Summary
Fluoroquinolone susceptibility-testing is an important step in the design of effective treatment regimens for multidrug-resistant tuberculosis. Here we compare ciprofloxacin, ofloxacin and moxifloxacin resistance results from 226 multidrug-resistant samples. The low level of concordance observed supports that drug sensitivity tests should be performed for the specific FLQ planned for clinical use. The results also support the new WHO recommendation for testing moxifloxacin at a critical-concentration of 2.0μg/ml.
Keywords: drug susceptibility test, multidrug resistant tuberculosis, critical concentration
INTRODUCTION
Fluoroquinolones (FLQs) are among the most effective drugs available for the treatment of multidrug resistant tuberculosis (MDR-TB)1. There is, however, uncertainty about how to interpret results from resistance testing of FLQs. Although the third- and fourth-generation agents (e.g. levofloxacin and moxifloxacin (MOXI)) are considered to have the most activity against Mycobacterium tuberculosis (MTB), in vitro FLQ drug susceptibility testing (DST) may still be performed using second-generation FLQs (e.g. ofloxacin (OFLX) and ciprofloxacin (CIP)). Resistance to OFLX or CIP is inferred to mean resistance to any FLQ2. This extrapolation of resistance across the FLQ class is implicit in how extensively drug resistant (XDR) TB is defined, i.e. as MDR-TB that is resistant to an injectable agent and any FLQ. Yet, the World Health Organization (WHO) now recommends against extrapolating resistance results from second- to third-generation FLQs3.
Interpretation of DSTs is further complicated by the need to choose a “critical concentration” (CC) for testing. If bacterial growth occurs in the presence of a drug at this CC, the bacteria are considered to be resistant. Currently used CCs are often lower than the serum concentrations achievable for the drug. In some cases, this means that isolates are classified as drug-resistant when they may still respond to treatment4. For MOXI, data on the optimal CC is limited5. A MOXI CC of 0.125-0.5 μg/ml is widely used to both guide patient care and in clinical trials2,6. In 2012, based on the experience of several supranational reference laboratories, the WHO proposed an interim recommendation to increase the MOXI CC from 0.5 to 2.0 μg/ml, however, the WHO did not proceed with formal policy guidance recommending the revised concentrations because of the limited data available3. A more refined resistance measure is the FLQ minimum inhibitory concentration (MIC), and this involves testing at a series of increasing concentrations of drug. MICs are seldom measured clinically due to the cost and labor involved.
To determine whether early-generation DST results can predict resistance to later-generation FLQs, we compared DST results for three FLQs: CIP, OFLX and MOXI. We also compared the distribution of MICs for MOXI to achievable serum drug concentrations to assess the validity of the current standard CC.
METHODS
Using an archive of M. tuberculosis samples from patients referred for individualized M/XDR-TB treatment in Lima, Peru, between February 1, 1997, and July 31, 20031, we selected isolates that had undergone CIP DST using the indirect agar proportion method on 7H10 media (Table 1). In 2001, the testing laboratory altered its standard CIP CC, reducing it from 2μg/ml to 1μg/ml. We randomly selected 175 CIP resistant and 100 CIP sensitive isolates from this archive for OFLX and MOXI MIC testing (Table 1) using the indirect proportion method on 7H10 agar. Cutoffs for sensitivity and resistance were chosen based on peak serum concentration data (Table 1), and the prevalent CCs of 2.0μg/ml for OFLX and 0.25-0.50μg/ml for MOXI7,8.
Table 1.
