Abstract
Maternally transferred antibodies have been documented in a wide range of taxa and are thought to adaptively provide protection against parasites and pathogens while the offspring immune system is developing. In most birds, transfer occurs when females deposit immunoglobulin Y into the egg yolk, and it is proportional to the amount in the female’s plasma. Maternal antibodies can provide short-term passive protection as well as specific and nonspecific immunological priming, but high levels of maternal antibody can result in suppression of the offspring’s humoral immune response. We injected adult female zebra finches (Taeniopygia guttata) with one of two antigens (lipo-polysaccharide [LPS] or keyhole limpet hemocyanin [KLH]) or a control and then injected offspring with LPS, KLH, or a control on days 5 and 28 posthatch to examine the impact of maternally transferred antibodies on the ontogeny of the offspring’s humoral immune system. We found that offspring of females exposed to KLH had elevated levels of KLH-reactive antibody over the first 17–28 days posthatch but reduced KLH-specific antibody production between days 28 and 36. We also found that offspring exposed to either LPS or KLH exhibited reduced total antibody levels, compared to offspring that received a control injection. These results indicate that high levels of maternal antibodies or antigen exposure during development can have negative repercussions on short-term antibody production and may have long-term fitness repercussions for the offspring.
Introduction
Inducible immune defenses provide protection against invading parasites and pathogens while allowing organisms to avoid the costs of mounting an immune response until challenged with infection (Tollrian and Harvell 1999). Among the most important inducible defenses for vertebrates is the adaptive humoral (or “antibody-mediated”) immune system, in which immunoglobulins (Ig) are produced in response to the presence of foreign antigen.
In birds, a fully functioning humoral immune response depends on B-cell colonization and maturation in the bursa of Fabricius and maturation and differentiation of T-cells in the thymus (Apanius 1998). Production of specific antibodies as part of a primary response appears to be possible in the first week posthatch in both precocial and altricial species (Koppenheffer and Robertson 1980; Grindstaff et al. 2006; Davison et al. 2008; Smits and Bortolotti 2008). However, the adaptive humoral immune system does not become fully functional until a few weeks or even months after hatching (Tizard 2002; Davison et al. 2008), depending on the species (Apanius 1998). To help offspring combat infections during the vulnerable period between hatching and maturation of the humoral immune response, females can transfer antibodies to the egg for protection (Janeway et al. 2001).
Neonates are exposed to a suite of novel environmental antigens (Solomon 1971), and their naive immune systems are not capable of mounting full responses to these invaders (Lawrence et al. 1981; Davison et al. 2008). Maternal antibodies can thus provide immediate protection against antigens of potentially pathogenic organisms (Solomon 1971; Davison et al. 2008). They may also protect offspring from having to mount an energetically expensive immune response, energy that can otherwise be invested in growth and development (Grindstaff et al. 2003; Lee 2006; Pihlaja et al. 2006). The production of maternal antibodies is dependent on maternal antigen exposure (Roitt et al. 1998; Lemke et al. 2003), and the repertoire of antibodies passed to offspring represents the combined exposure history of the female (Lemke et al. 2003). In birds, the amount of antibody transferred to the egg is proportional to the amount circulating in the female’s serum (Al-Natour et al. 2004; Hamal et al. 2006). Maternal antibodies are actively transferred to the yolk during yolk formation (Linden and Roth 1978; West et al. 2004). Antibody assimilation begins in the developing embryo following incubation and continues after hatching (Davison et al. 2008). Maternal antibodies are catabolized by the developing chick (Brambell 1970; Grindstaff et al. 2003), and the rate of loss is thought to be tied to developmental rate, with fast-developing species catabolizing maternal antibodies more rapidly than slowly developing species (Garnier et al. 2012). In addition, maternal antibodies persist for a longer period of time in the chick when initial maternal antibody levels are high (Solomon 1971; Grindstaff 2010). The capacity for short-term passive protection is thus determined by the amount of antibody transferred from the mother to her offspring and the length of time those antibodies persist in the offspring.
In addition to short-term passive protection against infection, maternal antibodies can help prime the offspring’s immune system for future antigen exposure (Anderson 1995; Carlier and Truyens 1995; Grindstaff et al. 2006; reviewed in Lemke et al. 2009). Immunological priming results in a more robust specific response (Anderson 1995; Carlier and Truyens 1995; Reid et al. 2006) and/or a stronger nonspecific response (Grindstaff et al. 2003). Nonspecific priming of the offspring’s immune system occurs when maternal antibodies opsonize antigen, which then leads to improved antigen recognition by macrophages (Janeway et al. 2001). Similarly, there is accumulating evidence that a lack of maternal antibodies has long-lasting consequences for offspring (reviewed in Lemke et al. 2004). As with antigen-specific priming, nonspecific priming is not well understood, and the factors that determine the occurrence and degree of nonspecific priming are likely tied to antigen type, the amount of antibody transferred, and the developmental rate of the humoral immune system.
Maternal antibodies can also block or suppress antibody production in the offspring (Lancaster 1964; Staszewski et al. 2007; Elazab et al. 2010). At high levels, maternal antibodies may coat the antigen present in the offspring, thus preventing specific B-cells from recognizing the antigen and impeding proper B-cell development (Carlier and Truyens 1995; Elazab et al. 2010). This blocking effect could negatively affect the short-term immunological response of the offspring (Staszewski et al. 2007; Elazab et al. 2010) as well as the ability to mount an antibody response later in life (Carlier and Truyens 1995). The degree of endogenous antibody suppression is thought to be dependent on the amount of maternal antibody transferred to the offspring as well as on the amount of antigen the offspring is exposed to (Carlier and Truyens 1995; Staszewski et al. 2007; Elazab et al. 2010).
Maternal antibodies may thus have important fitness consequences for offspring, but this has not been well studied. In this study, we examined the effects of maternal exposure to one of two antigens, lipopolysaccharide (LPS) and keyhole limpet hemocyanin (KLH), or a control on the primary and secondary antibody responses of nestling zebra finches (Taeniopygia guttata). Zebra finches are small passerine birds with altricial young that undergo rapid growth and development. We used LPS and KLH because they activate antibody production in distinct ways. LPS is a thymus-independent type-1 antigen, meaning that antibody production is not dependent on T-cell assistance (Janeway et al. 2001). KLH is a thymus-dependent antigen, and production of specific antibodies to KLH requires the presence of armed helper T-cells (Janeway et al. 2001).
We measured total antibody levels (total Ig) and antigen-specific antibody levels in nestlings at five standardized time points between days 5 and 36 posthatch. These multiple measures allowed us to track the production of antigen-specific antibodies following primary and secondary injections of LPS and KLH, as well as the production of total Ig, over the course of posthatch development. We tested whether maternal antigen exposure resulted in passive immunity, specific or nonspecific priming, and/or suppressive effects on the humoral immune system of developing birds. Grindstaff et al. (2006) previously documented a priming effect of maternal exposure to LPS on antibody production in the offspring of a wild population of pied flycatchers, and we predicted that maternal exposure to LPS or KLH would result in enhanced primary and secondary responses to the antigens in the nestlings. We also predicted that maternal exposure to LPS or KLH would result in an enhanced response to the other antigen via nonspecific priming and that total Ig levels would be highest in nestlings exposed to the same antigen as their mother.
Methods
Study Population
Research was approved by the Oklahoma State University Institutional Animal Care and Use Committee under protocol AS107, in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council 1985). For details on zebra finch care, as well as hatching and cross fostering of offspring, see Grindstaff et al. (2012). In short, finches were housed in randomly assigned pairs. During breeding, nest boxes were checked one or two times per day for eggs and/or young. To facilitate synchronization of egg laying for cross fostering, clutches were removed during incubation to stimulate production of a replacement clutch. Hatching order was assigned whenever possible, and young were individually marked and weighed to the nearest 0.01 g. Cross fostering occurred within 72 h of hatching. Within natal nests, young were divided into three treatment groups (see below). Young within foster nests did not differ by more than 60 h in age. Clutch and brood size were matched such that foster brood size was within 1 of clutch size. In all, 134 young survived until at least 11 d posthatch. These young originated from 44 different females (13 treated with KLH, 14 treated with LPS, and 17 treated with phosphate-buffered saline [PBS; control]).
