Abstract
Purpose
To compare the clinical outcomes in patients with chronic renal insufficiency (CRI) and renal artery stenosis (RAS) following renal artery (RA) stent placement with and without embolic protection device (EPD) usage.
Materials and Methods
Eighteen patients who had RA stent placement with EPD were matched to control patients (RA stent only). Blood pressure, number of hypertensive medications, and estimated glomerular filtration rate (eGFR) at 3 months before the procedure and after 12 months were determined. An increase of ≥ 20% in eGFR at 12 months from baseline was defined as “improvement,” decrease of ≥20% as “deterioration,” and an eGFR change between those values as “stabilization” at 12 months.
Results
At 12 months, stage 4 patients treated with EPD had significantly higher eGFR than controls (P = .01). There was no statistical difference in blood pressure outcomes between the 2 groups.
Conclusions
Patients with stage 4 CRI did significantly better with EPD than those treated without it.
Keywords: renal artery stenosis, embolic protection disease, clinical outcomes
Introduction
The primary therapy for the treatment of symptomatic renal artery stenosis (RAS) has become percutaneous renal artery angioplasty with stent placement. The reported procedural success rates are 98% to 100%.1 Despite these excellent technical results, predicting which patients will benefit from improvement in renal function after renal artery stent placement is difficult. Several studies have reported that kidney function will improve in 30% to 40%of the patients, stabilize in another 30%to 40%of patients, and decrease in 20% to 30% of patients.2,3 A number of explanations have been given including deterioration in kidney function, contrast medium-induced nephrotoxicity, progressive concomitant nephrosclerosis from multiple cardiovascular risk factors, diffuse distal atherosclerosis, and atheroembolism.4
It is thought that atheroembolism is caused by the release of microscopic plaque fragments and cholesterol crystals from the RAS or atherosclerotic aorta.4,5 Such releases can occur during manipulation of the catheter in the suprarenal aorta or while crossing the RAS that results in subsequent embolization into the renal parenchyma.4,5 A number of studies have suggested that postprocedural renal function decrease is caused by intraprocedure and postprocedural atheroembolic complications.4 The advent of newer balloons and stents on low profile system such as a 0.014-in platform may reduce the risk of atheroembolism.
Embolic protection devices (EPDs) have been employed to prevent atheroembolism when treating stenosis involving saphenous vein bypass grafts that are being used as coronary arterial conduits and during carotid artery stent placement.6 Several reports have suggested that these devices may prevent atheroembolism during renal artery stent placement.7–18 These studies have shown that technical feasibility and early clinical outcomes of renal and hypertensive responses in patients undergoing renal artery stent placement with concomitant use of EPD are good. There has been one randomized study Prospective Randomized Study Comparing Renal Artery Stenting With/Without Distal Protection (RESIST) performed comparing stent placement with EPD and use of abciximab in patients with stage 2 and stage 3 disease which found that stenting plus EPD with abciximab was significantly better than the other 3 groups but no difference between EPD and non-EPD groups was noted.17 Furthermore, only one case control-matched study has been performed using the Angioguard EPD in patients with RAS and worsening renal function compared with patients who were stented for the same indication without the use of an EPD and found increased benefit in kidney function.8 In the present study, we present our clinical outcomes of kidney function and hypertensive response after the placement of renal artery stent placement in patients who received concomitant EPD compared with a matched group of patients who underwent renal artery stent placement without the use of an EPD for renal insufficiency (Kidney Disease Outcome and Quality Initiative [KDOQI] stage 3 or worse) and followed for a minimum of 1 year.
Materials and Methods
Patient Selection, Definitions, and End Points
Our institutional review board approval was obtained prior to conducting this study. A retrospective review was conducted for all patients who had chronic renal insufficiency (CRI) and RAS that underwent renal artery angioplasty/stent placement with concomitant use of an EPD between June 2005 and September 2009. The primary end points were changes in estimated glomerular filtration rate (eGFR), proteinuria, and hypertension at time of follow-up obtained through our medical records ending in January 2011.
Each of the patients (n = 25) who had CRI with RAS with a renal artery stent placed with the use of an EPD was matched to patientswho had CRI and underwent renal artery angioplasty/stent placement without the use of an EPD. The following criteria were used to match the patients: patients had the same gender, presence of diabetes (type II in all patients), age within 5 years, eGFR with in 5mL/min when calculated using the Modification of Diet in Renal disease (MDRD) equation (see below), and matching high or low proteinuria in the urine (high was defined as estimated 24 hour proteinuria above 300 mg/24 hours), measured at maximum 6 months before the procedure. In order to ensure similar lengths of follow-up, patients were matched based on when the procedure was done, typically within 8 months of each other.
Matches for 5 patients could not be obtained for the following reason and were excluded from the present study: 2 patients were younger than 50 years old and there were no patients who underwent treatment of RAS of the same age during the same time period. Three patients could not be matched because we could not identify patients with similar eGFRs during the same time period, leaving 18 in the EPD group and 18 in the control group.
