications such as hypertension in deliveries, with sensitivity as low as 58%.28 This analysis found that <2% of all deliveries were affected by hypertension, but other studies reported that hypertension occurred in 5%–10% of pregnancies.29
This study found that increases in PDM were pervasive across demographic groups. Strikingly, this analysis found that PDM deliveries are also becoming more complicated as the proportion with hypertension, preeclampsia or pre-conception, is also increasing, a combination that places children born to women with diabetes at risk for serious adverse outcomes. Meanwhile, publicly funded healthcare programs such as Medicare and Medicaid paid for an increasing number of PDM deliveries, while private insurance bore the cost of fewer, representing a shift in the financial burden of PDM. Finally, the rate of PDM is increasing among Hispanic women who represent a growing proportion of the population in which the risk of developing diabetes is also increasing. The increase in overweight prevalence is likely to be driving these increases. Effective diabetes prevention and control strategies for women of child-bearing age may help protect the health of women and their newborns.
Footnotes
Dr. Barbara Bardenheier is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. B.H.B. and L.S.G. researched data, contributed to discussion, wrote the manuscript, reviewed and edited the manuscript, and approved submission of the manuscript for publication. S.Y.K. researched data, contributed to discussion, approved submission of the manuscript for publication, and reviewed and edited the manuscript. H.M.D. contributed to discussion, wrote the manuscript, reviewed and edited the manuscript, and approved submission of the manuscript for publication. G.I. and P.C. contributed to data analyses, discussed, reviewed, and edited the manuscript, and approved submission of the manuscript for publication. This study was possible because of the statewide data collection efforts of the following organizations: Arizona Department of Health Services, Arkansas Department of Health, California Office of Statewide Health Planning and Development, Colorado Hospital Association, Florida Agency for Health Care Administration, Hawaii Health Information Corporation, Iowa Hospital Association, Kentucky Cabinet for Health and Family Services, Maine Health Data Organization, Maryland Health Services Cost Review Commission, Massachusetts Division of Health Care Finance and Policy, Michigan Health & Hospital Association, Nevada Department of Health and Human Services, New Jersey Department of Health, New York State Department of Health, North Carolina Department of Health and Human Services, Oregon Health Policy and Research, Oregon Association of Hospitals and Health Systems, Rhode Island Department of Health, South Dakota Association of Healthcare Organizations, Utah Department of Health, Vermont Association of Hospitals and Health Systems, Washington State Department of Health, and Wisconsin Department of Health Services.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
No potential conflicts of interest relevant to this article were reported.
No financial disclosures were reported by the authors of this paper.
References
- 1.Cheng YJ, Imperatore G, Geiss LS, et al. secular changes in the agespecific prevalence of diabetes among U.S. adults: 1988-2010. Diabetes Care. 2013;36(9):2690–6. doi: 10.2337/dc12-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imperatore G, Boyle JP, Thompson TJ, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–20. doi: 10.2337/dc12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray JG, O'Brien TE, Chan WS. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. QJM. 2001;94(8):435–44. doi: 10.1093/qjmed/94.8.435. [DOI] [PubMed] [Google Scholar]
- 4.Langer O, Conway DL. Level of glycemia and perinatal outcome in pregestational diabetes. J Matern Fetal Med. 2000;9(1):35–41. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<35::AID-MFM8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Casele HL, Laifer SA. Factors influencing preconception control of glycemia in diabetic women. Arch Intern Med. 1998;158(12):1321–4. doi: 10.1001/archinte.158.12.1321. [DOI] [PubMed] [Google Scholar]
