Abstract
Our purpose was to study the safety, distribution, pharmacokinetics, immunogenicity and tumor response of intraperitoneal (IP) 212Pb-TCMC-trastuzumab (TCMC is S-2-(4-isothiocyantobenzl)-1, 4, 7, 10-tetraaza-1, 4, 7, 10=tetra (2-carbamoylmethl) cyclododecane) in patients with HER-2 expressing malignancy.
Methods
In a standard 3+3 Phase 1 design for dose escalation, 212Pb-TCMC-trastuzumab was delivered IP less than 4 hours after giving 4mg/kg IV trastuzumab to patients with peritoneal carcinomatosis who had failed standard therapies.
Results
Five dosage levels (7.4, 9.6, 12.6, 16.3, 21.1 MBq/m2) showed minimal toxicity at >1 year for the first group and >4 months for others. The lack of substantial toxicity was consistent with the dosimetry assessments (mean equivalent dose to marrow = 0.18 mSv/MBq). Radiation dosimetry assessment was performed using pharmacokinetics data obtained in the initial cohort (n=3). Limited redistribution of radioactivity out of the peritoneal cavity to circulating blood, which cleared via urinary excretion and no specific uptake in major organs was observed in 24 hours. Maximum serum concentration of the radiolabeled antibody was 22.9% at 24h (decay corrected to injection time) and 500 Bq/mL (decay corrected to collection time). Non-decay corrected cumulative urinary excretion was ≤6% in 24h (2.3 half lives). Dose rate measurements performed at 1m from the patient registered less than 5μSv/hr (using portable detectors) in the latest cohort, significantly less than what is normally observed using nuclear medicine imaging agents. Anti-drug antibody assays performed on serum from the first 4 cohorts were all negative.
Conclusions
Five dose levels of IP 212Pb-TCMC-trastuzumab treatment of patients with peritoneal carcinomatosis showed little agent related toxicity, consistent with the dosimetry calculations.
Keywords: radioimmunotherapy, dosimetry, radionuclide, alpha, 212Pb-TCMC-trastuzumab
Introduction
Spread of tumor in the peritoneal cavity is an adverse factor and therapeutic challenge for a variety of malignancies. Multiple prior experiences in ovarian cancer have shown that the high failure rate in the peritoneal cavity despite removal of all visible disease followed by adjuvant chemotherapy can be reduced by radionuclide therapy (1-5). Most intraperitoneal (IP) radionuclide therapies of ovarian cancer have used beta emitter antibody conjugates (radioimmunotherapy or RIT) and have resulted in dose-limiting marrow suppression (1-10). Less toxicity is projected using radionuclides with shorter half lives, because less radioactivity would distribute systemically (11). Additionally, application of the more radiobiologically potent alpha emitters such as the 212Pb/212Bi parent-daughter pair (212Pb half life=10.6 hours) or 211At (half-life = 7.2 hours) should improve efficacy over prior beta emitter radioimmunotherapy while limiting irradiation of neighboring healthy cells (12). This first in human Phase I trial of 212Pb-TCMC-trastuzumab provided a critical opportunity to assess the safety, toxicity, immunogenicity, serum pharmacokinetics, urinary excretion, imaging, body count biodistribution, dosimetry, and tumor response to this agent. Among other alpha emitters in clinical trials, dose escalations have been well tolerated, without dose-limiting toxicity(13). Intraperitoneal administration of 211At-Mx (Fab') 2 has been under study as treatment of ovarian cancer for several years (14). These studies have shown low risk of adverse events through the highest dose level, which was a 24 hour dwell of 1.5L at 200MBq/L. The low toxicity was predicted by calculated effective dose of <2Sv (15). Although there is no current laboratory or imaging measure of efficacy, the pre-clinical investigation suggests that dose is adequate for control of targeted microscopic disease clusters(16).
Materials and Methods
Trial Design
This phase I trial, sponsored by AREVA Med (Bethesda, Maryland) and conducted at a single clinical site (University of Alabama at Birmingham, first five cohorts), used a single IP injection of the investigational agent, 212Pb-TCMC-trastuzumab, in patients with HER-2 expressing malignancies mainly confined to the peritoneal cavity who had failed standard therapy. The clinical protocol was approved by the Western Institutional Review Board and was conducted under an investigational new drug application. Three patients were to be treated at each level, with expansion to six patients if dose limiting toxicity developed. Dose escalation (radioactivity per m2 body surface) was 30% per cohort. HER-2 expression of at least 1+ by immunohistochemistry in more than 10% of the cells was deemed acceptable for gastric cancer. Initially, reactivity in 30% of cells was required for non-gastric malignancies but was reduced to 10% or more after patient #10. This trial uses trastuzumab as a targeting agent in the IP cavity, not as a primary therapy where +2 expression is desired for breast cancer patients. The less stringent requirement of 1+ is consistent with expression of 100,000 receptors/cell, which may be approximately 100x higher than on the normal cells.
Alternatively, elevated HER-2 serum level by ELISA was sufficient and one patient was allowed to be treated whose expression value met serum criteria when rounded off to nearest whole number. Patients needed to have free flow of fluid in the peritoneal cavity and were excluded for serious cardiac dysfunction, left ventricular ejection fraction less than 50%, poor organ function (defined as any of the following: elevated creatinine, total bilirubin greater than 1.5 times normal, AST and ALT greater than 2.5 times normal, absolute neutrophil counts less than 1,500/μL, platelets less than 100,000/μL) or other conditions that might compromise safety. Other exclusion criteria included ECOG performance status greater than 2, pregnancy or breast feeding, evidence of bowel obstruction or transmural involvement, prior radiation to the whole abdomen, prior IP radionuclide therapy, stem cell transplant, history of HIV or Hepatitis B antibody positivity, or detectable antibody to trastuzumab. Patients were excluded for a history of cumulative anthracycline therapy exceeding 200 mg/m2 for doxorubicin or comparable low dose of other anthracyclines.