Resistance classification, and concentrations tested for the three fluoroquinolones using the Agar Proportions Method on 7H10 media.
| Drug Tested | Concentrations Tested |
Peak Drug Serum Concentration |
Sensitive | Intermediate Resistance |
Resistant |
|---|---|---|---|---|---|
| OFLX (MIC μg/mL) | 1.0, 2.0, 4.0, 6.0, 8.0, 10.0 |
2-104 | <2.0 | 2.0 | ≥4.0 |
| MOXI (MIC μg/mL) | 0.125, 0.25, 0.5, 1.0, 4.0, 8.0 |
4-911 | <0.5 | 0.5,1.0 | ≥4.0 |
| CIP* (μg/mL) | 1.0 or 2.0 | - | ≤1 or ≤2 | NA | >1 or >2 |
OFLX: ofloxacin, MOXI: moxifloxacin, CIP: ciprofloxacin.
Prior to 2001 isolates were tested at a critical concentration of 2.0μg/ml, from 2001 onwards the critical concentration was decreased to 1.0μg/ml.
We excluded 49 isolates from the analysis that were found to be previous samples from the same patient. Of the 45 total isolates that were CIP resistant but MOXI and OFLX sensitive, 12 (25%) were randomly selected for repeat MIC testing and 10 were reconfirmed to be MOXI and OFLX sensitive. For 2 isolates the measured MOXI MIC increased from 0.25μg/ml to 0.5μg/ml. In eight randomly selected concordant isolates, repeat testing confirmed the exact MIC except in 2 isolates. For the first the MOXI MIC changed from (<=0.125 to 0.25) and for the second the MOXI MIC decreased from 8 to 4 and OFLX MIC from 8 to 6. The repeat MIC results were retained for all data analysis.
We used the Fisher exact test using R version 3.1.0 for statistical testing. This study was deemed not to constitute human subjects research by the Partners Human Research Committee.
RESULTS
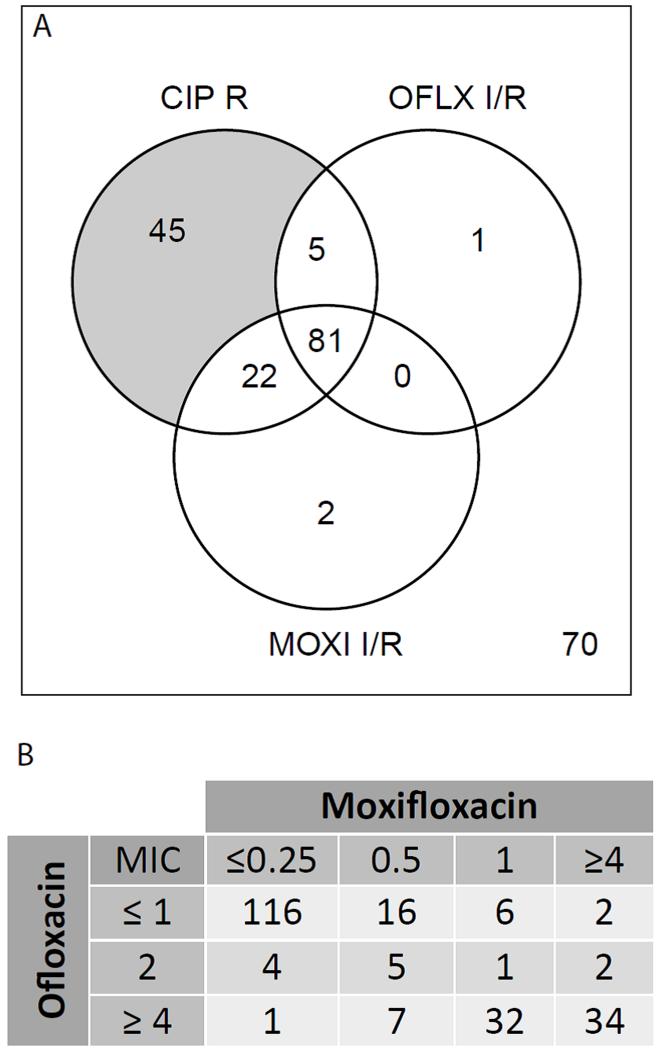
We analyzed 226 MDR-TB patient isolates, of which 153 were CIP resistant and 73 CIP sensitive. Among these, 56% (86/153) of CIP-resistant isolates had an OFLX MIC in the intermediate or resistant range, and 67% of the 153 had a MIC in the intermediate or resistant range for MOXI (Fig 1A). The proportion of strains that were intermediate or resistant was not different before and after 2001 when the CIP CC was decreased from 2.0 to 1.0μg/ml (Fisher p-value 0.7 for CIP/MOXI and 0.8 for CIP/OFLX). None of the 73 CIP sensitive strains was fully resistant to MOXI or OFLX, while three were of intermediate MIC to one of these two drugs.