Maternal Treatment
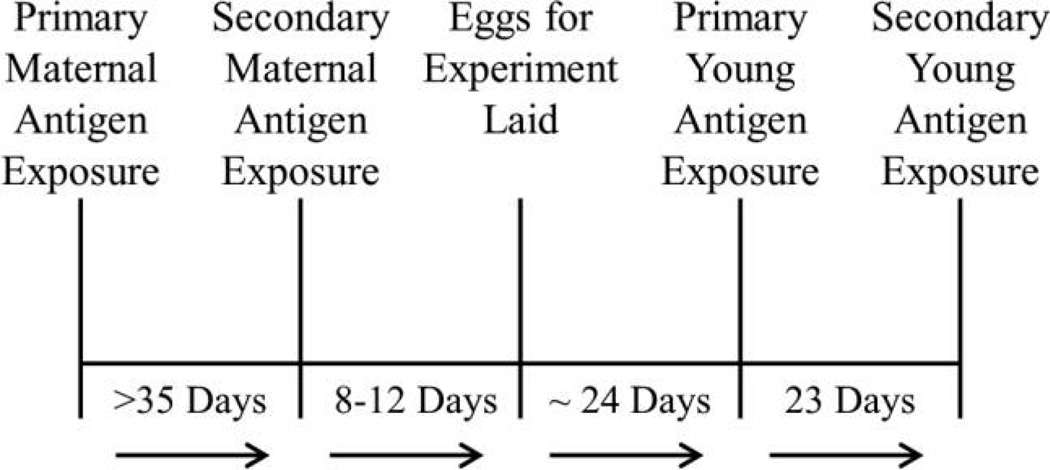
Adult females (N = 60) were randomly assigned to one of three groups, one control group and two antigen treatment groups (fig. 1). The control group was injected with 50 µL sterile PBS (Sigma, P5368). Birds in the first antigen treatment group were injected with LPS derived from Salmonella typhimurium (Sigma, L7261; 1.0 mg LPS/kg body weight in 50 µL of PBS; Owen-Ashley et al. 2006). Birds in the second antigen treatment group were injected with KLH (Calbiochem, 374817; 50 µg KLH in 50 µL PBS; Hasselquist et al. 1999). Treatments were injected intra-abdominally after swabbing with 70% isopropyl alcohol. Females were immunized for the first time before the production of the first clutch. The second, booster immunization was given at least 35 d after the primary challenge, shortly before production of the replacement clutch. The mean number of days between the secondary challenge and laying of the first egg in the replacement clutch was 18 d (range: 7–59 d).
Figure 1.
Time line for pre- and postnatal experimental procedures. Adult female zebra finches were exposed to one of three experimental treatments (keyhole limpet hemocyanin, lipopolysaccharide, or phosphate-buffered saline) before egg laying. Females were then given a secondary injection of the same treatment and allowed to lay a clutch of eggs that were replaced with dummy eggs. Dummy-egg clutches were removed 7 d after the secondary injection, and females laid replacement clutches over the subsequent 4–5 d, on average. Eggs hatched approximately 19 d later, and the offspring were given a primary injection on day 5 and a secondary injection on day 28.
Offspring Treatment and Blood Sampling
Nestlings were given a primary immunization on day 5. All young within a foster nest received the same treatment as the foster mother and differed in whether they received the same treatment as their natal mother or one of the other two treatments. Control offspring were given an injection of 25 µL of sterile PBS. LPS-challenged young were given an injection of 0.5 mg LPS/kg body weight in 25 µL sterile PBS. KLH-challenged young were given an injection of 12.5 µg KLH in 25 µL sterile PBS. Young received a secondary immunization with the adult-female doses on day 28.
On day 5 immediately before immunization, 20 µL of blood was collected from the brachial vein of nestlings to assess total and/or antigen-specific antibody levels. Blood samples were also collected from all young on days 10 and 17 to quantify residual maternal antibody levels and possible endogenous antibody production. On day 28, blood was collected immediately before challenge. A final blood sample was collected on day 36 to quantify the secondary antibody response.
Total Ig and Antigen-Reactive Antibody Enzyme-Linked Immunosorbent Assays (ELISAs)
Total Ig concentrations and antigen-reactive antibody titers were quantified with ELISAs, as described previously (Grind-staff et al. 2005; Grindstaff 2008). For details, see the appendix.
Statistical Analyses
All variables were checked for normality of residuals and homogeneity of variance before analyses. Antibody titer data were log + 1 transformed to achieve normality before analysis. Data were analyzed with general linear mixed models in which maternal identity and day × maternal identity were included as random factors. To examine the effect of maternal antigen exposure on offspring primary and secondary antibody responses, we ran mixed models with nine independent variables of interest (maternal treatment, young treatment, maternal ID, day, sex, latency to lay, egg mass, hatch order, and foster-nest hatch order) and three dependent variables (antibody levels at day 5 posthatch, primary antibody response, and secondary antibody response) for total Ig levels, LPS-reactive antibodies, and KLH-reactive antibodies. Latency to lay is the number of days from secondary maternal antigen exposure to egg laying. Hatch order coincided with lay order in more than 95% of the eggs laid (J. L. Grindstaff, unpublished data). Foster-nest hatch order is the age of the cross-fostered young relative to that of other young in the foster nest.
Not all individuals were tested for all dependent variables because plasma volume limitations restricted the sample sizes (table 1). For LPS-reactive antibody levels we sampled offspring directly exposed to LPS and/or those whose mothers had been LPS challenged, and for KLH-reactive antibody levels we sampled offspring directly exposed to KLH and/or those whose mothers had been KLH challenged. However, as a reference point to assess the strength of the response following the primary and secondary injections, we also compared antibody levels from control offspring of either control mothers or mothers that received the other antigen (i.e., for analyses of LPS-reactive antibodies, females that received KLH) to offspring that were exposed to the antigen of interest but whose mothers were not. Sample sizes for these reference analyses were small, however, and provide limited power to detect differences between controls and experimentals.
Table 1.
Experimental design and offspring sample size for blood samples from each treatment on days 5, 10, 17, 28, and 36
| Maternal treatment, young treatment |
Day 5* | Day 10 | Day 17 | Day 28* | Day 36 |
|---|---|---|---|---|---|
| KLH: | |||||
| KLH | 7 | 8 | 8 | 8 | 8 |
| LPS | 7 | 12 | 13 | 12 | 12 |
| Control | 13 | 14 | 14 | 13 | 13 |
| LPS: | |||||
| KLH | 14 | 16 | 16 | 16 | 16 |
| LPS | 14 | 16 | 16 | 15 | 15 |
| Control | 10 | 13 | 12 | 13 | 13 |
| Control: | |||||
| KLH | 10 | 12 | 12 | 11 | 11 |
| LPS | 15 | 17 | 16 | 17 | 16 |
| Control | 16 | 17 | 19 | 16 | 18 |
Note. Blood samples were collected from the birds on each of these days, and the asterisks indicate days on which the primary and secondary injections occurred.
KLH = keyhole limpet hemocyanin; LPS = lipopolysaccharide; control = phosphate-buffered saline.
Antibody levels were measured on day 5 to assess maternally transferred antibody levels and gauge the capacity for short-term passive protection. These data were analyzed independently from the primary and secondary antibody responses. To examine day 5 antibody levels for each antibody type, we used linear mixed models (SAS proc “Mixed”) in which maternal ID was included as a random effect; maternal treatment, sex, latency to egg laying, hatch order, foster-nest hatch order, and egg mass were included as fixed effects; and the denominator degrees of freedom were approximated with the containment method (Littell et al. 2006). The sex of each bird was determined visually once they reached maturity.