The severity of preinterventional renal impairment was classified using the KDOQI by eGFR calculated using MDRD equation: eGFR = 186 × serum creatinine−1.154 × age−0.203 × 0.742 (females) ×1.210 (African Americans).20 For the purposes of a more detailed analysis, we divided class 3 into 3A (eGFR 45–59 mL/min per 1.73 m2) and 3B (eGFR 30–44 mL/min per 1.73 m2).20 After revascularization, an increase of ≥ 20% in eGFR from baseline was defined as “improvement,” decrease of >20% in eGFR from baseline as “deterioration,” and a change less than 20% in eGFR was defined as “stabilization.”
Technical success of the stent procedure was defined as less than 30% residual stenosis by visual estimation after deployment. Technical success of the EPD was defined as successful deployment of the device, retrieval of the device, and adequate filtration. This was reported as the number of successful EPD deployments per number of total attempted EPD deployments. “Complete protection” of the kidney was defined as complete protection of the renal artery with the device being deployed in the main renal artery. Partial protection of the kidney was when the device was deployed in the main branch of the renal artery. All complications were recorded according to the guidelines published by the Society of Interventional Radiology.21
Renal Duplex Ultrasound
Renal duplex ultrasound was used for screening and quantification of the severity of the RAS.21 Baseline and follow-up studies were performed with a 2.5 to 4.5 MHz phased array transducer (128/XP; Acuson, Mountain View, California or Sequoia 512; Siemens, Erlangen, Germany). Renal artery stenosis or restenosis was defined on ultrasound using angle-corrected peak systolic velocity aortic velocity ratios (aortic velocity measured near the origin of the renal artery) at a Doppler insinuation angle not greater than 60°.21 A peak systolic velocity greater than 180 cm/s or ratios greater than 3.5 ratio indicated an RAS of at least 60%. Average of 2 to 3 intrarenal segmental artery indices were used to calculate the mean index.
Renal Artery Stent Placement
All patients were admitted to the hospital the day before the procedure for overnight hydration with bicarbonate and or oral N-acetylcysteine (600 mg twice daily, Mucomyst, Bristol-Myers Squibb, Princeton, New Jersey); unless patients had contraindications, such as congestive heart failure or asthma, patients were prehydrated with oral fluids or intravenous crystalloids with or without N-acetylcysteine to prevent potential nephrotoxic effects of contrast medium.22 Prior to angiography, informed consent was obtained from the patient for potential renal angioplasty and stent implantation. All procedures were performed with use of the same technique. If the patient had magnetic resonance angiography or computed tomography angiography (CTA) imaging demonstrating the renal arteries, only selective angiography was performed, otherwise we performed abdominal aortogram followed by selective renal artery angiography. The right common femoral artery was accessed with 18 gauge, 7-cm angiographic needle (Cook, Bloomington, Indiana). A 6F or 7F vascular sheath (Terumo, Somerset, New Jersey) was placed in the artery over a Bentsen guide wire (Cook). A 6F or 7F internal mammary guide catheter (Boston Scientific, Natick, Massachusetts) was used to engage the origin of the renal artery. Angiography was performed with 4 to 8 mL of Visipaque 320 (GE Healthcare, Princeton, New Jersey) or 4 to 8 mL of gadopentetate dimeglumine (Magnevist, Bayer, Wayne, New Jersey) to determine the extent of stenosis. Gadolinium was used before the 2006 Food and Drug Administration (FDA) warning and the reports of its association with nephrogenic fibrosing dermopathy in patients with reduced kidney function.23 Patients were typically given a bolus of heparin intravenously (50 U/kg of body weight) prior to the placement of the stent. Renal artery stenosis was defined as a diameter reduction of greater than 60% by visual estimate when compared to its immediate, distal nondilated main renal arterial segment at the time of intervention as defined by the guidelines published by the Society of Interventional Radiology.21 Renal artery stenosis was defined as “ostial” if they were located within less or equal to 5-mm distance away from renal artery origin from the aorta and defined as “nonostial” if it was located more than 5-mm away from renal artery origin.
Embolic Protection Devices Used
Embolic protection devices were selected and used at the discretion of the operator. The first 8 patients had the FilterWire EZ (Boston Scientific, Natick, Massachusetts) as their EPD. FilterWire EZ has one size and can be used in reference vessel diameters of 3.5 to 5.5 mm with a pore size of 120 to 180 µm. The next 10 patients had SpideRX as their EPD. The SpideRX (ev3, Plymouth, Minnesota) can be used for reference vessel diameters of 2.5 to 7.0 mm with a pore size of 180 to 300 µm.
When using the FilterWire EZ device, the device was manipulated across the stenosis and then unsheathed distally to the RAS, and the filter was allowed to appose the vessel wall by allowing it to be manipulated into a distal renal artery. If the device could not be advanced across the stenosis, the “buddy wire technique” was used, with a second 0.014-in wire advanced across the stenosis to provide support to deliver the device. A Reflex wire (Cordis, Miami, Florida) was typically used to cross the RAS when using the SpideRX device and the device was brought on the Reflex wire and deployed distal to the stenosis. Diameters of the devices were typically greater than the diameter of the nondiseased artery. Thus, 5 mm devices were used for renal artery diameters less than 5 mm.