Eligible, consenting, adult patients were housed in a Clinical Research Unit where they received a single IP injection of 212Pb-TCMC-trastuzumab in 50 mL less than 4 hours following 4mg/kg IV trastuzumab. The standard IV loading dose of 4mg/kg trastuzumab was given to saturate systemic receptors as the tumor targets were in the peritoneal cavity. This procedure was used to minimize the chance of any radioactivity localization to tissues outside the peritoneal cavity, especially the heart, since trastuzumab has been associated with cardiac toxicity. Additional saline was instilled into the peritoneal cavity before and after 212Pb-TCMC-trastuzumab, for a total volume of up to1, 000mL.
Pharmacokinetics
Post-treatment serum pharmacokinetics, urinary excretion and biodistribution studies were performed. Blood samples were obtained at 2 and 24 hours on all patients. In the first cohort, additional samples were taken immediately post-infusion and at 8, 12, 18, and 63 hours; complete urine was collected for 24 hours. Details of methods have been reported previously (17). Whole body anterior and posterior gamma camera images (peak window at 238.6 keV) were acquired post-treatment and repeated at 18-24 hours as reported (17). Biodistribution of 212Pb-TCMC-trastuzumab was confirmed with probe counts immediately post treatment and at three additional times over 24 hours. Probe measurements were taken at the axilla, the mid femur, the umbilicus and over the sternum using the Inspector 1000 portable radiation detector (Canberra Industries, Meriden, Connecticut).
Radiation Dosimetry
Dosimetry data included measurements of fraction of administered 212Pb activity remaining in the whole body, blood serum, peritoneal cavity (injection site), and urine for various time points post-infusion. These data represented the decay-corrected (biological) retentions. Whole body imaging did not demonstrate significant radionuclide uptake in any major organ or tissue for separate quantization. The standard MIRD dosimetry approach was used to calculate organ doses, as follows (18), (19). Uptake and retention in the peritoneal cavity and in the whole body were plotted. Mathematical functions were fit to the effective (not decay-corrected) data. The remainder of whole body activity, assumed to be distributed uniformly at low levels in all other organs and tissues, was determined by subtracting activity in the peritoneal cavity from that in the total body. The functions representing the time-activity curves were then integrated to determine the total disintegrations assigned to the major source regions. Correction were made for patient weight, then the radiation absorbed doses to the major organs and the whole body were calculated using the software OLINDA- Organ Level Dosimetry, Internal Dose Assessment Code (Version 1.1, copyright Vanderbilt University, 2007) (20).
The dynamic bladder model was not used; the bladder wall was assumed to be part of the “remainder tissues,” and only gamma cross-organ dose was considered.
Dosimetry Assumptions
Administered activity in the peritoneal fluid lies external to the organs, with radioactivity moving by gradual transfer via blood to the circulatory system and urinary excretion. Blood is a transfer compartment that is uniformly distributed in every organ or tissue and the whole body, and does not represent a separate organ or tissue. Therefore, circulating radioactivity imparts a radiation dose to the remainder of whole body outside the peritoneal cavity before excretion. A second component of dose involves gamma radiation to remainder of whole body from activity in the peritoneal fluid. We calculated radiation doses for the alpha, beta, and photon components of 212Pb through its complete decay chain. 212Pb resides in equilibrium with its daughters at all time points; daughters include 212Bi, 212Po, and 208Tl (Figure 1) (21). The equilibrium dose constant for 212Pb in equilibrium with daughter products is 18.061 (g-cGy/μCi-hr) or 5.38×10-10 kg-Gy/Bq-hr (= 4.96×10-8 kg-Gy/Bq through complete decay). Radiation dose to the peritoneal fluid was calculated by integrating the total number of radioactive transformations, converting to energy units, and dividing by the mass using standard absorbed dose equations and accounting for all emissions for 212Pb/212Bi and their daughter products through complete decay.
Figure 1.
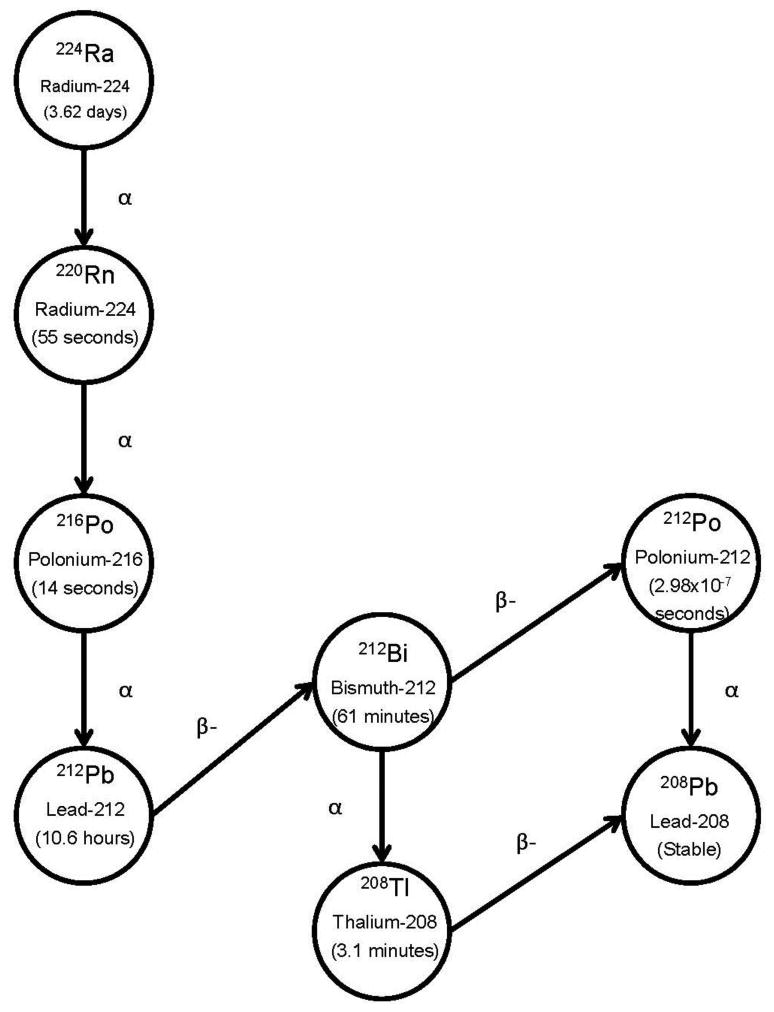
The decay scheme of 212Pb includes abundant alpha emissions of 6.1- and 8.8MeV, respectively, from its daughters 212Bi and 212Po.