To test for specific priming and/or suppressive effects of maternal antibodies on offspring humoral immune responses, we examined antibody levels in the offspring following primary and secondary exposure to the antigen. For the period following primary exposure to the antigen on day 5, we used repeated-measures mixed models to examine antibody levels on days 10, 17, and 28. We did not include day 5 because of variable and limited sample sizes for that day. We examined antibody levels between days 28 and 36 to assess the antibody response following secondary exposure on day 28. For the repeated-measures models, maternal ID and day × maternal ID were included as random factors; maternal treatment, young treatment, latency, hatch order, day, and the interactions between day and both maternal and young treatments were included as fixed effects. We tested the effects of sex, egg mass, foster-nest hatch order, the interaction between maternal and young treatment, and the interaction between egg mass and young treatment, but none of these factors contributed significantly to the models and were not retained (see description of Akaike Information Criterion [AIC] model selection below). The covariance structures between repeated measurements of the same individuals were explicitly modeled. A spatial power-law covariance model was used to account for unequally spaced longitudinal measurements in which correlations were expected to decline as a function of time (Littell et al. 2006). The spatial power-law covariance model is a generalization of an autoregressive error model (Littell et al. 2006), which allows for accurate assessment of within-subject slopes (Schielzeth and Forstmeier 2009). Denominator degrees of freedom were approximated with the Kenward-Rodgers method (Littell et al. 2006).
For antigen-specific antibody responses following primary and secondary injections, we ran the repeated-measures mixed models as described above but grouped offspring by whether their mother was exposed to the focal antigen or not, resulting in two maternal treatments for each antigen. This approach provided more power to detect differences between offspring that received specific maternal antibodies and those that did not for the two antigens. To explicitly test for nonspecific priming effects of maternal antibodies, we ran repeated-measures mixed models as described above within offspring treatment groups. For example, among offspring treated with KLH, we compared KLH-reactive antibody titers in offspring of females injected with LPS to those of offspring of control females. If maternal antibodies induce nonspecific priming, offspring of LPS-treated females should have higher levels of KLH-reactive antibodies than offspring of control-treated females.
To compare competing models we used the AIC (Symonds and Moussalli 2011). We examined all permutations of the fixed effects until we found the model with the lowest AIC score. We used post hoc pairwise tests of least squares means to compare significant terms and a sequential Bonferroni correction for multiple tests of the same hypothesis. We tested for differences among antibody levels within a time period (i.e., following primary injection), using pairwise t-tests comparing least squares means generated in the full models. For day 5 models, we used one-way t-tests for post hoc comparisons between maternal treatments, because of the expectation that females exposed to a specific antigen would transfer antigen-specific antibodies, whereas females that were not exposed to that antigen would not. All other pairwise comparisons were two-tailed, and α was set at 0.05 for all tests. All analyses were run in SAS (ver. 9.3; SAS Institute, Cary, NC) and JMP (ver. 10.0; SAS Institute).
Results
Total Ig
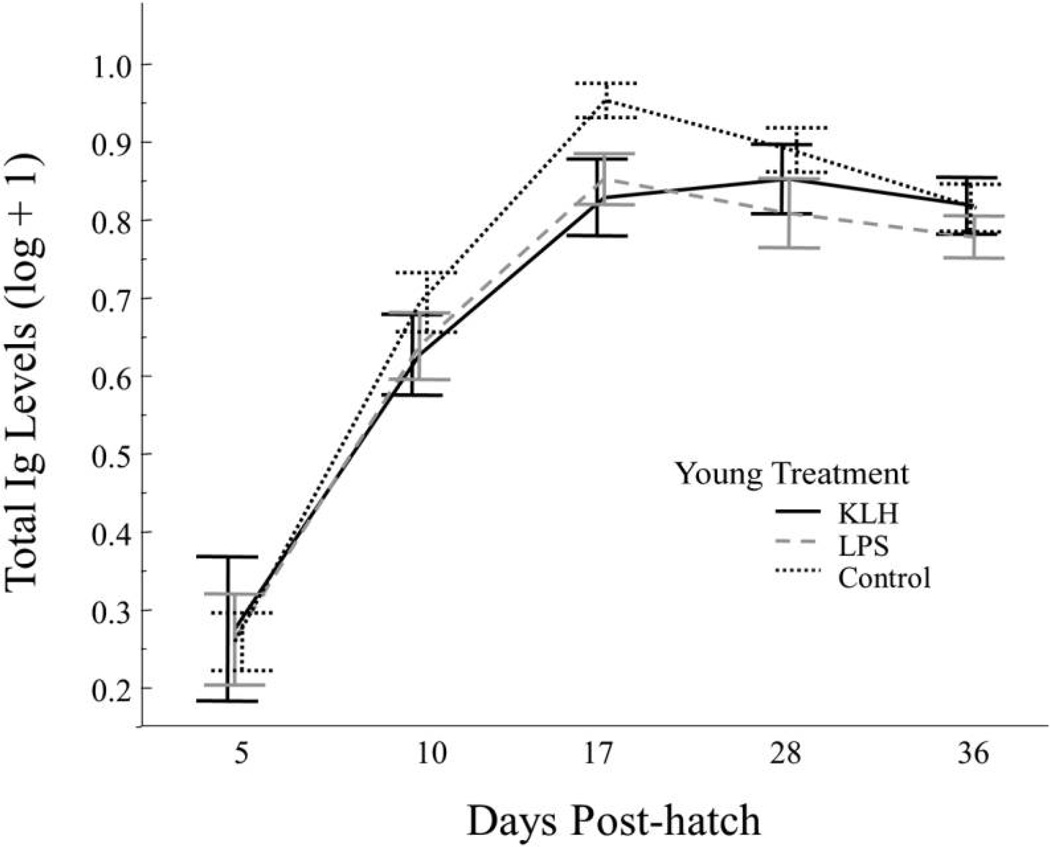
There were no significant predictor variables for total Ig levels at day 5 (table 2), but we found significant effects of young treatment and day on total Ig levels from day 10 to day 28 (table 2), in which total Ig levels increased from day 10 to day 28. Offspring that received a control injection had higher total Ig levels than those that received KLH (T2,74.8 = 2.2, P = 0.03) or LPS (T2, 79.5 = 2.2, P = 0.03; fig. 2). Latency also influenced total Ig levels following primary injection, with a longer delay between injection and laying being associated with higher total Ig levels. This result appears to be driven strongly by one female with a substantially longer latency period (11 d longer). When this outlier was removed, latency no longer contributed significantly, but there were no other qualitative differences in the final model, so latency was retained. Day was the only significant factor influencing total Ig levels between day 28 and day 36 (table 2), and there was a decrease in total Ig levels from day 28 to day 36 (T1,89.7 = 2.93, P< 0.01).
Table 2.