Diameters of the device and stent were chosen based on the visual estimate of the diameter of the nondiseased renal artery distal to the stenosis. Pressure gradients were only measured if there was question of lesion severity. A mean gradient of 10 mm Hg measured simultaneously between aorta and distal renal artery or a systolic gradient of more than 20 mm Hg was considered hemodynamically significant measured simultaneously using an 0.018-in microcatheter (Turbo catheter, Terumo, Tokyo, Japan) distal to the RAS and the 6F guide in the aorta proximal to the RAS.21 Follow-up angiograms were obtained after stent inflation to the diameter of nondiseased renal artery. The device was removed and captured completely with the catheter proved for that purpose and was then visually examined for any debris.
After the procedure, patients were given 300 mg oral clopidogrel (Bristol-Meyers Squibb/ Sanofi, Bridgewater, New Jersey) after the sheath was removed and then started on clopidogrel, 75 mg/d for a minimum of 3 months.
Control Group
Patients undergoing stent placement for RAS went through the same procedure as noted above for EPD patients except for the deployment and retrieval of the EPD.
Follow-Up
Follow-up was advised at 3-, 6-, 12-month intervals after the procedure. Manual brachial blood pressure measurement was performed in the sitting position by taking the average of 6 blood pressures and the number and type of blood pressure medications were recorded. Serum creatinine and blood urea nitrogen were used to evaluate the renal function.
Statistical Methods
Categorical variables are expressed as ratios and percentages. Continuous variables are expressed as means ± standard deviation or medians with ranges. Statistical comparison for continuous variables before and after renal artery intervention was performed using a Student t test. P ≤ .05 was considered statistically significant. SAS software (version 9, SAS, Cary, North Carolina) was used for all statistical analyses.
Results
Study Groups
Patient demographics are included in Table 1. There were no major differences between the EPD and control groups with respect to several important factors including coronary artery disease, hypertension, and CRI. However, there were significant differences in the incidence of peripheral atherosclerotic disease which was higher in the control group (P = .02) and the incidence of history of smoking that was higher in the EPD group (P = .01).
Table 1.
Demographics of the EPD and Control Groups
| EPD Group, N (%) |
Control Group, N (%) |
P Value |
|
|---|---|---|---|
| Total patients | 18 | 18 | NS |
| Number of males | 14 (78) | 14 (78) | NS |
| Age at intervention (years old) | 73 ±7 | 73 ±7 | NS |
| Caucasian | 18 (100) | 18 (100) | NS |
| Risk factors | |||
| Family history of CAD | 10 (56) | 14 (78) | NS |
| Smoking | 13 (72) | 5 (28) | .01 |
| Hypertension | 17 (945) | 16 (89) | NS |
| Chronic renal insufficiency | 18 (100) | 18 (100) | NS |
| Diabetes | 9 (50) | 9 (50) | NS |
| Hyperlipidemia | 16 (89) | 14 (78) | NS |
| CAD | 14 (78) | 12 (67) | NS |
| PAD | 7 (39) | 15 (83) | .02 |
| Stroke | 5 (28) | 6 (33) | NS |
| Carotid disease | 7 (39) | 6 (33) | NS |
| Baseline statin use | 14 (78) | 11 (61) | NS |
| High proteinuria | 8 (44) | 8 (44) | NS |
| Indications | |||
| Chronic renal insufficiency | 14 (78) | 13 (72) | NS |
| Hypertension | 4 (22) | 5 (28) | NS |
| Length of follow-up (months) | 24 ± 17 | 25 ± 19 | NS |
Abbreviations: CAD, coronary artery disease; PAD, peripheral artery disease; NS, not significant EPD, embolic protection device.
Treatment With Renal Artery Stent Placement
The EPD group had 28 ostial renal artery stenoses treated, while the control group had 25 ostial renal artery stenoses (Table 2). There were a higher percentage of patients with solitary kidneys and RAS in the control group (n = 3, 17%), while there were none in the EPD group. There were a higher percentage of patients with bilateral RAS in the EPD group (n = 8, 44%) compared with the control group (n = 5, 38%). In the control group with bilateral renal artery intervention, angioplasty was performed in 1 patient for fibromuscular dysplasia and the contralateral side had a stent placed for ostial stenosis. In another patient in the same group, one side was treated for ostial stenosis with a stent and the contralateral side was treated with angioplasty for recurrent stenosis in a bypass graft to the artery. In 1 patient undergoing unilateral intervention from both groups, 2 renal arteries were treated on the same side. Except as described above, all stenoses were treated with low profile balloon expandable stents such as the Herculink biliary stent (Abbot Vascular, Santa Clara, California) or Palmaz blue (Cordis). Two drug-eluting stents were used for arteries with diameters less than 4 mm such as the Taxus Express 2 (Boston Scientific, Natick, Massechusetts) or Cypher (Cordis). The choice of stent was based on the preference of the operator based on the length and diameter of stenosis.
Table 2.