Toxicity monitoring
The patients were followed for toxicity as defined in the Common Terminology Criteria for Adverse Events (NCI CTCAE v.4.03). As a precautionary measure (based on prior studies of other alpha-emitter conjugates) adjuvant medications were initially used. A saturated solution of potassium iodide (SSKI) was initiated the evening before treatment and continued for 3 days. Furosemide (40mg) was also started the day before 212Pb-TCMC-trastuzumab and used for 10 days, then followed by spironolactone (100mg) daily for 6 months as renal protective agents. After 10 patients, the SSKI was discontinued and the diuretic use was shortened. ELISA testing of 6 week serum samples was performed to determine if there was any evidence of an immune response to TCMC-trastuzumab.
Patient Population
Sixteen patients with HER-2 expressing malignancies who had failed standard therapy were treated at five dose levels. Fifteen of the 16 patients were females with recurrent ovarian cancer (or primary peritoneal carcinomatosis) and the only male patient, #6, had colon cancer. Patient #15 was African American; all others were Caucasian of age 46-83 and had undergone definitive surgery plus standard adjuvant chemotherapy as initial treatment. After relapse, patients had a variety of other therapies as salvage prior to 212Pb-TCMC-trastuzumab. All patients met the eligibility criteria as described above (or described as exception below); some had symptomatic ascites, but only one had required paracentesis for relief.
Investigational Agent 212Pb-TCMC-trastuzumab
Trastuzumab is an FDA-approved humanized monoclonal antibody (Genentech) which has therapeutic efficacy by immunologic mechanisms in tumors that over-express the HER-2 receptor (22). TCMC-Trastuzumab was provided in a form for further manufacturing of an investigational drug product for human use. Radiolabeling and release testing was performed at UAB. Details of manufacturing and quality assurance have been reported (17).
Results
Three to four patients were treated at each of 5 dose levels (7.4, 9.6, 12.6, 16.3, 21.1 MBq/m2) with 30% increase per level. Although there was no toxicity that triggered expansion of any cohort to more than three patients, the administered activity for patient #8 fell short of the desired level. Since the administered activity was consistent with that of the prior cohort, she was added as a fourth patient to cohort 2.
Toxicity Profile
The IP 212Pb-TCMC-trastuzumab treatment was well tolerated with most of the adverse events attributed to other medications and conditions rather than the investigational agent. Complaints were considered unrelated if ongoing prior to therapy and not worsened, or if they developed post therapy but were more likely related to other medications and conditions. The majority of early adverse events not present pre-therapy were related/attributed to the adjuvant diuretics or IV trastuzumab, rather than the investigational agent212Pb-TCMC-trastuzumab. All patients had placement of a temporary 8 French catheter that was removed less than one week post therapy. One patient had perforation of the bowel at catheter placement; this patient required hospitalization for peritonitis which delayed her therapy for one week. No sequelae were observed in the subsequent six months.
Table 1 lists the grade of each adverse event for individual patients as related, unrelated or of unknown relationship to study drug and the total percent (%) affected at the right. For example, unrelated Grade 1 abdominal pain or tenderness was noted in three patients and Grade 2 in one patient. 212Pb-TCMC-trastuzumab related Grade 1 was noted in two patients, for a total of 37.5% affected.
Table 1. Severity and Relation of Adverse Events to Investigational Agent 212Pb-TCMC-trastuzumab *.
| Event | Unrelated | Related | Unknown | % affected |
|---|---|---|---|---|
| Abdominal Pain/Tenderness | 1,1,1,2 | 1,1 | 37.5 | |
| Infection | 2, 2, 3, 3, 3 | 38 | ||
| Rash Or Skin Burn | 1,1,2,2, 3 | 31 | ||
| Nausea | 1,1,1,2 | 25 | ||
| Short Of Breath | 1,3, 3,3,3 | 31 | ||
| Fatigue | 1,1,2 | 18.75 | ||
| Short Of Breath | 1,3, 3 | 18.75 | ||
| Small Bowel Obstruction | 3 | 18.75 | ||
| Fever/Chills | 1 | 12.5 | ||
| Decreased Appetite | 1 | 12.5 | ||
| Diarrhea | 1 | 1 | 12.5 | |
| Muscle Cramps | 2 | 6 | ||
| Cutaneous Fistula | 3 | 6 | ||
| Thrombosis | 2 | 6 | ||
| Perforation With Peritonitis | 3 | 6 | ||
| Facial Flushing | 1 | 6 | ||
| Renal Stone | 3 | 6 | ||
| Weight Loss | 1 | 6 | ||
| Sore Throat | 1 | 6 | ||
| Indigestion | 1 | 6 | ||
| Sinus Congestion | 2 | 6 |
For the patients with small bowel obstruction, associated symptoms including nausea, vomiting, flatus and abdominal pain are not reported separately unless they occurred at another time.