Total Ig, LPS-reactive, and KLH-reactive antibodies on day 5, following primary injection (days 10–28), and following secondary injection (days 28–36)
| Day 5 |
Days 10–28 |
Days 28–36 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Independent variables | F | df | P | F | df | P | F | df | P |
| Total Ig: | |||||||||
| Maternal treatment | 1.6 | 2, 23.6 | .22 | .04 | 2, 39.4 | .96 | 1.09 | 2, 35.7 | .35 |
| Young treatment | NA | NA | NA | 3.29 | 2, 78.2 | .04 | 2.15 | 2, 85.8 | .12 |
| Hatch order | 1.32 | 4, 41.3 | .28 | NR | NR | NR | NR | NR | NR |
| Latency | NR | NR | NR | 5.63 | 1, 45.7 | .02 | NR | NR | NR |
| Day | NA | NA | NA | 32.04 | 2, 62.1 | <.001 | 6.58 | 1, 29.9 | .02 |
| Day × maternal treatment | NA | NA | NA | 3.02 | 4, 62.1 | .02 | 1.08 | 2, 28.5 | .35 |
| Day × young treatment | NA | NA | NA | NR | NR | NR | .53 | 2, 83.7 | .59 |
| LPS-reactive antibodies: | |||||||||
| Maternal treatment | 5.18 | 1, 28.5 | .03 | 10.11 | 1, 68.6 | <.01 | 4 | 1, 49 | .051 |
| Young treatment | NA | NA | NA | NR | NR | NR | 3.2 | 1, 49 | .08 |
| Latency | NR | NR | NR | .71 | 1, 66.7 | .4 | 1.56 | 1, 49 | .22 |
| Day | NA | NA | NA | .53 | 2, 57.4 | .59 | 24.25 | 1, 51 | <.001 |
| Day × maternal treatment | NA | NA | NA | 2.33 | 2, 57.4 | .17 | 2.13 | 1, 51 | .15 |
| KLH-reactive antibodies: | |||||||||
| Maternal treatment | 15.04 | 1, 18 | <.001 | 8.29 | 1, 32.2 | <.01 | 2.15 | 1, 39.6 | .15 |
| Young treatment | NA | NA | NA | 5.36 | 1, 27.4 | .03 | NR | NR | NR |
| Day | NA | NA | NA | 2.53 | 2, 39.4 | <.01 | 30.68 | 1, 30.5 | <.001 |
| Day × maternal treatment | NA | NA | NA | 26.36 | 2, 39.4 | <.001 | 8.28 | 1, 30.5 | <.01 |
Note. Results of the mixed models for total immunoglobulin (Ig) and lipopolysaccharide (LPS)-reactive and keyhole limpet hemocyanin (KLH)-reactive antibody levels on day 5, the period following primary exposure to antigen (days 10–28), and the period following secondary exposure to antigen (days 28–36). For the LPS-reactive antibody results, the two maternal treatments were LPS and (KLH + control [phosphate-buffered saline]) and the young treatments were LPS and control. For the KLH-reactive antibody results, the two maternal treatments were KLH and (LPS + control) and the young treatments were KLH and control. Each model included maternal ID as a random effect.
NA = not applicable; NR = not retained in final model; boldface values indicate significant effects.
Figure 2.
Total immunoglobulin (Ig) levels for each young treatment group from day 5 to day 36. Treatments were keyhole limpet hemocyanin (KLH), lipopolysaccharide (LPS), or phosphate-buffered saline (control). Birds received a primary injection of LPS, KLH, or a control on day 5 and a secondary injection with the same substance on day 28. Error bars represent mean ± SE.
LPS-Reactive Antibodies
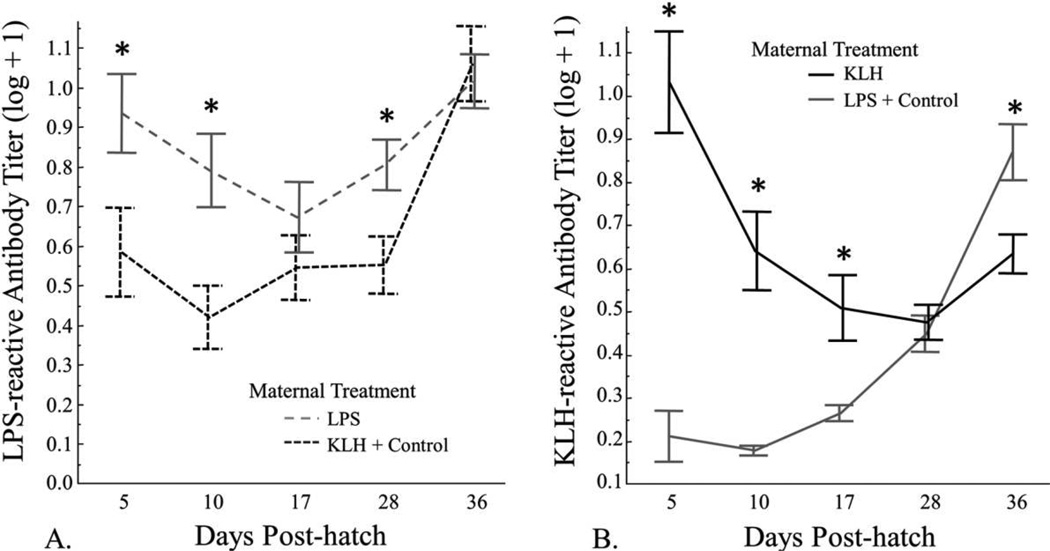
There was a significant effect of maternal treatment on day 5 antibody levels and during the period following the primary injection (table 2), in which offspring of females exposed to LPS had significantly higher levels of LPS-reactive antibodies on day 5 and during the period following primary exposure (fig. 3A). For the period following the secondary injection, there was a significant effect of day (table 2), in which levels of LPS-reactive antibodies increased significantly from day 28 to day 36 (fig. 3A; T1,55.9 = 5.71, P< 0.001). There was no difference in LPS-reactive antibodies between the maternal treatment groups on day 36 (T1,86.7 = 0.26, P = 0.8).
Figure 3.
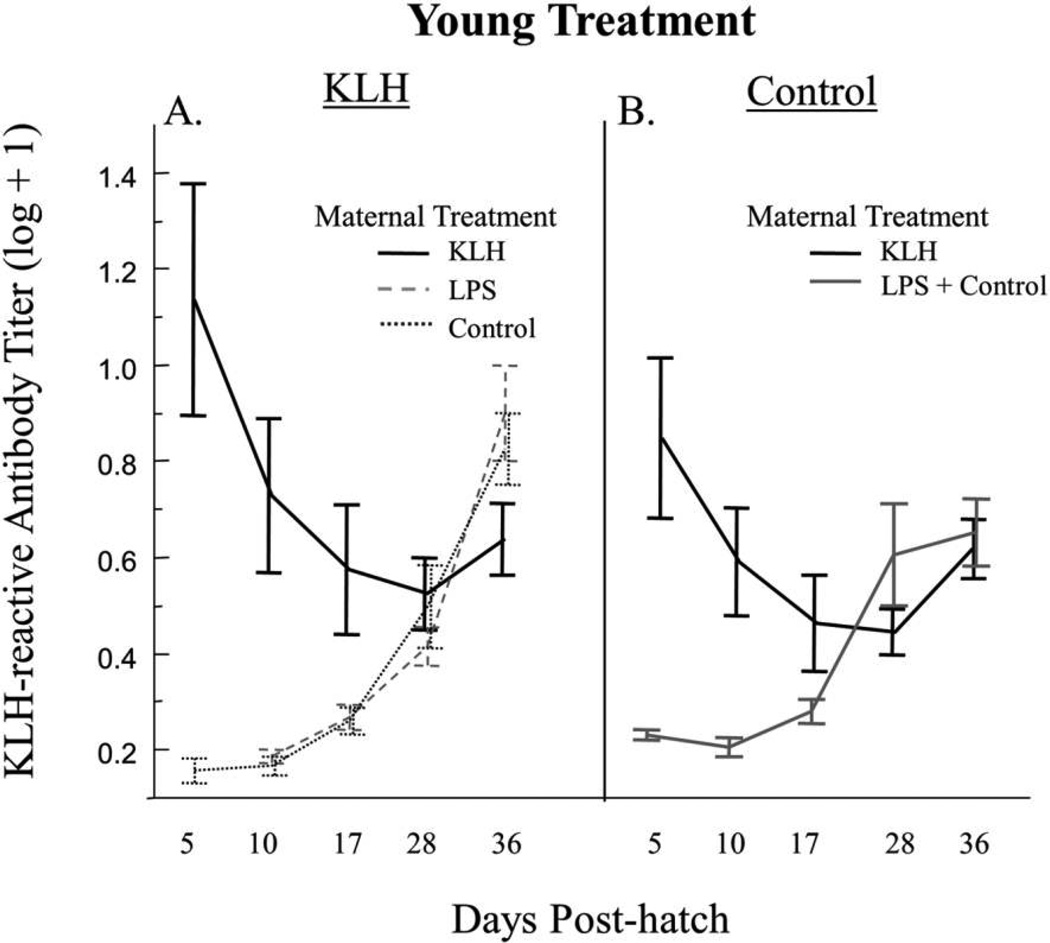
Relative lipopolysaccharide (LPS)-reactive antibody levels (A) and relative keyhole limpet hemocyanin (KLH)-reactive antibody levels (B) from day 5 to day 36. A, LPS-reactive antibody levels for offspring of female zebra finches exposed to LPS and offspring of females exposed to KLH or phosphate-buffered saline (control). B, KLH-reactive antibody levels for offspring of female zebra finches exposed to KLH and offspring of females exposed to LPS or control. Birds received a primary injection of LPS, KLH, or control on day 5 and a secondary injection on day 28. In A, data are from birds whose mothers were exposed to LPS and/or who were exposed to LPS themselves, whereas in B, data are from birds whose mothers were exposed to KLH and/or who were exposed to KLH themselves. Error bars represent mean ± SE, and an asterisk denotes a significant (P < 0.05) difference between groups for that day via post hoc t-tests.