Figure 1.
DST results. A- Venn diagram of a subset of the DST results showing overlap between ciprofloxacin (CIP), moxifloxacin (MOXI) and ofloxacin (OFLX) resistance (number outside the circle represents the number of isolates sensitive by testing for all three drugs). I or R designations are as defined in Table 1. B- Cross tabulation of MOXI and OFLX MIC results, all drug concentrations are in μg/ml. Numbers in the cells represent strain counts, total=226 strains.
MOXI and OFLX MIC results were in full agreement in 69% (156/226) of samples. Among the remaining 70 (31%) samples, 56% of the discrepancies (39/70) were among isolates with high-level resistance to OFLX but intermediate resistance to MOXI. Of the 226 isolates, 30% had MOXI MICs in the intermediate range (Fig 1B) in contrast to only 5% of the isolates with intermediate OFLX MIC. One third (22/67) of the MOXI intermediate isolates had an OFLX MIC<2 and this explains most of the other discrepancies (22/70, 31%) between the OFLX and MOXI MIC results (Fig 1B).
We determined the sensitivity and specificity of the MOXI 0.5μg/ml MIC as a proxy for the OFLX MIC of 2.0μg/ml, given the recent WHO recommendation to use these interchangeably3. We determined the sensitivity to be 89.1% (66/74 strains with OFLX MIC>2.0 have a MOXI MIC >0.5) and the specificity to be 92.8% (141/152 strains with OFLX MIC ≤2 have an MOXI MIC ≤0.5).
DISCUSSION
Concordance in resistance among the FLQ agents tested was lower than expected with one third to one half of strains showing no agreement among the three agents. The discrepancy between in vitro resistance results of CIP, OFLX and MOXI, and in particular the high rates of CIP/OFLX resistant isolates that were intermediate or sensitive to MOXI, confirms findings from previous smaller studies9, and could account for the continued clinical efficacy of later generation FLQs in XDR-TB treatment10. This discordance among agents within the FLQ class calls into questions the value of defining XDR-TB as TB resistant to one or more FLQ rather than TB that is resistant to all—or at least the most active—class members. The latter definition will arguably carry better prognostic information.
We also found that a substantial proportion (30%) of strains had MOXI MICs in the intermediate range. Peak serum concentrations for MOXI are reported to be upwards of 4μg/ml11, and although serum concentration may not directly correlate with the intracellular concentrations needed for TB bactericidal activity, the high serum concentrations may indicate that intermediate isolates (MIC 0.5-1.0 μg/ml) can be clinically treatable with standard doses of MOXI (400mg/day)2. This finding should be supported with the direct measurement of outcomes in patients infected with MOXI intermediate isolates. This finding is consistent with prior reports2 and supports the recent interim change in WHO’s guidelines suggesting the use of a MOXI critical concentration of 2.0 μg/ml. In sum, our results indicate that CIP or OFLX DST results should not be used clinically to guide the use of MOXI, and support the WHO recommendation to test MOXI at a critical concentration of 2.0 μg/ml.
Acknowledgments
We would like to thank the Peruvian team for their patient care and for providing the clinical isolates that made this study possible.
