1. FETAL AND EARLY ADULT DEVELOPMENT OF THE ADRENAL CORTEX
1.1 Formation of the adrenal cortex
1.1.1 Origin of the adrenogonadal primordium (AGP)
The adrenal glands develop from two separate embryological tissues: the medulla is derived from neural crest cells originating in proximity to the dorsal aorta, while the cortex develops from the intermediate mesoderm 1. The appearance of the adrenal gland in the form of the adrenogonadal primordium (AGP) at 28–30 days post conception (dpc) in humans (embryonic day (E) 9.0 in mice) is marked by the expression of steroidogenic factor 1 (SF1, NR5A1), a nuclear receptor essential for adrenal development and steroidogenesis 2, 3. The bilateral AGP first appears as a thickening of the coelomic epithelium between the urogenital ridge and the dorsal mesentery. Each AGP contains a mixed population of adrenocortical and somatic gonadal progenitor cells. SF1 positive AGP cells then delaminate from the epithelium and invade the underlying mesenchyme of the intermediate mesoderm 4.
1.1.2 Separation of AGP (formation of the adrenal gland)
Following delamination, the majority of AGP cells migrate dorsolaterally to form the gonadal anlagen (gonadal primordial; GP). A subset of AGP cells that express higher levels of SF1 migrate dorsomedially to form the adrenal anlagen (adrenal primordial; AP or adrenal fetal zone; FZ), ultimately settling ventrolateral to the dorsal aorta 3. At about 48 dpc in humans (E11.5-E13.5 in mice), neural crest cells migrate from the dorsal midline just lateral to the neural tube to the area where the AP is developing 5. These cells persist as discrete islands scattered throughout the embryonic adrenal until birth and ultimately coalesce and differentiate into the catecholamine-producing chromaffin cells of the adrenal medulla 6, 7. Meanwhile, the adrenal gland starts to separate from surrounding mesenchyme and becomes encapsulated with the formation of a fibrous layer overlying the developing cortical cells, a process largely complete by 52 dpc in humans (E14.5 in mice) 8.
Adrenocortical and chromaffin cells have an intimate relationship during embryonic development and postnatal homeostasis. Adrenal glucocorticoids play an essential role in chromaffin cell hormone production by regulating the expression of phenylethanolamine N-methyltransferase (PNMT) that results in epinephrine (as opposed to nor-epinephrine) being the dominant catecholamine produced in the post-natal adrenal medulla9, 10. However, mutant mice lacking the glucocorticoid receptor exhibit normal embryonic neural crest cell migration to the adrenal and normal early fetal chromaffin cell development 11. Similarly, even in the setting of a hypoplastic (Sf1 heterozygous (Sf1+/−) mice) or aplastic (Sf1 null (Sf1−/−) mice) adrenal cortex, a rudimentary adrenal medulla develops12, albeit in an ectopic location in the hypoplastic gland12,13. Further studies are needed to yield insights into the molecular mechanisms that dictate the interplay between the steroidogenic adrenocortical cells and the catecholamine-producing chromaffin cells of the adrenal gland 14, 15.
1.2 Fetal development of the adrenal cortex
1.2.1 Fetal zone formation and function
After encapsulation, the embryonic adrenal cortex expands rapidly. In humans, the enlargement of the fetal cortex (fetal zone; FZ) accounts for the majority of the prenatal growth, especially during the last 6 weeks of gestation. Indeed, the human fetal adrenal is one of the largest organs at term (0.2 % of total body weight and nearly the size of the kidney), with 80% of the gland composed of FZ cells16. These large steroidogenic cells (20–50mm) exhibit a high cytoplasmic/nuclear ratio and robustly express cytochrome P450 17 alpha (CYP17), a bifunctional enzyme with both 17 hydroxylase and 17,20 lyase activities that converts pregnenolone to dehydroepiandrosterone (DHEA). Due to the high activity of CYP17 at this stage, the human fetal adrenal cortex produces large amounts of DHEA and DHEA-S, which is then converted by the placenta to oestrogens for the maintenance of normal pregnancy. While large amounts of other sulphated Δ5 steroids, including pregnenolone sulphate and 17α-hydroxypregnenolone sulphate are also produced by FZ cells, it is unclear if such steroids play a functional role in human biology.