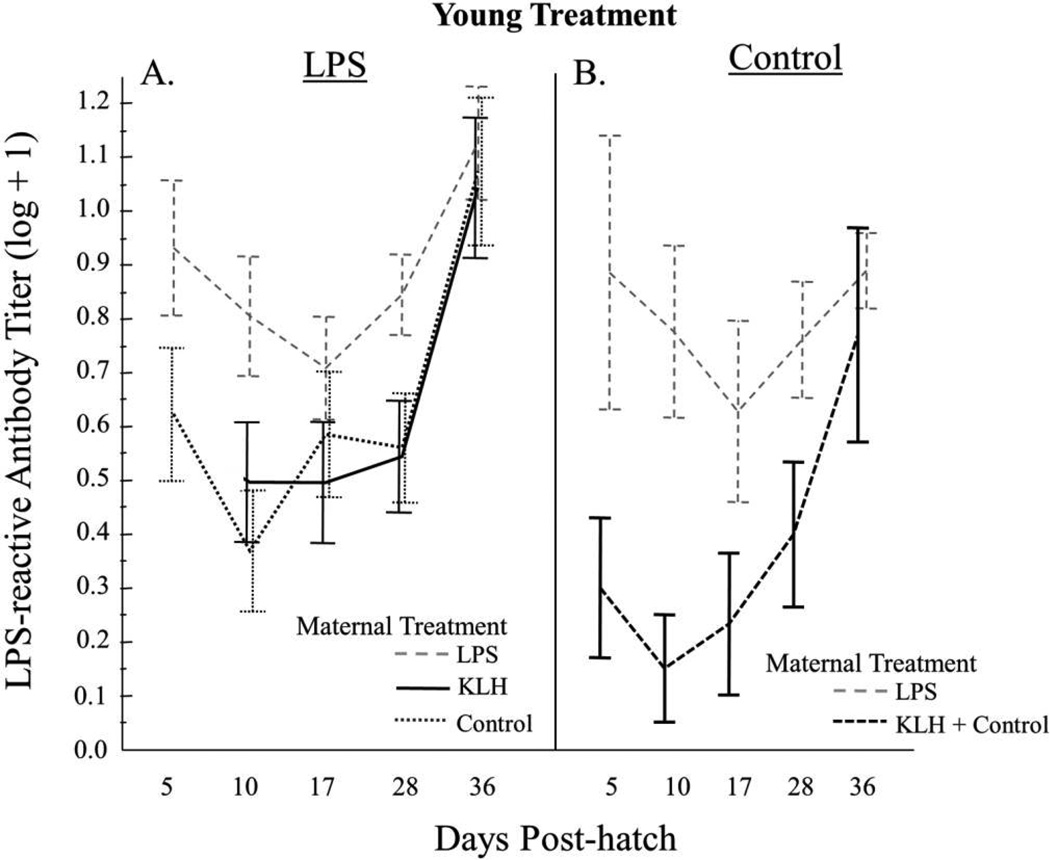
Among offspring challenged with LPS, we found that LPS-treated offspring of LPS-treated females had higher levels of LPS-reactive antibodies than LPS-treated offspring of KLH- and control-treated females following the primary (F1,18 = 15.35, P = 0.001) and secondary (F1,27 = 4.60, P = 0.04; fig. 4A) injections. However, levels of LPS-reactive antibodies did not differ between LPS-treated offspring of LPS-treated females and control-treated offspring of LPS-treated females following the primary (F1,27.24 = 0.25, P = 0.62) or secondary (F1,26 = 2.49, P = 0.13; fig. 4) exposures. There were no differences in LPS-reactive antibody levels between offspring of KLH-treated females and control-treated females for the period following primary injection (fig. 4A; F1,28.5 = 0.00, P = 0.97) or that following secondary injection (F1,27.2 = 0.02, P = 0.88).
Figure 4.
Relative lipopolysaccharide (LPS)-reactive antibody levels for each maternal treatment, by young treatment, from day 5 to day 36. Birds received a primary injection of LPS (A) or phosphate-buffered saline (control; B) on day 5 and a secondary injection on day 28. Error bars represent mean ± SE. KLH = keyhole limpet hemocyanin.
We detected LPS-reactive antibodies in control offspring of control mothers and mothers exposed to KLH, and these levels increased over time (fig. 4B). Levels of LPS-reactive antibodies did not differ significantly between offspring that were never exposed to LPS (i.e., neither maternal exposure nor young exposure) and LPS-treated offspring of control and KLH mothers following primary injection (F1,35.5 = 3.83, P = 0.058) or secondary injection (F1,34 = 2.24, P = 0.14; fig. 4). Power to detect differences was limited, however, because of small sample sizes of control/non-target antigen birds.
KLH-Reactive Antibodies
Maternal treatment was a significant factor affecting KLH-reactive antibodies on day 5 (table 2). Offspring of females that received KLH had significantly higher levels of KLH-reactive antibodies than offspring of females that did not receive KLH (fig. 3B). For the period following the primary injection, there was a significant effect of maternal treatment (table 2), in which offspring of females exposed to KLH had significantly higher levels of KLH-reactive antibody than offspring of females that did not receive KLH (fig. 3B). In addition, there was a significant effect of young treatment following primary injection, in which offspring treated with KLH had significantly lower levels of KLH-reactive antibodies than control-treated offspring (table 2). This effect is driven primarily by the fact that many of the control offspring had mothers previously challenged with KLH and therefore had high levels of maternally transferred KLH-reactive antibodies for the period following the primary injection (fig. 5B). There was also a significant effect of day (table 2) as well as a significant interaction between day and maternal treatment (table 2), in which offspring of females injected with KLH exhibited a decrease in KLH-reactive antibodies, whereas offspring of females that received LPS or a control exhibited an increase in KLH-reactive antibodies (fig. 5A). Day and the interaction between day and maternal treatment were significant factors for the period following secondary injection (table 2); levels of KLH-reactive antibodies increased from day 28 to day 36 across all treatments, but the offspring of females treated with KLH exhibited a significantly smaller increase than offspring of females not treated with KLH (fig. 3B).
Figure 5.
Relative keyhole limpet hemocyanin (KLH)-reactive antibody levels for each maternal treatment, by young treatment, from day 5 to day 36. Birds received a primary injection of KLH (A) or phosphate-buffered saline (control; B) on day 5 and a secondary injection on day 28. Error bars represent mean ± SE. LPS = lipopolysaccharide.