In addition to the adverse events in Table 1, five patients developed asymptomatic, transient elevation in one or more liver function tests in the initial 6 weeks, while #14 developed elevation that persisted until drainage of fluid around the liver. These enzyme changes were considered related because no other etiology was judged to be more likely. However, the abnormality occurred with an episode of dehydration in patient #9, with steatosis in #11 and cleared in #14 after drainage of fluid around the liver. Three others had per-existing enzyme elevations but Patient #5 is the only one with continued, progressive abnormalities that coincided with an increase of her liver metastases. Four patients had other laboratory abnormalities including hyper- or hypokalemia, hypomagnesemia, hyponatremia and elevated creatinine that were judged unrelated to investigational agent but most were likely related to diuretics and dehydration. One patient also had an unrelated Grade 3 coagulopathy.
Initial 6 week renal monitoring showed transient, increased creatinine to 1.4mg/dL in an 80 year old patient and 1.3 in a 74 year old who became dehydrated. Monitoring renal function up to 1 year for late toxicity revealed one patient who had elevation of creatinine to 1.4mg/dL after prolonged hydronephrosis from renal and ureteral stones; she subsequently had stent placement and creatinine returned to normal. One patient, #5, was allowed to be treated despite recently elevated creatinine because she had undergone stent placement for relief of ureteral obstruction. Her creatinine returned to normal by five weeks post-treatment. No proteinuria was observed. Regarding hematologic toxicity, six of the 15 females had Grade 1-2 anemia prior to treatment with hemoglobin 9.8-11.9 g/dL. Four of the six remained anemic at 6 weeks. Overall, the majority had the same or higher hemoglobin levels at 6 weeks.
One patient had a sub-normal white blood cell count (WBC) pre-treatment and one other patient in cohort 2 developed a transient WBC drop (without neutropenia) to 3500/μL at week 4. A summary of neutropenia monitoring post-treatment is presented in Figure 2 as the mean of all 16 patients compared to the upper and lower limits of normal. The neutrophil and total WBC counts showed little variation and the mean remained within normal limits. Platelet levels were above the lower limits of normal in all patients pre-treatment and remained such at 6 weeks in 15 of the 16 patients. One patient developed a transient grade 1 thrombocytopenia drop to 142,000/μL on day 28 which normalized by day 35. The mean platelet counts for all patients are shown in Figure 2.
Figure 2.
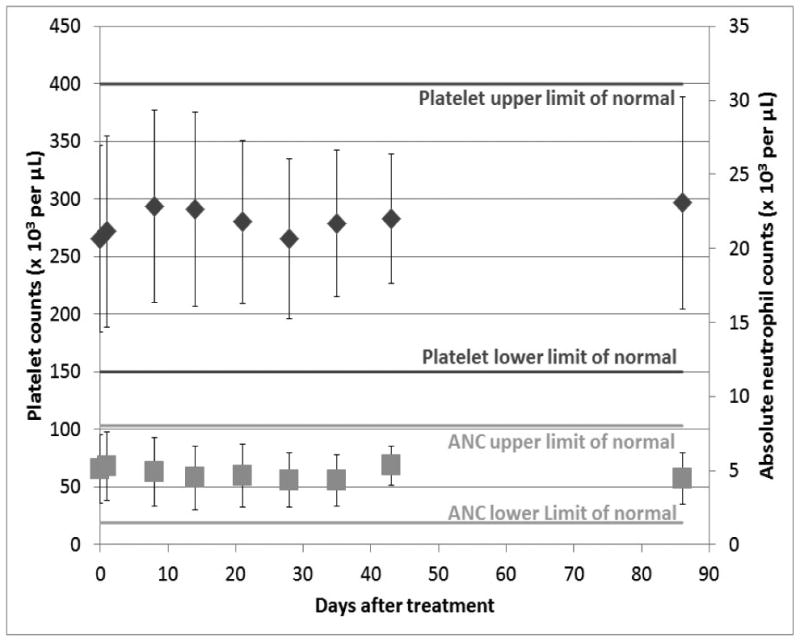
The absolute neutrophil counts and platelet counts (mean ± standard deviation) for all patients are compared over time of early follow-up. All means were within the normal range.
To date, follow-up cardiac toxicity monitoring has not shown any arrhythmia on electrocardiogram or decrease in ejection fraction by echo cardiography. One patient who had bilateral pleural effusions developed a small pericardial effusion at 12 weeks but ejection fraction did not drop.
No evidence of an immune response to the TCMC-trastuzumab was found in 11 patients at 6 weeks post therapy (3 did not have 6 week samples available and 2 remain to be tested). No patients had signs or symptoms of serum sickness at any time during follow-up.
Pharmacokinetics and Dosimetry
More extensive pharmacokinetics and imaging data were obtained on the first cohort as previously reported than for the current study (17). Whole body gamma camera images 18-24 hours after 212Pb-TCMC-trastuzumab infusion showed no visible redistribution of activity from the peritoneal cavity and no evidence of uptake in any major organ or tissue by direct counting. This observation of prolonged peritoneal cavity localization was confirmed by the serial body counts, which showed the highest external dose-rates over the abdomen. Dose rate measurements performed with hand-held instruments at 1m over the patient recorded less than 5μSv/hr (0.5mRem/hr) in latest cohort, significantly less than what is normally observed with nuclear medicine imaging agents.