- 6.Holing EV, Beyer CS, Brown ZA, Connell FA. Why don't women with diabetes plan their pregnancies? Diabetes Care. 1998;21(6):889–95. doi: 10.2337/diacare.21.6.889. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Geiss LS, Cheng YJ, et al. Long-term and recent progress in blood pressure levels among U.S. adults with diagnosed diabetes, 1988–2008. Diabetes Care. 2011;34(7):1579–81. doi: 10.2337/dc11-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agency for Healthcare Research and Quality, DHHS. HCUP facts and figures. 2007 www.hcup-us.ahrq.gov/reports/factsandfigures/2007/definitions.jsp. [PubMed]
- 9.Kuklina EV, Meikle SF, Jamieson DJ, et al. Severe obstetric morbidity in the United States: 1998-2005. Obstet Gynecol. 2009;113(2 Pt 1):293–9. doi: 10.1097/AOG.0b013e3181954e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality. DHHS. HCUPnet home page. doi: 10.1080/15360280802537332. hcupnet.ahrq.gov. [DOI] [PubMed]
- 11.Lee JW, Brancati FL, Yeh HC. Trends in the prevalence of type 2 diabetes in Asians versus whites: results from the United States National Health Interview Survey, 1997–2008. Diabetes Care. 2011;34(2):353–7. doi: 10.2337/dc10-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC. Increasing prevalence of diagnosed diabetes—United States and Puerto Rico, 1995-2010. MMWR Morb Mortal Wkly Rep. 2012;61(45):918–21. [PubMed] [Google Scholar]
- 13.CDC, DHHS. Diabetes data and trends. apps.nccd.cdc.gov/DDTSTRS/default.aspx#div4.
- 14.Balsells M, Garcia-Patterson A, Gich I, Corcoy R. Maternal and fetal outcome in women with type 2 versus type 1 diabetes mellitus: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94(11):4284–91. doi: 10.1210/jc.2009-1231. [DOI] [PubMed] [Google Scholar]
- 15.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–33. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 16.Holing EV. Preconception care of women with diabetes: the unrevealed obstacles. J Matern Fetal Med. 2000;9(1):10–3. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<10::AID-MFM4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136(5):664–72. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 18.Troiano RP, Flegal KM. Overweight children and adolescents: description, epidemiology, and demographics. Pediatrics. 1998;101(3 Pt 2):497–504. [PubMed] [Google Scholar]
- 19.Caton AR, Bell EM, Druschel CM, et al. Antihypertensive medication use during pregnancy and the risk of cardiovascular malformations. Hypertension. 2009;54(1):63–70. doi: 10.1161/HYPERTENSIONAHA.109.129098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbins JM, Vaccarino V, Zhang H, Kasl SV. Socioeconomic status and diagnosed diabetes incidence. Diabetes Res Clin Pract. 2005;68(3):230–6. doi: 10.1016/j.diabres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Glied S, Jack K, Rachlin J. Women's health insurance coverage 1980-2005. Womens Health Issues. 2008;18(1):7–16. doi: 10.1016/j.whi.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 23.CDC. National Center for Health Statistics. Health data interactive. www.cdc.gov/nchs/hdi.htm.
- 24.Gupta LS, Wu CC, Young S, Perlman SE. Prevalence of diabetes in New York City, 2002-2008: comparing foreign-born South Asians and other Asians with U.S.-born whites, blacks, and Hispanics. Diabetes Care. 2011;34(8):1791–3. doi: 10.2337/dc11-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barker LE, Kirtland KA, Gregg EW, Geiss LS, Thompson TJ. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med. 2011;40(4):434–9. doi: 10.1016/j.amepre.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Kim C, Kim SY, Sappenfield W, Wilson HG, Salihu HM, Sharma AJ. Are gestational diabetes mellitus and preconception diabetes mellitus less common in non-Hispanic black women than in non-Hispanic white women? Matern Child Health J. 2014;18(3):698–706. doi: 10.1007/s10995-013-1295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devlin HM, Desai J, Walaszek A. Reviewing performance of birth certificate and hospital discharge data to identify births complicated by maternal diabetes. Matern Child Health J. 2009;13(5):660–6. doi: 10.1007/s10995-008-0390-9. [DOI] [PubMed] [Google Scholar]
- 28.Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. Am J Obstet Gynecol. 2006;194(4):992–1001. doi: 10.1016/j.ajog.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 29.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]