Among offspring challenged with KLH, we found that there were no differences in KLH-reactive antibody levels between offspring of LPS-treated females and those of control-treated females for the periods following primary injection (F1,18.5 = 0.16, P = 0.7) or secondary injection (F1,231 = 0.00, P = 0.95; fig. 5A). This indicates that there was no nonspecific priming effect of maternal exposure to LPS on the response to KLH treatment, at least over this period of development.
As with the LPS treatment, we detected KLH-reactive antibodies in control offspring of control mothers and mothers exposed to LPS, and these levels increased from day 10 to day 36 (fig. 5B). Levels of KLH-reactive antibodies did not differ significantly between control-treated offspring of LPS- or control-treated females and KLH-treated offspring of LPS- or control-treated females following primary injection (F1,27.21 = 3.26, P = 0.08) or secondary injection (F1,31.35 = 0.1, P = 0.76). There was, however, a significant day × young treatment interaction, (F1,28 = 7.63, P = 0.01), in which antibody levels increased at a significantly greater rate from day 28 to day 36 in KLH-treated offspring of control- or LPS-treated females than in control offspring of control mothers or mothers exposed to LPS (fig. 5). This provides some evidence for a response to KLH after challenge on day 28. Power to detect differences was again limited by small sample sizes of control/ nontarget birds.
Discussion
We documented short-term passive immunity as well as suppressive effects of maternal antibodies on levels of antigen-specific antibodies in developing zebra finches. Short-term passive immunity was evident at day 5 and persisted through the period following primary antigen exposure for both KLH-reactive and LPS-reactive antibodies. We found no evidence for either specific or nonspecific immunological priming due to maternal transfer of antibodies. In contrast, we found a suppressive effect of maternally derived KLH-reactive antibodies on the antibody response of offspring following secondary exposure to KLH. We also documented a negative effect of offspring antigen exposure on levels of total Ig following primary injection, suggesting a reduction in total antibody production for offspring exposed to antigen.
We found no evidence that birds were able to mount a primary response to an injection of LPS or KLH on day 5 post-hatch. Whether they were able to mount a response following secondary exposure on day 28 is unclear. Antibody levels increased from day 28 to day 36 for offspring exposed to the focal antigen, but not significantly above background levels (i.e., those produced by control offspring of control females or those exposed to the nonfocal antigen). These results indicate that all offspring produced antibody capable of cross-reacting with LPS and KLH and that production of these antibodies increased after day 17. These results are in line with previous work on altricial species in which primary exposure to antigen before day 7 posthatch failed to stimulate primary or secondary antibody responses (Grindstaff et al. 2006 and Killpack and Karasov 2012, respectively).
Total Ig Levels
Total Ig levels increased significantly from day 5 to day 17 and then began to decrease on day 28 and continued to decline through day 36 across all treatments. These data likely document the pattern of endogenous production of total Ig in developing zebra finches rather than changes in maternally transferred antibody or endogenous production of LPS- or KLH-reactive IgY. Maternal antibodies typically peak in the first 2–5 d posthatch (reviewed in Davison et al. 2008) and are then catabolized over the first 14 d posthatch in most altricial species studied (Lozano and Ydenberg 2002; Nemeth et al. 2008; King et al. 2010). The subsequent increase in total Ig levels likely reflects production of natural antibodies, which does not require antigen exposure (Davison et al. 2008). In addition, control offspring of control females exhibited the same pattern and absolute levels of total Ig similar to, if not higher than, those of offspring exposed to antigen. These results suggest that both maternally transferred IgY and the antigen-reactive antibody produced by developing chicks comprise a small proportion of total Ig in circulation, at least on day 5 and after.
During the period following primary injection, offspring receiving a control injection had higher total Ig levels than offspring exposed to either LPS or KLH. This difference disappeared in the period following the secondary injections, but it suggests that exposure to high doses of antigen during development can temporarily suppress total Ig production. Whether this suppression occurs in levels of IgM or IgY is not clear. The reduction in total Ig may be a result of resource constraint imposed by the immune system if the chicks utilize either cell-mediated responses, enhanced complement activity, or some combination of both following antigen exposure. These responses require protein, which may be allocated away from natural antibody production in the rapidly growing chicks. Conversely, the lower levels of total Ig could be a product of decreased antibody synthesis due to maternal antibody-induced suppression (sensu Carlier and Truyens 1995). There is evidence that high levels of maternal antibodies impede proper B-cell development (Carlier and Truyens 1995; Elazab et al. 2010) and can block production of endogenous IgM (Möller and Wigzell 1965), which could lead to a reduction in natural antibodies as well as in antigen-specific antibodies.
LPS-Reactive Antibody Response
In offspring of females exposed to LPS, we found evidence of short-term passive immunity provided by maternally transferred antibodies and no evidence of specific or nonspecific priming. Levels of LPS-reactive antibodies in offspring of females not exposed to LPS were relatively high, as was the variance in LPS-reactive antibody levels across days and among treatments (fig. 4). LPS is found in the cell walls of gram-negative bacteria (e.g., Escherichia coli), which are ubiquitous in the environment. The high variance across days and the high levels in day-5 birds from control and KLH-treated females may stem, in part, from individual differences in maternal exposure to environmental LPS.
LPS is a thymus-independent type-1 antigen that activates antibody production in the absence of T-cells (Janeway et al. 2001). The antibody response can, therefore, occur more rapidly than responses to thymus-dependent antigens such as KLH (Janeway et al. 2001). In addition, because thymus-independent antigens do not require T-cell assistance, developmentally immature individuals may be more capable of mounting a response against those antigens than against thymus-dependent antigens (Janeway et al. 2001). However, we did not detect a primary response to LPS injection in any of the treatments. This provides further evidence that at 5 d posthatch, zebra finches are primarily dependent on maternal antibodies for humoral responses (Killpack and Karasov 2012). Offspring of LPS-treated females exhibited significantly higher levels of LPS-reactive antibodies following the primary injection than offspring of females exposed to KLH or a control, even though there was no significant change in antibody levels over time (fig. 3A). This result suggests that offspring of females exposed to LPS retained maternally derived LPS-reactive antibody for 2–3 wk posthatch and that the maternal antibodies were supplemented with endogenously produced antibodies. The pattern of decreasing LPS-reactive antibodies from day 5 to day 17 and subsequent increase after day 28 in offspring of females injected with LPS (fig. 3A) indicates that endogenous production of LPS-reactive antibody offsets catabolism of maternal antibody between days 17 and 28. The increase in LPS-reactive antibodies in offspring that were exposed to KLH neither directly nor indirectly likely reflects production of natural antibodies capable of recognizing LPS (Davison et al. 2008).
There was no evidence of specific or nonspecific priming. Had priming occurred, there would have been an increase in LPS-reactive antibodies in LPS-treated offspring of LPS-treated females (specific) or those of KLH-treated females (nonspecific) following primary and secondary exposures (fig. 4). The lack of nonspecific priming is potentially due to differences between the antigens: LPS is a covalently joined lipid-polysaccharide molecule and a thymus-independent antigen, whereas KLH is a large oxygen-carrying protein and a thymus-dependent antigen (Janeway et al. 2001). However, other factors, such as maternal antibody concentration, antigen amount, and developmental stage of the immune system, can all influence whether maternal antibodies are protective or suppressive (Carlier and Truyens 1995; Staszewski et al. 2007; Gasparini et al. 2009; Elazab et al. 2010).