Multiple post–treatment blood samples were obtained on the first cohort (n=3) up to 63 hours (more than 5 half-lives of 212Pb). Serum radioactivity levels for these patients were compared with 2- and 24 hour samples from each of the remaining patients in Figure 3. The activity range of all subsequent patients was similar to that of the first cohort. Maximum serum concentration of the radiolabeled antibody was 22.9% at 24 hours (decay corrected to injection time) and 500 Bq/mL (decay corrected to collection time). The four patients with the lowest serum radioactivity levels at 24 hours all had ascites. Ascites is usually associated with peritoneal carcinomatosis with tumor obstructing lymphatic channels such that the normal rate of flow from the abdominal cavity is diminished.
Figure 3.
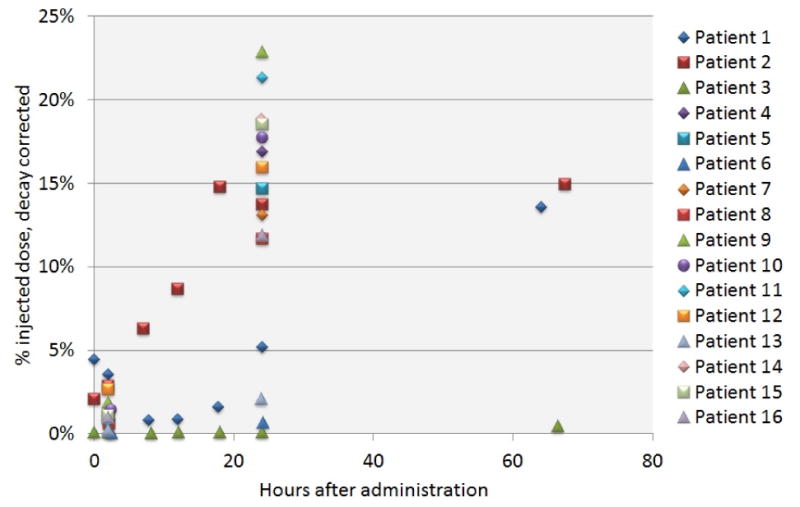
Serum levels of radioactivity after IP 212Pb-TCMC-trastuzumab are compared as decay corrected percent injected dose at 2- and 24h, and up to 63h for the 1st cohort.
The whole body 212Pb- retention data fit by linear least-squares analysis to a single exponential showed an effective (not decay-corrected) retention half-time of 9.56 to 10.4 hours and a biological half-time of 118 hours to infinity (r = 0.999) for time points within 24 hours. The peritoneal cavity 212Pb retention data fit a single exponential having an effective retention half-time of 8.8 to 10.4 hours and a biological half-time of 60.9 hours to infinity (r = 0.999). The integrated residence time (“normalized number of disintegrations” or “time-integrated activity coefficient”), the input value to OLINDA-EXM in Bq-hr/Bq, was found to be 12.8 to 15.0 hours.
The remainder of body activity was plotted by linear least-splines and was integrated. The tail of the remainder activity curve was fit to a single exponential having a maximum retention half-time of 10.6 hours (physical half-life of 212Pb), yielding integral area-under-curve residence time values of 0.015 hours to 1.323 hours.
Table 2 provides organ equivalent doses in units of mSv/MBq administered and include contribution from daughters 212Bi, 212Po, and 208Tl, assuming using an alpha particle relative biological effectiveness (RBE) of 3.0.
Table 2. Organ equivalent dose (mSv/MBq) for alpha RBE (weighting factor) = 3.0.
| Target Organ | Patient 001 | Patient 002 | Patient 003 | Average |
|---|---|---|---|---|
| Adrenals | 1.13E-01 | 5.64E-01 | 1.32E-02 | 2.30E-01 |
| Brain | 1.09E-01 | 5.58E-01 | 6.98E-03 | 2.25E-01 |
| Breasts | 1.09E-01 | 5.59E-01 | 7.60E-03 | 2.25E-01 |
| Gallbladder Wall | 1.32E-01 | 5.87E-01 | 3.99E-02 | 2.53E-01 |
| Lower Lg. Int. Wall | 1.41E-01 | 5.99E-01 | 5.43E-02 | 2.65E-01 |
| Small Intestine | 1.14E+00 | 6.39E-01 | 1.01E-01 | 6.27E-01 |
| Stomach Wall | 1.20E-01 | 5.73E-01 | 2.36E-02 | 2.39E-01 |
| Upper Lg. Int. Wall | 1.86E-01 | 6.48E-01 | 1.12E-01 | 3.15E-01 |
| Heart Wall | 1.10E-01 | 5.60E-01 | 8.78E-03 | 2.26E-01 |
| Kidneys | 1.20E-01 | 5.72E-01 | 2.32E-02 | 2.38E-01 |
| Liver | 1.15E-01 | 5.66E-01 | 1.58E-02 | 2.32E-01 |
| Lungs | 1.09E-01 | 5.59E-01 | 8.39E-03 | 2.25E-01 |
| Muscle | 1.15E-01 | 5.65E-01 | 1.53E-02 | 2.32E-01 |
| Ovaries | 1.59E-01 | 6.18E-01 | 7.64E-02 | 2.84E-01 |
| Pancreas | 1.16E-01 | 5.68E-01 | 1.82E-02 | 2.34E-01 |
| Peritoneal Fluid | 1.89E+02 | 3.52E+02 | 2.07E+02 | 2.49E+02 |
| Red Marrow | 9.32E-02 | 4.42E-01 | 1.79E-02 | 1.84E-01 |
| Osteogenic Cells | 3.86E-01 | 2.08E+00 | 3.51E-02 | 8.34E-01 |
| Skin | 1.10E-01 | 5.60E-01 | 9.31E-03 | 2.26E-01 |
| Spleen | 1.14E-01 | 5.65E-01 | 1.51E-02 | 2.31E-01 |
| Thymus | 1.09E-01 | 5.59E-01 | 7.50E-03 | 2.25E-01 |
| Thyroid | 1.09E-01 | 5.58E-01 | 7.06E-03 | 2.25E-01 |
| Urinary Bladder Wall | 1.20E-01 | 5.73E-01 | 2.43E-02 | 2.39E-01 |
| Uterus | 1.54E-01 | 6.10E-01 | 6.78E-02 | 2.77E-01 |
| Total Body | 2.54E+00 | 4.89E+00 | 5.57E+00 | 4.33E+00 |
As the actual activity per unit volume may be highly variable over time, only dose to a standard reference volume was provided. All values may be multiplied by the administered activity to obtain actual equivalent dose values. All results were rounded to three significant figures. The actual patient weight was used, and organ masses were assumed to match those of a standard reference phantom of similar weight.