Procedural Data of the Embolic Protection Device (EPD) and Control Groupsa
| EPD Group, N (%) |
Control Group, N (%) |
|
|---|---|---|
| Number of stenoses treated | 28 | 25 |
| Unilateral interventions | 10 (56)b | 13 (72)c |
| Bilateral interventions | 8 (44)d | 5 (38)e |
| Solitary kidney | 0 | 3 (17) |
| Number of stenoses protected | 20 (71) | NA |
| SpideRx | 10 (58) | NA |
| FilterWire | 8 (42) | NA |
| Technical success of EPD | 21/24 (88) | |
| FilterWire could not cross stenosis | 3 | NA |
| Diameter of artery ≤4.0 mm | 4 | NA |
| Early bifurcation | 1 | NA |
| Gadolinium used | 17 (94) | 9 (50) |
| Procedure complications | 2 (11) | 1 (6) |
No significant difference between EPD group and control group.
One patient had 2 renal arteries treated on the right side with stent placement.
One patient had 2 renal arteries treated on the left side with stent placement.
One patient had 3 stenoses treated: 2 on one side and one on the contralateral side which were all stented.
One patient with bilateral renal artery stenosis had a stent placed for atherosclerotic ostial disease and angioplasty on the contralateral side of fibromuscular dysplasia.
A second patient had bilateral renal artery stenosis involving an atherosclerotic ostial lesion which was stented and a bypass graft to the contralateral kidney which was treated with angioplasty.
Types of EPDs Used
In the EPD group, 10 (56%) patients had the SpideRX deployed while 8 (44%) had the FilterWire EZ device deployed (Table 2). The use of an EPD was attempted in 24 stenoses with a successful protection in 21 of the 24 RAS, giving a technical success rate of 88%. In 3 stenoses, the FilterWire EZ device could not cross the stenosis. The use of the EPD was not attempted in stenosis with a diameter less than 4 mm (n = 4) and in 1 stenosis because the main renal artery was too short. Therefore, of the 24 treated renal artery stenoses, 21 were protected using an EPD.
Contrast Used
Of the 36 patients, 26 (EPD and non-EPD; 72%) of the patients had gadolinium angiography. We were using gadolinium as a contrast agent prior to the 2006 FDA warning of nephrogenic fibrosing dermopathy warning in patients with reduced kidney function. We observed no evidence of nephrogenic fibrosing dermopathy in these patients.
Kidney Function
In the EPD group, the primary indication for stent placement was renal insufficiency (n = 14, 78%) followed by uncontrolled hypertension (n = 4, 22%), while in the control group, it was renal insufficiency (n = 13, 72%) and uncontrolled hypertension (n = 5, 28%; Table 1). The baseline average eGFR for the EPD and control groups were 34.8 ± 13.6 mL/ min per 1.73 m2 and 33.6 ± 12.8, respectively (P = not significant [NS]). At 12-month follow-up, there was no difference in the average eGFR in the EPD group eGFR of 36 ± 13 mL/min when compared with the control group 35.8 ± 13.7 (P = NS). There was a higher percentage of patients who had ≥ 20% increase in the eGFR after the procedure when compared with baseline in the EPD group (7 of 18 [39%]) and the control group (4 of 18 [22%]). There was no change in eGFR at 12-month follow-up when compared with baseline in 11 of 18 (61%) patients in the EPD group and 13 of 18 (72%) patients in the control group. Finally, 1 patient (5.3%) had a ≥ 20% decrease in eGFR at 12-month follow-up when compared with baseline in the EPD group and 2 patients (11%) in the control group.