KLH-Reactive Antibody Response
We found evidence of short-term passive immunity for offspring of females exposed to KLH, no evidence of specific or nonspecific priming, and evidence of suppressive effects of maternal exposure to KLH. We found a significant difference between maternal treatments in KLH-reactive antibody levels on day 5, in which offspring of females exposed to KLH had significantly higher KLH-reactive antibody levels than offspring of females not exposed to KLH. Females exposed to KLH transferred high levels of KLH-reactive antibodies to their offspring (fig. 3B). Circulating levels of KLH-reactive antibodies declined in offspring of females treated with KLH until day 28, at which point they began to increase (fig. 3B). The latter increase coincided with the period of greatest increase in KLH-reactive antibodies for KLH-treated offspring of control females or LPS-treated females (fig. 5A) as well as for control offspring from control females or LPS-treated females (fig. 5B). Again, this increase in KLH-reactive antibodies in offspring that were not exposed to KLH likely reflects production of antibodies that recognize KLH, as has been documented previously in chickens (Parmentier et al. 2004).
The lack of evidence for a primary response suggests that B-and/or T-cell development was incomplete at the time of exposure (Janeway et al. 2001; Davison et al. 2008; Killpack and Karasov 2012). Previous research has documented that zebra finches are capable of mounting a detectable response to KLH by 14 d posthatch (Killpack and Karasov 2012), and we found some evidence that zebra finches responded to the KLH challenge on day 28. However, we had a small sample size that limited our power for this comparison. Transfer of maternal KLH-reactive antibody resulted in reduced production of KLH-reactive antibody following secondary exposure, compared to offspring treated with KLH whose mothers were not exposed to KLH. High levels of maternal IgY can result in suppression of the offspring’s immune system by coating the antigen in the offspring and blocking specific B-cells from recognizing the antigen (Carlier and Truyens 1995). Elazab et al. (2010) also documented suppressive effects of maternally derived KLH-specific antibodies on the endogenous production of KLH-specific antibodies through the first 6 wk of life in chickens. The authors found that suppression of the chicks’ antibody production depended on the dose of maternally derived antibodies as well as the dose of the antigen (Elazab et al. 2010).
Conclusions
Passive immunity from maternal antibodies has been well documented in many taxa (Solomon 1971; Carlier and Truyens 1995; Pravieux et al. 2007), but suppressive effects of maternal antibody have not been documented in a passerine bird to our knowledge (see Gasparini et al. 2009 for an example of suppression in the altricial tawny owl Strix aluco). In addition, we found a temporary negative effect on total Ig levels of exposure to antigen during early development.
The general trends for the maternal transfer and endogenous production of KLH- and LPS-reactive antibodies were similar, but maternal transfer of KLH-reactive antibodies resulted in the unequivocal suppression of endogenously produced KLH-reactive antibodies in the offspring. The lack of a suppressive effect of a maternal challenge with LPS may be related to differences in the amount of antigen-specific maternal antibody transferred. Females treated with LPS appeared to transfer lower amounts of LPS-reactive antibodies to their offspring, compared to the amount of KLH-reactive antibodies that females treated with KLH transferred to their offspring. This is supported by a comparison of the treated and control females at day 5 in figure 3.
Our data indicate that both maternal antibodies and antigen challenge early in life affect offspring immune function, with the potential for long-term fitness consequences.
Acknowledgments
Matt Anderson, Kent Andersson, Courtney Cupps, Sara Friedemann, Kaylee Hollingsworth, Lisa Hughes, Madeleine Naylor, Amanda Neujahr, Alecia Rains, Arielle Shanahan, and Matt Waselik provided invaluable assistance with zebra finch care and blood sampling. Thanks to Tara Stewart for edits and comments on the manuscript. The project described was supported by award R15HD066378 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. The authors declare no conflicts of interest for this study.
APPENDIX
ELISA Methods
Total-Ig ELISA
ELISA plates (Nunc, Maxi-Sorp) were coated with 100 µL of anti-bird IgG (goat anti-bird IgG Bethyl Labs A140–110A), at a concentration of 2 µg/mL, suspended in carbonate buffer (0.1 M, pH 9.6). Plasma samples were diluted 1 : 40 in diluent (1% milk powder, PBS-Tween-20). After washing, diluted samples were added to the plate in duplicate. At least two blank wells were included on each plate. The alkaline phosphatase-labeled secondary antibody (rabbit anti-chicken IgY alkaline phosphatase labeled; Sigma, A9171) was diluted 1 : 1,000. Plates were read on a BioTek microplate reader (ELx808) after a 20-min incubation period at room temperature with the substrate buffer. The mean value of the blanks was subtracted from the mean of duplicate values for each sample to account for nonspecific binding. A serial dilution of a chicken IgY standard (G116A, Promega) was included on all plates as a standard curve (1,000, 100, 50, 25, 12.5, 6.25, 3.125, 1.56, 0.78, 0.39, and 0.195 ng/mL). Standards were run in duplicate. Antibody concentrations were calculated on the basis of the standard curve run on the same plate as the unknown samples.
KLH-Reactive and LPS-Reactive IgY ELISAs
Titers of antigen-reactive antibodies were determined with the same general ELISA methods. To quantify KLH-reactive antibody titers, 100 µL of KLH, at a concentration of 50 µg/mL, suspended in carbonate buffer was added to all wells before incubation overnight at 4°C Plasma samples were diluted 1 : 40 in diluent, and the horseradish peroxidase-labeled secondary antibody (goat anti-bird IgG; Bethyl Labs, A140–110P) was diluted to a concentration of 1 : 1,000. Plates were read after a 25-min incubation at room temperature with the substrate buffer. To quantify LPS-reactive antibody titers, plates were coated with 100 µL of LPS, at a concentration of 50 µg/mL, suspended in carbonate buffer. Plasma samples were diluted 1 : 40 in diluent, and the secondary antibody (goat anti-bird IgG; Bethyl Labs) was diluted to a concentration of 1 : 1,000. Plates were read after a 30-min incubation at room temperature with the substrate buffer. On each plate, we included a dilution series of a standard pool that covered the range of antibody titers for female and offspring samples. We used the differences between the standard curves to account for between-plate variation.