1.2.2 Emergence of the definitive zone
By the 8th week of gestation, new adrenocortical cells emerge between the capsule and FZ, forming the definitive zone (DZ) that later develops into the adult cortex. The DZ is composed of SF1-positive, densely packed basophilic cells arranged in a narrow band of cellular clusters17, 18. Small in size (10–20 mm), DZ cells are lipid-poor during midgestation and exhibit structural characteristics typical of cells in a proliferative state. However as gestation advances, inner cells of the DZ form arched cords with finger-like columns of cells reaching the outer rim of the FZ. During the third trimester, the cells of these cords continue to expand and begin producing cortisol under the regulation of adrenocorticotropin hormone (ACTH) defining the emergence of the ZF of the adult adrenal cortex.
1.2.3 Factors involved in fetal adrenal development (regulatory mechanism)
SF1
The nuclear receptor SF1 (also known as NR5A1) has emerged as a pivotal factor for the initiation and fetal maturation of the adrenal cortex. In the absence of SF1 expression, the adrenal gland does not form (adrenal aplasia in Sf1-knockout mice19 and most human patients20, 21). Moreover, Sf1 haploinsufficiency (Sf1+/−) results in delayed and incomplete development of the adrenal gland in mice22, while overexpression of Sf1 results in aberrant proliferation, gonadal differentiation and ultimate neoplasia in the mouse adrenal23.
The regulation of Sf1 gene expression is complex, with different enhancer elements controlling the spatial and temporal expression in the developing adrenal. Current data indicate that while Wilms tumor 1 (WT1) regulates Sf1 expression in the AGP, Cbp/P300-Interacting transactivator, with Glu/Asp-Rich carboxy-terminal domain, 2 (CITED2) expression in the AGP is necessary for proper differentiation of the adrenal primordial (AP or fetal zone- FZ) 24, 25. Zubair, et al identified the mouse fetal adrenal-specific enhancer (FAdE) in the Sf1 gene as the critical mediator of Sf1 gene expression in the AP26. The transcription complex containing the homeobox protein PKNOX1 (Prep1), homeobox gene 9b (Hox9b) and pre B-cell leukemia transcription factor 1 (Pbx1), initiates FAdE-mediated Sf1 expression in the AGP26. Sf1 subsequently regulates itself by maintaining FAdE-mediated Sf1 expression in the AP. After E14.5 in mice, FAdE is no longer utilized27. In the emerging DZ, Sf1 regulation is shifted to a different definitive enhancer that has not yet been characterized. In humans, however, no similar FADE or DZ enhancers have yet been confirmed26.
Dax1
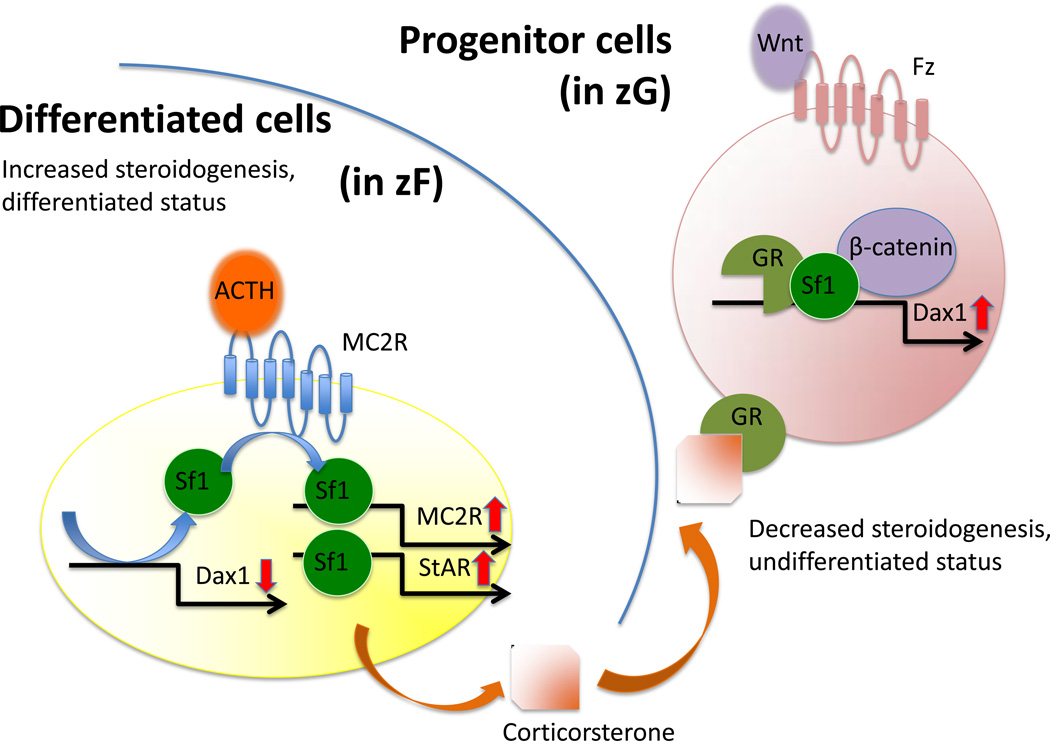
A Sf1 target gene, Dax1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1, Nr0b1), is an orphan nuclear receptor. DAX1 was first cloned as the gene responsible for X-linked Cytomegalic Adrenal Hypoplasia Congenita 28, 29. In the adrenal, Dax1 primarily serves as a co-repressor of Sf1-mediated transcription of steroidogenic genes 4, 20, 30. Consistent with this role and the enriched expression of Dax1 in the outer cortex, knockdown of Dax1 results in premature differentiation of mouse adrenocortical progenitor cells. However, this occurs at the expense of depleting this essential cell population, ultimately resulting in adrenal failure31. As such, Dax1 plays an essential role in the maintenance of stem/progenitor cell pluropotency. Moreover, Dax1 may serve as the repressor for FAdE activity in the mouse adrenal gland during the FZ to DZ transition 27. (The relationship between Dax1, Sf1, ACTH and Wnts are summarized in Figure 1)