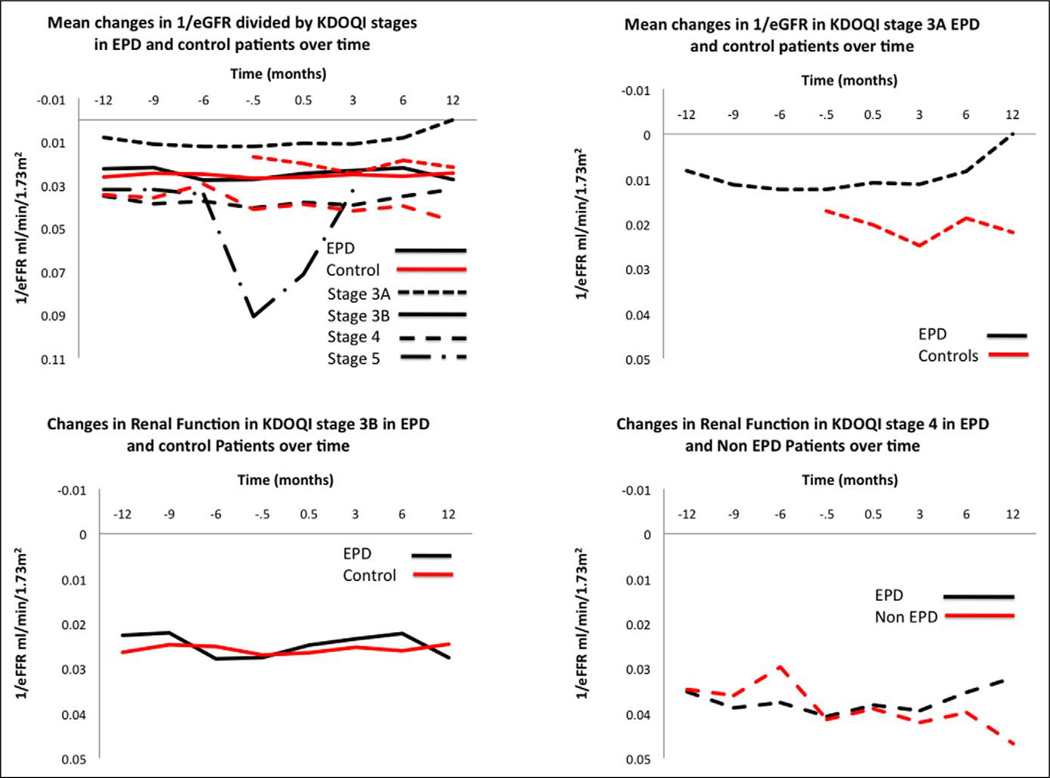
We next analyzed the similar change in eGFR based on the baseline KDOQI stages for both groups (Table 3). Patients were classified as 3A (eGFR 45–59 mL/min per 1.73 m2), classified as 3B (eGFR 30–44 mL/min per 1.73 m2), stage 4 (eGFR 15–29 mL/min per 1.73 m2), or stage 5 (≤15 mL/min per 1.73 m2). For stage 4 patients, only a significant improvement in the eGFR was observed at 12-month follow-up when compared with baseline for the EPD group and control group (P = .01). We next plotted 1/mean eGFR for the different KDOQI stages (Figure 1). This demonstrated that stage 3A and 4 patients benefited with EPD compared with non-EPD patients as evidenced by the return to baseline mean eGFR at 12-month follow-up.
Table 3.
Kidney Function
| EPD Group, N (%) | Control Group, N (%) | |||
|---|---|---|---|---|
| Improved | 7 (16) | 4 (22) | ||
| Unchanged | 11 (68) | 13 (72) | ||
| Decreased | 0 (0) | 1 (6) | ||
| Baseline | Follow-up | Baseline | Follow-up | |
| Serum creatinine (mg/dL) | 2.15 ± 0.72 | 1.99 ± 0.58 | 2.15 ± 0.64 | 2.11 ± 0.98 |
| eGFR (mL/min) | 34.8 ± 13.6 | 36.0 ± 13 | 33.6 ± 12.8 | 35.8 ± 13.7 |
| KDOQI class 2 | 0 | 0 | 1 | 2 |
| 3A | 5 | 5 | 3 | 2 |
| 3B | 6 | 8 | 8 | 6 |
| 4 | 7 | 4 | 7 | 6 |
| 5 | 1 | 0 | 0 | 0 |
| Hemodialysis | NA | 2 | NA | 3 |
| Deaths | NA | 5 | NA | 5 |
Abbreviations: EPD, embolic protection device; KDOQI, Kidney Disease Outcome and Quality Initiative; NA, not applicable.
Figure 1.
Mean changes in 1/eGFR divided by KDOQI stages in EPD and control patients over time. eGFR indicates estimated glomerular filtration rate; KDOQI, Kidney Disease Outcome and Quality Initiative; EPD, embolic protection device.
At baseline, the mean 24-hour proteinuria in the EPD group versus control group was 642.5 ± 968.3 mg/24 h versus 668.1 ± 824.8 mg/24 h, respectively, and at 12-month follow-up was 473.4 ± 489.04 mg/24 h versus 662.4 ± 889.01 mg/24 h, respectively (P = NS). There were 8 patients with high proteinuria in both the EPD group and control group prior to treatment. After treatment, this was reduced to 4 patients in each group. We performed an analysis for proteinuria before and after treatment for both groups and KDOQI stage and found no statistical differences in outcomes.
Hypertension Response
Preprocedure, the EPD patients had a systolic blood pressure of 147 ± 27 mm Hg and a diastolic pressure of 70 ± 13 mm Hg, measured in the sitting position and as an average of 6 readings. At follow-up, the systolic blood pressure improved significantly to 128 ± 20 mm Hg (P < .05) while diastolic blood pressure decreased to 68 ± 12 mm Hg. Patients in the control group had preprocedure systolic pressure of 156 ± 28 mm Hg that decreased to 139 ± 29 mm Hg (P = NS) postprocedure and a preprocedure diastolic pressure of 76 ± 16 mm Hg that decreased to 69 ± 15 mm Hg, a nonsignificant change from baseline. There were no differences in the blood pressure response or medication change when analyzed by the different KDOQI stages for the EPD group compared with control.
Complications
There were no complications in the control group. In the EPD group, there was 1 complication in which a pseudoaneurysm in the groin occurred (5%) treated with an ultrasound guided thrombin injection and resolved.