Literature Cited
- Al-Natour MQ, Ward LA, Saif YM, Stewart-Brown B, Keck LD. Effect of different levels of maternally derived antibodies on protection against infectious bursal disease virus. Avian Dis. 2004;48:177–182. doi: 10.1637/5319. [DOI] [PubMed] [Google Scholar]
- Anderson R. On the maternal transmission of immunity: a “molecular attention” hypothesis. Biosystems. 1995;34:87–105. doi: 10.1016/0303-2647(94)01444-c. [DOI] [PubMed] [Google Scholar]
- Apanius V. Ontogeny of immune function. In: Starck JM, Ricklefs RE, editors. Avian growth and development: evolution within the altricial-precocial spectrum. New York: Oxford University Press; 1998. pp. 203–222. [Google Scholar]
- Brambell FWR. Transmission of immunity in birds. In: Neuberger A, Tatum EL, editors. The transmission of passive immunity from mother to young. New York: Elsevier; 1970. pp. 20–41. [Google Scholar]
- Carlier Y, Truyens C. Influence of maternal infection on offspring resistance towards parasites. Parasitol Today. 1995;11:94–99. doi: 10.1016/0169-4758(95)80165-0. [DOI] [PubMed] [Google Scholar]
- Davison E, Kaspers B, Schat KA. Avian immunology. London: Elsevier; 2008. [Google Scholar]
- Elazab MFA, Fukushima Y, Fujita Y, Horiuchi H, Mat-suda H, Furusawa S. Induction of immune suppression in the chick by an optimal dose of an immunizing antigen in the presence of its specific maternal antibody. J Vet Med Sci. 2010;72:257–262. doi: 10.1292/jvms.09-0298. [DOI] [PubMed] [Google Scholar]
- Garnier R, Ramos R, Staszewski V, Militão T, Lobato E, González-Solís J, Boulinier T. Maternal antibody persistence: a neglected life-history trait with implication; from albatross conservation to comparative immunology. Proc R Soc B. 2012;279:2033–2041. doi: 10.1098/rspb.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini J, Piault R, Bize P, Roulin A. Pre-hatching maternal effects inhibit nestling humoral immune response in the tawny owl Strix aluco. J Avian Biol. 2009;40:271–278. [Google Scholar]
- Grindstaff JL. Maternal antibodies reduce costs of an immune response during development. J Exp Biol. 2008;211:654–660. doi: 10.1242/jeb.012344. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL. Initial levels of maternally derived antibodies predict persistence time in offspring circulation. J Ornithol. 2010;151:423–428. [Google Scholar]
- Grindstaff JL, Brodie ED, III, Ketterson ED. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc R Soc B. 2003;270:2309–2319. doi: 10.1098/rspb.2003.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff JL, Demas GE, Ketterson ED. Diet quality affects egg size and number but does not reduce maternal antibody transmission in Japanese quail Coturnix japonica. J Anim Ecol. 2005;74:1051–1058. [Google Scholar]
- Grindstaff JL, Hasselquist D, Nilsson J-Å, Sandell M, Smith HG, Stjernman M. Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc R Soc B. 2006;273:2551–2557. doi: 10.1098/rspb.2006.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff JL, Hunsaker VR, Cox SN. Maternal and developmental immune challenges alter behavior and learning ability of offspring. Horm Behav. 2012;62:337–344. doi: 10.1016/j.yhbeh.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamal KR, Burgess SC, Pevzner IY, Erf GF. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poult Sci. 2006;85:1364–1372. doi: 10.1093/ps/85.8.1364. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Marsh JA, Sherman PW, Wingfield JC. Is avian humoral immunocompetence suppressed by testosterone? Behav Ecol Sociobiol. 1999;45:167–175. [Google Scholar]
- Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology: the immune system in health and disease. New York: Garland; 2001. [Google Scholar]
- Killpack TL, Karasov WH. Ontogeny of adaptive antibody response to a model antigen in captive altricial zebra finches. PLoS ONE. 2012;7:e47294. doi: 10.1371/journal.pone.0047294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MO, Owen JP, Schwabl HG. Are maternal antibodies really that important? patterns in the immunologic development of altricial passerine house sparrows (Passer domesticus) PLoS ONE. 2010;5:e9639. doi: 10.1371/journal.pone.0009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenheffer T, Robertson P. The anti-SRBC response of pigeons exposed to sheep erythrocytes as squabs. Am Zool. 1980;20:864. [Google Scholar]
- Lancaster J. Newcastle disease control by vaccination. Vet Bull. 1964;34:57–76. [Google Scholar]
- Lawrence E, Arnaud-Battandier F, Grayson J, Koski I, Dooley N, Muchmore A, Blaese R. Ontogeny of humoral immune function in normal chickens: a comparison of immunoglobulin-secreting cells in bone-marrow, spleen, lungs and intestine. Clin Exp Immunol. 1981;43:450–457. [PMC free article] [PubMed] [Google Scholar]
- Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol. 2006;46:1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- Lemke H, Coutinho A, Lange H. Lamarckian inheritance by somatically acquired maternal IgG phenotypes. Trends Immunol. 2004;25:180–186. doi: 10.1016/j.it.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Lemke H, Hansen H, Lange H. Non-genetic inheritable potential of maternal antibodies. Vaccine. 2003;21:3428–3431. doi: 10.1016/s0264-410x(03)00394-3. [DOI] [PubMed] [Google Scholar]
- Lemke H, Tanasa RI, Trad A, Lange H. Benefits and burden of the maternally-mediated immunological imprinting. Autoimmun Rev. 2009;8:394–399. doi: 10.1016/j.autrev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Linden C, Roth T. IgG receptors on foetal chick yolk sac. J Cell Sci. 1978;33:317–328. doi: 10.1242/jcs.33.1.317. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2nd ed. Cary, NC: SAS Institute; 2006. [Google Scholar]
- Lozano GA, Ydenberg RC. Transgenerational effects of maternal immune challenge in tree swallows (Tachycineta bicolor) Can J Zool. 2002;80:918–925. [Google Scholar]
- Möller G, Wigzell H. Antibody synthesis at cellular level: antibody-induced suppression of 19s and 7s antibody response. J Exp Med. 1965;121:969–989. doi: 10.1084/jem.121.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. 6th ed. NIH Publ. No. 86-23. Washington, DC: Department of Health and Human Services; 1985. [Google Scholar]
- Nemeth NM, Oesterle PT, Bowen RA. Passive-immunity to West Nile virus provides limited protection in a common passerine species. Am J Trop Med Hyg. 2008;79:283–290. [PubMed] [Google Scholar]
- Owen-Ashley NT, Turner M, Hahn TP, Wingfield JC. Hormonal, behavioral, and thermoregulatory responses to bacterial lipopolysaccharide in captive and free living white-crowned sparrows (Zonotrichia leucophrys gambelii) Horm Behav. 2006;49:15–29. doi: 10.1016/j.yhbeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Parmentier HK, Lammers A, Hoekman JJ, De Vries Reilingh G, Zaanen IT, Savelkoul HF. Different levels of natural antibodies in chickens divergently selected for specific antibody responses. Dev Comp Immunol. 2004;28:39–49. doi: 10.1016/s0145-305x(03)00087-9. [DOI] [PubMed] [Google Scholar]
- Pihlaja M, Siitari H, Alatalo RV. Maternal antibodies in a wild altricial bird: effects on offspring immunity, growth and survival. J Anim Ecol. 2006;75:1154–1164. doi: 10.1111/j.1365-2656.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- Pravieux JJ, Poulet H, Charreyre C, Juillard V. Protection of newborn animals through maternal immunization. J Comp Pathol. 2007;137:S32–S34. doi: 10.1016/j.jcpa.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JM, Arcese P, Keller LF, Hasselquist D. Long-term maternal effect on offspring immune response in song sparrows Melospiza melodia. Biol Lett. 2006;2:573–576. doi: 10.1098/rsbl.2006.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitt I, Brostoff J, Male DK. Immunology. 5th ed. London: Mosby; 1998. [Google Scholar]
- Schielzeth H, Forstmeier W. Conclusions beyond support: overconfident estimates in mixed models. Behav Ecol. 2009;20:416–420. doi: 10.1093/beheco/arn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JEG, Bortolotti GR. Immunological development in nestling American kestrels Falco sparverius: post-hatching ontogeny of the antibody response. Comp Biochem Physiol A. 2008;151:711–716. doi: 10.1016/j.cbpa.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Solomon JB. Foetal and neonatal immunology. Frontiers of Biology, no. 20. North-Holland, Amsterdam: 1971. [Google Scholar]
- Staszewski V, Gasparini J, McCoy KD, Tveraa T, Boulinier T. Evidence of an interannual effect of maternal immunization on the immune response of juveniles in a long-lived colonial bird. J Anim Ecol. 2007;76:1215–1223. doi: 10.1111/j.1365-2656.2007.01293.x. [DOI] [PubMed] [Google Scholar]
- Symonds MRE, Moussalli A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol. 2011;65:13–21. [Google Scholar]
- Tizard I. The avian antibody response. Semin Avian Exot Pet Med. 2002;11:2–14. [Google Scholar]
- Tollrian R, Harvell CD. The evolution of inducible defenses: current ideas. In: Tollrian R, Harvell CD, editors. The ecology and evolution of inducible defenses. Princeton, NJ: Princeton University Press; 1999. pp. 306–321. [Google Scholar]
- West AP, Herr AB, Bjorkman PJ. The chicken yolk sac IgY receptor, a functional equivalent of the mammalian MHC-related Fc receptor, is a phospholipase A2 receptor homolog. Immunity. 2004;20:601–610. doi: 10.1016/s1074-7613(04)00113-x. [DOI] [PubMed] [Google Scholar]