Figure 1.
Interaction of Sf1, GR, β-catenin on Dax1 regulation
Accordingly, regulation of Dax1 expression is predicted to be a dynamic process, balancing progenitor renewal and adrenocortical differentiation/steroidogenesis. Sf1 activates Dax1 transcription in cooperation with paracrine Wnt signaling and glucocorticoids that are synthesized in the differentiated adult cortex32. Conversely, ACTH, the well-established glucocorticoid stimulator, has been shown to effect release of Sf1 complexes from the Dax1 promoter, thus leading to effective inhibition of Dax1 transcription. This is predicted to promote the response of Sf1-positive progenitor cells to ACTH and subsequently initiate steroidogenesis. In an analogous fashion, Dax1 plays a similar role in mouse embryonic stem (ES) cells. It is transcriptionally activated by the other member of the NR5A family, Liver Receptor Homolog 1 (LRH1), and the homeobox transcription factor Nanog 33. Moreover, knockdown of Dax1 leads to premature spontaneous differentiation of ES cells into cells of all three germ layers, establishing Dax1 as a critical mediator of stem/progenitor cell pluripotency.34
ACTH and other hormonal factors
ACTH is a 39 amino acid peptide secreted from the anterior pituitary gland under control of corticotropin-releasing hormone (CRH) 35. As a major component in the hypothalamic- pituitary-adrenal axis, ACTH binds to the transmembrane receptor melanocortin receptor 2 (MC2R) specifically in adrenocortical cells and exerts its effect through downstream cAMP and Ras/MEK/ERK signaling pathways36. While adrenocortical growth and differentiation are independent of ACTH during the first trimester of human pregnancy, ACTH begins to play an essential role in the morphological and functional development of the adrenal gland after 15 weeks of gestation37. The growth promoting effects of ACTH are mediated in part through the stimulation of locally produced growth factors such as insulin-like growth factor 2 (IGF2) and fibroblast growth factor beta (FGFβ) 38, 39. During development, as the outer DZ emerges, ACTH participates in the regulation of steroidogenesis, cell differentiation and cell growth40–42.
As mentioned above, CRH, a 41-amino acid peptide, is commonly considered to regulate adrenal function indirectly through the central nervous system (CNS) via the hypothalamic-pituitary-adrenal (HPA) axis43. Secreted by the parvocellular neurons of the paraventricular nucleus (PVN) in the hypothalamus in response to central and peripheral stimuli, CRH stimulates corticotroph cells in the anterior pituitary to produce POMC, which is subsequently cleaved to ACTH44.
Interestingly CRH, CRH-homologous peptides (Urocortin 1–3, UCN1-3) and their receptors (CRF1 and CRF2 receptor) are all found locally in the both adult and fetal adrenal gland with different distribution patterns46, 47. In adults, while CRH is found to be expressed throughout the gland, UCN1 mainly presents in chromaffin cells in the medulla44, 45. On the contrary, UCN2 is observed in all three zones of human cortex but is only weakly expressed in adrenal medulla, and UCN3 also appears to be expressed in human adrenals45, 46. The expression patterns of CRF and UCNs in fetal adrenals parallel the developmental changes in human, where UCN1, UCN3 and the CRF1 and CRF2 receptors all appear to be expressed in both FZ and DZ of the adrenal cortex47, 48.