Discussion
In the present study, we compared the use of EPD during the placement of RA stent in patients compared with patients who were treated with RA stent placement alone for the outcomes of renal function and hypertension response. In the EPD group, the kidney function improved in 7 patients (37%), remained stable in 10 (58%), and declined in 1 (5%). In the control group, the kidney function improved in 4 patients (21%), remained stable in 12 (68%), and declined in 2 (11%). Finally, a subset analysis based on KDOQI stage (3A vs 4) showed a significant improvement in mean eGFR at 12 months when compared with baseline in the EPD group when compared with controls (P = .01).
There have been several studies performed using different EPDs (GuardWire, SpideRX, FilterWire, and Angioguard) in patients with RAS for the treatment of CRI (eGFR <60 mL/min).7,9,15 Holden and Hill studied 57 patients with a median age of 72 (59–85) who were treated for CRI with renal artery stent placement in which 37 patients underwent treatment with Angioguard device and were compared with 20 patients without EPD use.7 The majority (75%) of the patients had moderate-to-severe renal failure (eGFR = 15–40 mL/min as estimated by Cockroft Gault equation). The authors defined changes in renal function after the procedure based on serum creatinine rather than eGFR. Based on changes in the serum creatinine levels, patients with EPD use had a 95% improvement or stabilization when compared with patients without embolic protection (80%). The major limitations of this study were that the renal outcomes were defined using serum creatinine instead of eGFR and that the control group was not matched to the embolic protection group, but rather a group of patients who had been treated with a renal artery stent without an EPD.
In the second article by the same group, the authors reported in 63 patients with a median age of 70 with moderate-to-severe renal failure in a majority of the patients with concomitant hypertension.8 The authors used eGFR and 2 different EPDs: Angioguard (88%) and FilterWire EZ (12%). The reported success rate was 97% at 6 months with the vast majority (92%) of patients having revascularization of RAS involving either bilateral or solitary functioning kidneys. These particular subgroups were reported to be associated with superior renal functional outcome rates compared with those with unilateral renal artery revascularization, thus limiting the observations of this study with a “selection bias”.24,25
There have been several studies using the GuardWire EPD.26 Edwards et al reported in 26 patients during renal artery revascularization in patients with CRI with a baseline serum creatinine 1.9 ± 0.7 mg/dL and refractory hypertension.26 The authors reported a 100% technical success rate with a renal functional benefit at 1 month. There are several limitations for this study including that there was a very short follow-up and 7 patients (30%) had RAS treated for in-stent restenosis, which has a presumed lower incidence of distal embolization potential. In addition, patients with bilateral RAS were staged and only 1 side was treated followed by contralateral treatment 1 month later, which may account for their superior renal functional outcomes. Finally, an unknown number of patients with renal artery diameters > 6 mm were not excluded from the final analysis, in which the efficacy of the GuardWire could not be used because of the currently available sizes. A CTA study in which renal arteries were measured with different anatomical measures for determining which EPDs could be used showed that 50% of main renal arteries on right side and 59% on the left side were > 6 mm in their largest diameter, which limits the potential use of GuardWire EPDs in a significant proportion of patients.16
A second study using the GuardWire EPD was performed in 63 patients (men = 54%) with a mean age of 75.2 ± 7.7 years.26 Renal function was determined by calculating the eGFR using MDRD formula and participants were divided into KDOQI classes based on baseline eGFR. The mean baseline serum creatinine and eGFR were 1.87 ± 0.6 mg/dL and 36.63 ± 11.42 mL/min per 1.73 m2, respectively, and at last clinical follow-up was 1.96 ± 0.72 mg/dL and 38.75 ± 13.25 mg/mL per 1.73 m2, respectively (P = NS). After revascularization, an improvement from baseline of ≥1 KDOQI class was defined as improvement, unchanged KDOQI class as stabilization, and worsening of ≥1 KDOQI class as deterioration. Over a mean follow-up period of 16 ± 12 months, 14 patients (25%) improved, 33 (58%) remained stable, and 10 (18%) deteriorated. There was one GuardWire related dissection, which was successfully stented.
A recent study was performed using either the SpideRX or FilterWire EPDs while performing renal artery stent placement in 23 patients treated for 29 RAS with KDOQI stages 3 to 5 CRI.17 Kidney function change after stent placement was defined as above in the GuardWire study. After stent placement, there was stabilization or improvement in kidney function in 96% of the patients with significant decrease in systolic blood pressure. In 35% of the renal artery stent placements performed using a SpideRX device, debris was observed within the device after stent placement. Taken collectively, all these studies show that there is benefit of using an EPD while performing renal artery stent placement, however, it is unclear how much benefit is derived using the device with the stent placement compared with just the stent placed alone in this group of patients.