Funding:
This work was supported by the Harvard Center for AIDS Research (CFAR), an NIH-funded program (P30 AI060354), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, NIDDK, NIGMS, FIC, and OAR. This work was also supported by the American Lung Association Biomedical Research Grant (RG-270912-N) and the Parker B. Francis Fellowship (M.R.F), the NIH/Fogarty International Center (K01 TW009213 K.R.J) and NIH NIAID (U19-AI109755, M.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions:
M.R.F and K.R.J. designed the study with key contributions from C.D.M. and M.M. A.S. and D.K. maintained the strain bank and performed the MIC measurement. M.R.F performed the data analysis with key input from M.F.F. and wrote the first draft of the manuscript. M.M., C.D.M., M.F.F. and K.R.J. provided key edits of the manuscript.
References
- 1.Mitnick CD, Franke MF, Rich ML, Alcantara Viru FA, Appleton SC, Atwood SS, et al. Aggressive Regimens for Multidrug-Resistant Tuberculosis Decrease All-Cause Mortality. PLoS ONE. 2013 Mar 13;8(3):e58664. doi: 10.1371/journal.pone.0058664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sirgel FA, Warren RM, Streicher EM, Victor TC, van Helden PD, Böttger EC. gyrA mutations and phenotypic susceptibility levels to ofloxacin and moxifloxacin in clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother. 2012 May;67(5):1088–93. doi: 10.1093/jac/dks033. [DOI] [PubMed] [Google Scholar]
- 3.Companion handbook: to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. [Internet] WHO Press; Geneva: 2014. [cited 2014 Sep 23]. Available from: http://apps.who.int/iris/bitstream/10665/130918/1/9789241548809_eng.pdf?ua=1&ua=1. [PubMed] [Google Scholar]
- 4.Böttger EC. The ins and outs of Mycobacterium tuberculosis drug susceptibility testing. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2011 Aug;17(8):1128–34. doi: 10.1111/j.1469-0691.2011.03551.x. [DOI] [PubMed] [Google Scholar]
- 5.Horne DJ, Pinto LM, Arentz M, Lin S-YG, Desmond E, Flores LL, et al. Diagnostic Accuracy and Reproducibility of WHO-Endorsed Phenotypic Drug Susceptibility Testing Methods for First-Line and Second-Line Antituberculosis Drugs. J Clin Microbiol. 2013 Feb;51(2):393–401. doi: 10.1128/JCM.02724-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, et al. Four-Month Moxifloxacin-Based Regimens for Drug-Sensitive Tuberculosis. N Engl J Med. 2014 Sep 7; doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaeva Y, Bukatina A, Krylova L, Nosova E, Makarova M, Moroz A. Determination of critical concentrations of moxifloxacin and gatifloxacin for drug susceptibility testing of Mycobacterium tuberculosis in the BACTEC MGIT 960 system. J Antimicrob Chemother. 2013 Oct;68(10):2274–81. doi: 10.1093/jac/dkt202. [DOI] [PubMed] [Google Scholar]
- 8.Global Laboratory Initiative Mycobacteriology Lab Manual. gli_mycobacteriology_lab_manual_quadri.indd-gli_mycobacteriology_lab_manual_web.pdf [Internet]. [cited 2014 Oct 10]. Available from: http://www.stoptb.org/wg/gli/assets/documents/gli_mycobacteriology_lab_manual_web.pdf.
- 9.Sulochana S, Rahman F, Paramasivan CN. In vitro activity of fluoroquinolones against Mycobacterium tuberculosis. J Chemother Florence Italy. 2005 Apr;17(2):169–73. doi: 10.1179/joc.2005.17.2.169. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2010 Jul 1;51(1):6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peloquin CA, Hadad DJ, Molino LPD, Palaci M, Boom WH, Dietze R, et al. Population Pharmacokinetics of Levofloxacin, Gatifloxacin, and Moxifloxacin in Adults with Pulmonary Tuberculosis. Antimicrob Agents Chemother. 2008 Mar;52(3):852–7. doi: 10.1128/AAC.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]