Response Assessment
Tumor assessment has been followed by serial standard CT scans and tumor markers. Some measurable lesions decreased but no patient met criteria for a partial response by RECIST criteria at 6 weeks. Outcome in consecutive patients is compared to characteristics and details of therapy in Table 3. Tumor assessment by standard CT using 5mm slice thickness has not been ideal to assess response as most patient tumor sizes were less than 15mm, Comparing pre-treatment, weeks 4 and 6 post-treatment CA 125 levels did not correlate with other clinical parameters. The majority had increasing CA 125 levels despite some having regression of measurable disease. Only two patients had a consistent decrease and their levels fell within normal limits despite one having a large tumor burden and extensive ascites. CA125 decreased by week 12 for two patients who had elevated levels at 4 and 6 weeks, but no intervening additional therapy.
Table 3. Patient Characteristics Are Compared With Outcome.
| MBq infused | Age (y) | No. of Chemotherapy regimens* | Ascites | Largest tumor (cm) (includes nodes) | No. of masses > 1 cm | HER-2 immuno-histochemistry score | ELISA | Response 6 wk | Mo. Since treatment |
|---|---|---|---|---|---|---|---|---|---|
| 15 | 46 | 5 | limited | 3.4 | 5 | 2+; >30% | 10.3 | S | 9† |
| 11 | 67 | 5 | limited | 2.8 | 5 | 1+; >30% | N/A | S | 24 |
| 11 | 83 | 3 | large | 4 | 2 | 2+; >30% | 9.6 | P | 14† |
| 13 | 60 | 3 | 0 | 1.8 | 5 | 1+; >30% | 12.3 | S | 18 |
| 17 | 62 | 1 | 0 | 2.5 | 3 | 2+; >30% | 13.7 | P | 2† |
| 21 | 77 | 2 | large | 2.9 | 1 | 1+; <10% | 14.6 | P | 2† |
| 21 | 66 | 9 | 0 | 2.6 | 4 | 1+; >30% | 12.3 | S | 11† |
| 19 | 59 | 4 | 0 | 4.3 | 3 | 1+; >30% | 14.7 | S | 14 |
| 25 | 80 | 2 | 0 | 7.2 | 5 | 1+; >10% | 13.7 | S | 4† |
| 19 | 71 | 3 | 0 | ne | 0 | 1+; >30% | 12.2 | S | 11 |
| 31 | 54 | 8 | 0 | 3.9 | 4 | 1+; >30% | N/A | S | 9 |
| 28 | 68 | 5 | 0 | 4.5 | 2 | N/A | 16.4 | P | 9 |
| 31 | 72 | 2 | large | 5.1 | 5 | 1+; >30% | 18.9 | S | 8 |
| 39 | 59 | 4 | limited | 1.2 | 3 | 1+; >30% | 15.2 | S | 5 |
| 40 | 73 | 2 | 0 | 1.5 | 1 | 2+; >10% | N/A | S | 4 |
| 38 | 61 | 1 | 0 | 1.3 | 2 | 1+; >30% | 9.2 | S | 4 |
Number of chemotherapy cycles ranged from 6 to 64. Most common regimens contained taxane or platinum, followed by anthracycline, gemcitabine, and topotecan as cytotoxic chemotherapy. Some regimens included bevacizumab, other antibody, vaccine, molecularly targeted inhibitor, or adenoviral vector therapy. Hormonal agents were not considered as regimens.
Deaths.
S = stable; P = disease progression; NA = not applicable.
Discussion
This first-in-human Phase I trial of intraperitoneal 212Pb-TCMC-trastuzumab provides safety data and extensive analyses of radioimmunoconjugate biodistribution/pharmacokinetics and radiation dosimetry, which are consistent with prior non-human model results (23, 24). We observed limited redistribution of 212Pb-TCMC-trastuzumab out of the peritoneal cavity and minimal toxicity with five dose level escalation. Thus, this study validated projected medical application, and feasibility of on-site manufacture for final product using a radionuclide that requires international shipment.
The rate of the conjugate traversing into the systemic circulation from the peritoneal cavity was found to be consistent with that previously demonstrated after IP administration of other antibody radionuclide conjugates (3, 9, 14, 25). However, due to the quicker decay of 212Pb, the amount of radioactivity arriving in the systemic circulation was much less than that of most other agents. This was expected, and was anticipated to result in less hematologic toxicity than that observed previously with beta-emitter conjugates (12).
In this study, we found no significant myelosuppression; only one patient each developed grade 1 leukopenia (nadir WBC=3500/ μL, without neutropenia) and grade 1 thrombocytopenia (platelet nadir=142,000/ μL).