More recently, the RESIST trial results were reported in which 100 patients at 7 centers were randomly assigned to an open-label EPD (AngioGuard), or double-blind use of a platelet glycoprotein IIb/IIIa inhibitor, abciximab, in a 2 × 2 factorial design.17 The primary end point was absolute change in MDRD derived eGFR and percentage change from baseline to 30 days after renal artery stenting plus abciximab, filter-based embolic protection, or both. The mean eGFRs in these patients ranged from 52 to 66 mL/min prior to the intervention, mostly stages 3A and 2. The average eGFR decreased significantly (P < .05) in the stent alone (−7 ± 16/−10 ± 20) stenting and EPD (−9 ± 16/−12 ± 21), and stent with abciximab alone (−7 ± 13/−10 ± 0) groups, but did not decline in the group with stenting under combined EPD with abciximab protection (+2 ± 14/ +9 ± 30). Abciximab reduced the occurrence of platelet-rich emboli in the filters from 42% to 7% (P < .01) but no difference was observed in the capture of atheromatous debris (21% vs 19%; P = NS).
Although the previous reported results using EPDs while performing renal artery stent placement have been encouraging, these studies have several limitations because of selection criteria, study end point definitions, proportion of patients with advanced renal failure, and distribution of interventions on bilateral/solitary functioning kidneys. Because of these important differences in study design and other technical details including the use of different devices with differing pore sizes, it has become difficult to draw any conclusions. Finally, it is also speculative to attribute the reported renal functional benefit to the use of the EPD alone since stent placement alone has been shown to improve renal function. Some authors have reported renal function in terms of serum creatinine change from baseline, which is confounded by anthropometric and demographic variables such as age, diet, gender, race, and skeletal muscle mass. In contrast, renal function represented by GFR to some extent compensates for these confounding variables. Moreover, the definitions of improvement, stabilization, or deterioration of renal function following renal revascularization have similarly not been standardized. Published studies defined these terms as changes in absolute values of serum creatinine (eg, >0.2 mg/dL increase from the baseline defined as “failure”). However, changes in the percentage, instead of the absolute value from baseline, for defining “success” or “failure” is ideal, since the percentage change method nullifies the effect of the denominator (ie, the absolute value of the baseline renal function). The percentage change in renal function from baseline, and the reported time period of that change from baseline has also been reported in various ways in previous studies, making the comparison of results between studies, as well as with the current study, complicated and confusing. In the present study, we defined improvement, stabilization, and deterioration as a percentage change from baseline.
In the current study, we tried to overcome many limitations of previous studies by careful design and application of rigorous selection criteria. Contrary to prior studies, patients with in-stent restenosis, which presumably have a lower risk of atheroembolism, were excluded from our study. We defined renal functional benefit in study patients as stable or improved based upon the baseline based on change from baseline using 20% cutoff.21 We chose to match the groups for gender, age, presence, or absence of diabetes and high or low proteinuria. We also matched them to make sure the procedures were done within 8 months of each other and that both groups of patients used low profile stents. Using the current criteria for matches, the EPD and control groups were matched evenly for many comorbidities and had the same average follow-up. We observed in the EPD group that a higher percentage of patients stabilized or improved their eGFR after treatment when compared with control group. In addition, we observed a significant improvement in eGFR for patients with stage 4 CKD at followup when compared with controls. We did not observe any changes in hypertension consistent with recent observations made by Singer et al.27
There are several limitations to the current study. Due to its retrospective nature, our report is associated with all the inherent limitations of its design. Since the study sample is a referred population to a tertiary care center, the results are subjected to “selection bias,” which limits the power of our observations. A formal histologic analysis of aspirate was not performed.
This study shows that when comparing similar patient groups undergoing renal artery stent placement, those using EPD have better outcomes than patients in whom EPD was not used. Renal function based on MDRD-derived stages showed significant improvement in patients with stage 4 CKD treated using EPD when compared with controls. It suggests that EPDs may offer some advantage in patients with RAS with CRI, however, a randomized trial needs to be performed.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Sapoval M, Zahringer M, Pattynama P, et al. Low-profile stent system for treatment of atherosclerotic renal artery stenosis: the GREAT trial. J Vasc Interv Radiol. 2005;16(9):1195–2002. doi: 10.1097/01.RVI.0000171765.67665.D3. [DOI] [PubMed] [Google Scholar]
- 2.Dorros G, Jaff M, Jain A, Dufek C, Mathiak L. Follow-up of primary Palmaz-Schatz stent placement for atherosclerotic renal artery stenosis. Am J Cardiol. 1995;75(15):1051–1055. doi: 10.1016/s0002-9149(99)80723-1. [DOI] [PubMed] [Google Scholar]
- 3.Dorros G, Jaff M, Mathiak L, et al. Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation. 1998;98(7):642–647. doi: 10.1161/01.cir.98.7.642. [DOI] [PubMed] [Google Scholar]
- 4.Meyrier A. Renal vascular lesions in the elderly: nephrosclerosis or atheromatous renal disease? Nephrol Dial Transplant. 1996;11(suppl 9):45–52. doi: 10.1093/ndt/11.supp9.45. [DOI] [PubMed] [Google Scholar]
- 5.Scolari F, Bracchi M, Valzorio B, et al. Cholesterol atheromatous embolism: an increasingly recognized cause of acute renal failure. Nephrol Dial Transplant. 1996;11(8):1607–1612. [PubMed] [Google Scholar]
- 6.Stone GW, Rogers C, Hermiller J, et al. Randomized comparison of distal protection with a filter-based catheter and a balloon occlusion and aspiration system during percutaneous intervention of diseased saphenous vein aorto-coronary bypass grafts. Circulation. 2003;108(5):548–553. doi: 10.1161/01.CIR.0000080894.51311.0A. [DOI] [PubMed] [Google Scholar]
- 7.Holden A, Hill A. Renal angioplasty and stenting with distal protection of the main renal artery in ischemic nephropathy: early experience. J Vasc Surg. 2003;38(5):962–968. doi: 10.1016/s0741-5214(03)00606-2. [DOI] [PubMed] [Google Scholar]
- 8.Holden A, Hill A, Jaff MR, Pilmore H. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int. 2006;70(5):948–955. doi: 10.1038/sj.ki.5001671. [DOI] [PubMed] [Google Scholar]
- 9.Hagspiel KD, Stone JR, Leung DA. Renal angioplasty and stent placement with distal protection: preliminary experience with the FilterWire EX. J Vasc Interv Radiol. 2005;16(1):125–131. doi: 10.1097/01.RVI.0000147070.43605.76. [DOI] [PubMed] [Google Scholar]
- 10.Henry M, Henry I, Klonaris C, et al. Renal angioplasty and stenting under protection: the way for the future? Catheter Cardiovasc Interv. 2003;60(3):299–312. doi: 10.1002/ccd.10669. [DOI] [PubMed] [Google Scholar]
- 11.Henry M, Klonaris C, Henry I, et al. Protected renal stenting with the PercuSurge GuardWire device: a pilot study. J Endovasc Ther. 2001;8(3):227–327. doi: 10.1177/152660280100800301. [DOI] [PubMed] [Google Scholar]
- 12.Misra S, Gomes MT, Mathew V, et al. Embolic protection devices in patients with renal artery stenosis with chronic renal insufficiency: a clinical study. J Vasc Interv Radiol. 2008;19(11):1639–1645. doi: 10.1016/j.jvir.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Misselt AJ, McKusick MA, Stockland A, Misra S. Use of kissing embolic protection devices in the treatment of early bifurcation renal artery stenosis: a case report. J Vasc Interv Radiol. 2009;20(9):1240–1243. doi: 10.1016/j.jvir.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 14.Thatipelli M, Misra S. Endovascular intervention for renal artery stenosis. Abdom Imaging. 2010;35(5):612–621. doi: 10.1007/s00261-009-9572-1. [DOI] [PubMed] [Google Scholar]
- 15.Thatipelli MR, Misra S, Sanikommu SR, Schainfeld RM, Sharma SK, Soukas PA. Embolic protection device use in renal artery stent placement. J Vasc Interv Radiol. 2009;20(5):580–586. doi: 10.1016/j.jvir.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thatipelli MR, Sabater EA, Bjarnason H, McKusick MA, Misra S. CT angiography of renal artery anatomy for evaluating embolic protection devices. J Vasc Interv Radiol. 2007;18(7):842–846. doi: 10.1016/j.jvir.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Cooper CJ, Haller ST, Colyer W, et al. Embolic protection and platelet inhibition during renal artery stenting. Circulation. 2008;117(21):2752–2760. doi: 10.1161/CIRCULATIONAHA.107.730259. [DOI] [PubMed] [Google Scholar]
- 18.Kanjwal K, Haller S, Steffes M, et al. Complete versus partial distal embolic protection during renal artery stenting. Catheter Cardiovasc Interv. 2009;73(6):725–730. doi: 10.1002/ccd.21932. [DOI] [PubMed] [Google Scholar]
- 19.Cooper CJ, Murphy TP, Matsumoto A, et al. Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J. 2006;152(1):59–66. doi: 10.1016/j.ahj.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Rundback JH, Sacks D, Kent KC, et al. Guidelines for the reporting of renal artery revascularization in clinical trials. J Vasc Interv Radiol. 2003;14(9):S477–S492. doi: 10.1097/01.rvi.0000094621.61428.d5. [DOI] [PubMed] [Google Scholar]
- 22.Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291(19):2328–2334. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- 23.Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 24.Zeller T, Frank U, Muller C, et al. Stent-supported angioplasty of severe atherosclerotic renal artery stenosis preserves renal function and improves blood pressure control: long-term results from a prospective registry of 456 lesions. J Endovasc Ther. 2004;11(2):95–106. doi: 10.1583/03-1062.1. [DOI] [PubMed] [Google Scholar]
- 25.Campo A, Boero R, Stratta P, Quarello F. Selective stenting and the course of atherosclerotic renovascular nephropathy. J Nephrol. 2002;15(5):525–529. [PubMed] [Google Scholar]
- 26.Edwards MS, Craven BL, Stafford J, et al. Distal embolic protection during renal artery angioplasty and stenting. J Vasc Surg. 2006;44(1):128–135. doi: 10.1016/j.jvs.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Singer GM, Setaro JF, Curtis JP, Remetz MS. Distal embolic protection during renal artery stenting: impact on hypertensive patients with renal dysfunction. J Clin Hypertens (Greenwich) 2008;10(11):830–836. doi: 10.1111/j.1751-7176.2008.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]