The absorbed radiation dose per MBq administered for organs reported here is comparable to those of the non-human primate study with 212Pb-TCMC-trastuzumab where tissue samples were available for correlation with the gamma scans and body counts (21). The relatively low radiation dose reported here from the dosimetry calculations were consistent with the minimal toxicity observed. These data are closely comparable to dosimetry reported after IP administration of another alpha-emitter-radionuclide conjugate, 211At-Mx (Fab') 2(14) (15).
Neither CA125 nor CEA were helpful in assessing response. CEA was within normal limits for all 15 ovarian cancer patients. CA125 was abnormal in most of these patients but did not correlate with change in tumor size which one might hypothesize could have been due to RIT related peritoneal inflammation as mesothelial cells are known to be a source of CA-125 found in ascites fluid (26) (27). The intraperitoneal radioimmunotherapy may have resulted in sufficient surface disturbance to elevate the CA125 level even if there was a therapeutic effect. In the future, newer markers specific to ovarian cancer may also contribute to efficacy evaluation (28).
Two patients had disease progression with bowel obstruction; the remainder maintained or improved their functional status during the initial 6-week follow-up. The maximum benefit of IP alpha emitter therapy is expected to be for microscopic disease whereas monitoring in this study was limited by measures of gross disease. Efficacy determined by reduction in size of measurable masses and prolonged progression free outcome should be sufficient to allow additional study to optimize this form of therapy
The initial patients in this study received thyroid protection with SSKI. This requirement was based on experience of others with the alpha-emitter 211At where thyroid uptake without blocking was higher than any normal organ or tumor after IP administration (14). In the 211At study, the thyroid uptake was not unexpected due to the observed properties of 211At that are consistent with a heavier analog of iodine. The effect of blocking in later patients, reduced the uptake of 211At by >15 fold compared to the initial patients who did not receive blocking. Thyroid uptake was not predicted in the current trial due to the basic 212Pb properties, and had not been observed in animal studies but was included as a precautionary measure in the initial patients. After gamma imaging of the initial patients confirmed no evidence of uptake, and thyroid function showed no change, the adjuvant SSKI was discontinued.
Conclusions
Overall, the properties of 212Pb-TCMC-trastuzumab were expected to provide more potent radiation to targeted malignant cells, while limiting radiation exposure to normal tissues as compared to beta-emitter conjugates, due to the shorter half life and path length of 212Pb alpha radiation. This first in human dose escalation study confirmed the predicted dosimetry and low toxicity. These results were consistent with the prior animal model studies and human experience with alpha-emitter 211At-conjugate. Further study plans for dose escalation and evaluation of optimal timing/integration and potential synergy with other treatment modalities.
Acknowledgments
Appreciation is also expressed to Lolinda Brown, Andres Forero, Charles Landen, Ronald Alvarez, Daniel Yoder, Souheil Saddekni, Denise Charlotte Jeffers, Kurt Zinn, Mack Barnes, Robert Oster, Martin Brechbiel, Christine White and the late Michael Azure for their contributions
Grant support: Sponsored by AREVA Med and NIH CCTS grant 1UL1RR025777
Research Support: AREVA Med, NIH 1UL1RR025777-01
Footnotes
No Conflict of Interest for: Ruby Meredith, Sui Shen, Patty Bunch, Desiree Morgan, J. Michael Straughn Jr. or Jinda Fan. Darrell Fisher has served on the scientific Advisory Board; Eileen Banaga and Julien Torgue are employed by the sponsor of the clinical trial (AREVA Med LLC, Bethesda, MD.
References
- 1.Aarts F, Bleichrodt RP, Oyen WJ, Boerman OC. Intracavitary radioimmunotherapy to treat solid tumors. Cancer Biother Radiopharm. 2008 Feb;23:92–107. doi: 10.1089/cbr.2007.0412. [DOI] [PubMed] [Google Scholar]
- 2.Epenetos AA, Hird V, Lambert H, Mason P, Coulter C. Long term survival of patients with advanced ovarian cancer treated with intraperitoneal radioimmunotherapy. Int J Gynecol Cancer. 2000 Jan;10:44–46. doi: 10.1046/j.1525-1438.2000.99510.x. [DOI] [PubMed] [Google Scholar]
- 3.Meredith R, You Z, Alvarez R, Partridge E, Grizzle W, LoBuglio A. Predictors of long-term outcome from intraperitoneal radioimmunotherapy for ovarian cancer. Cancer Biother Radiopharm. 2012 Feb;27:36–40. doi: 10.1089/cbr.2011.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oei AL, Verheijen RH, Seiden MV, Benigno BB, Lopes A, Soper JT. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine Hmfg1 without improvement in overall survival. IntJCancer. 2007;120:2710–2714. doi: 10.1002/ijc.22663. [DOI] [PubMed] [Google Scholar]
- 5.Vergote IB, Vergote-De Vos LN, Abeler VM, et al. Randomized trial comparing cisplatin with radioactive phosphorus or whole-abdomen irradiation as adjuvant treatment of ovarian cancer. Cancer. 1992 Feb 1;69:741–749. doi: 10.1002/1097-0142(19920201)69:3<741::aid-cncr2820690322>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Young RC, Brady MF, Nieberg RK, et al. Adjuvant treatment for early ovarian cancer: a randomized phase III trial of intraperitoneal 32P or intravenous cyclophosphamide and cisplatin--a gynecologic oncology group study. J Clin Oncol. 2003 Dec 1;21:4350–4355. doi: 10.1200/JCO.2003.02.154. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson S, Gooden CS, Hird V, et al. Radioimmunotherapy after chemotherapy compared to chemotherapy alone in the treatment of advanced ovarian cancer: a matched analysis. Oncol Rep. 1998 Jan-Feb;5:223–226. doi: 10.3892/or.5.1.223. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez RD, Huh WK, Khazaeli MB, et al. A phase I study of combined modality (90)Yttrium-CC49 intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res. 2002 Sep;8:2806–2811. [PubMed] [Google Scholar]
- 9.Alvarez RD, Partridge EE, Khazaeli MB, et al. Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: a phase I/II study. Gynecol Oncol. 1997 Apr;65:94–101. doi: 10.1006/gyno.1996.4577. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JS, Hird V, Snook D, et al. Intraperitoneal radioimmunotherapy for ovarian cancer: pharmacokinetics, toxicity, and efficacy of I-131 labeled monoclonal antibodies. Int J Radiat Oncol Biol Phys. 1989 Feb;16:405–413. doi: 10.1016/0360-3016(89)90337-4. [DOI] [PubMed] [Google Scholar]
- 11.Macey DJ, Meredith RF. A strategy to reduce red marrow dose for intraperitoneal radioimmunotherapy. Clin Cancer Res. 1999 Oct;5:3044s–3047s. [PubMed] [Google Scholar]
- 12.Elgqvist J, Frost S, Pouget JP, Albertsson P. The potential and hurdles of targeted alpha therapy - clinical trials and beyond. Front Oncol. 2014 Jan 14;3:324–329. doi: 10.3389/fonc.2013.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baidoo KE, Yong K, Brechbiel MW. Molecular pathways: targeted alpha-particle radiation therapy. Clin Cancer Res. 2013 Feb 1;19:530–537. doi: 10.1158/1078-0432.CCR-12-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson H, Cederkrantz E, Back T, et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of (211)at-Mx35 F(Ab')2--a phase I study. J Nucl Med. 2009 Jul;50:1153–1160. doi: 10.2967/jnumed.109.062604. [DOI] [PubMed] [Google Scholar]
- 15.Cederkrantz E, Back T, Jacobsson L, et al. Effective dose of intraperitoneal {alpha}-radioimmunotherapy with 211-At. J NUCL MED MEETING ABSTRACTS. 2014 May 1;55:214. 2014. [Google Scholar]
- 16.Palm S, Back T, Lindegren S, Jensen H, Albertsson P, Jacobsson L. Modelling the biokinetics of intra-peritoneally administered targeted alpha therapy on patients for improved dosimetry. J NUCL MED MEETING ABSTRACTS. 2014 May 1;55:1131. 2014. [Google Scholar]
- 17.Meredith RF, Torgue J, Azure MT, et al. Pharmacokinetics and imaging of 212pb-Tcmc-Trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother Radiopharm. 2014 Feb;29:12–17. doi: 10.1089/cbr.2013.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loevinger R, B TF, Watson EE. Mird primer for absorbed dose calculations, Revised Edition. The Society of Nuclear Medicine. 1991 1991. [Google Scholar]
- 19.Bolch WE, Eckerman KF, Sgouros G, Thomas SR. Mird Pamphlet No. 21: A generalized schema for radiopharmaceutical dosimetry--standardization of nomenclature. J Nucl Med. 2009 Mar;50:477–484. doi: 10.2967/jnumed.108.056036. [DOI] [PubMed] [Google Scholar]
- 20.Stabin MG, Sparks RB, Crowe E. Olinda/Exm: The second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005 Jun;46:1023–1027. [PubMed] [Google Scholar]
- 21.Azure M. Radiolabeling and imaging of 212pb-TCMC-trastuzumab. Paper presented at: World Molecular Imaging Congress; Kyoto, Japan. 2010. [Google Scholar]
- 22.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of Her2-positive advanced gastric or Gastro-oesophageal junction cancer (Toga): a phase 3, open-label, randomised controlled trial. Lancet. Aug 28;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 23.Milenic D, Garmestani K, Dadachova E, et al. Radioimmunotherapy of human colon carcinoma xenografts using a 213Bi-labeled domain-deleted humanized monoclonal antibody. Cancer Biother Radiopharm. 2004 Apr;19:135–147. doi: 10.1089/108497804323071904. [DOI] [PubMed] [Google Scholar]
- 24.Milenic DE, Baidoo KE, Shih JH, Wong KJ, Brechbiel MW. Evaluation of platinum chemotherapy in combination with Her2-targeted alpha-particle radiation. Cancer Biother Radiopharm. 2013 Jun 11;28:441–449. doi: 10.1089/cbr.2012.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenblum MG, Kavanagh JJ, Burke TW, et al. Clinical pharmacology, metabolism, and tissue distribution of 90Y-labeled monoclonal antibody B72.3 after intraperitoneal administration. J Natl Cancer Inst. 1991 Nov 20;83:1629–1636. doi: 10.1093/jnci/83.22.1629. [DOI] [PubMed] [Google Scholar]
- 26.Moss EL, Hollingworth J, Reynolds TM. The role of Ca125 in clinical practice. J Clin Pathol. 2005 Mar;58:308–312. doi: 10.1136/jcp.2004.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarandakou A, Protonotariou E, Rizos D. Tumor markers in biological fluids associated with pregnancy. Crit Rev Clin Lab Sci. 2007;44:151–178. doi: 10.1080/10408360601003143. [DOI] [PubMed] [Google Scholar]
- 28.Shapira I, Oswald M, Lovecchio J, et al. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br J Cancer. 2013 Dec 24;110:976–983. doi: 10.1038/bjc.2013.795. [DOI] [PMC free article] [PubMed] [Google Scholar]