The presence of all CRH peptides and their receptors in the adrenal suggests that the CRH system can function locally within human and rodent adrenals. Another important source of CRH is the human placenta, which releases CRH into the fetal circulation. As gestation advances CRH concentration increases: the remarkable increase in placental CRH production at the end of gestation has been suggested to contribute to the parturition process by forming a feed-forward loop that leads to increased productions of cortisol and DHEA/ DHEA-S in human fetal adrenals. Studies have shown that CRH stimulates cortisol production in primary cultures of fetal adrenocortical cells by elevating the mRNA levels of steroidogenic acute regulatory protein and other steroidogenic enzymes, including 3β-HSD2, CYP21 and CYP11B149. In addition, CRH enhances the adrenal response to ACTH, further driving the production of cortisol and DHEA/DHEA-S49, 50. A further study has shown that the stimulatory effect of CRH on cortisol production may require the presence of chromaffin cells51, consistent with the potential paracrine relationship between cortex and medulla.
Insulin-like growth factor 2 (IGF2)
Both IGF1 and IGF2 are mitogens expressed in the adrenal gland. IGFs bind dimeric/heterodimeric cell surface receptors (insulin receptor (IR) or IGF receptor 1 (IGF1R)) and induce autophophorylation of the intracellular portion of the receptor, leading to activation of Ras/MEK/ERK and PI3K/AKT52, 53. Although both IGF1 and IGF2 are present during human adrenal development, IGF2 is more highly expressed in early fetal development, where it likely plays a major role 54–56. In contrast, IGF1 is dominant in the adult adrenal gland, functioning as a regulator of postnatal growth maintenance. Although at lower levels compared to fetal adrenal cortex, IGF2 is expressed in the adult adrenal gland with expression restricted to the peripheral cortex and capsule of the adult adrenal, coinciding with the stem/progenitor cell niche 53. In the mouse however, the expression pattern is a little different: in the fertilized mouse embryo, mRNA transcripts encoding Igf2 are present in low amounts almost as soon as the embryonic genome is activated, at the 2- to 4-cell stage, while the Igf1 mRNA appears later, around the time of implantation at 8 to 9 d of gestation. Besides their mitogenic effects, IGFs and FGF have been shown to be essential factors for maintenance of the stem cell niche in multiple other organ systems 56, 57, supporting a potential role for IGF2 in adrenal stem/progenitor cell maintenance. A more recent study found the IGF pathway essential in adrenogonadal development and primary sex determination, since knocking down the receptors causes adrenal agenesis, possibly by decreased expression of Sf1 in AGP and subsequent failure of adrenal primordia specification. Growth retardation, male-to-female sex reversal and delayed ovarian development are additionally observed in these mice58.
1.2.4 Transition of FZ to DZ/ adult cortex
Studies by Zubair, et al provided significant insights into the relationship between the FZ and the DZ/adult cortex 26, 27, 59, 60. Using the FAdE-Cre mouse model, these investigators observed proliferating cells in a scattered pattern throughout the adrenal gland until E13.5 13, 60. At later time points, proliferating cells assembled in the periphery of the adrenal gland 61. Similar to the developing human adrenal cortex, the mouse FZ is first encapsulated by surrounding cells of the intermediate mesoderm. Following encapsulation a second group of cells emerge as the densely packed DZ or definitive (adult) cortex. As previously noted, Sf1 is activated by FAdE only in the FZ before E14.5 but this enhancer does not activate Sf1 expression in the DZ. By breeding a Rosa26 mouse with a transgenic mouse harboring the Cre-recombinase gene driven by a basal Sf1 promoter and the FAdE enhancer, Zubair et al traced the fate of FZ cells during development. Their results demonstrated that all DZ cells are derived from FAdE-expressing cells of the fetal adrenal 60. To complement these experiments, Zubair et al also utilized a tamoxifen-inducible FAdE-cre mouse model. DZ staining was only observed when tamoxifen was administrated early in embryogenesis (E11.5-E12.5). No LacZ-positive cells were found when tamoxifen was administered after E14.5. These results are consistent with the absence of FAdE activity during later developmental stages and support the conclusion that the FZ gives rise to the DZ.
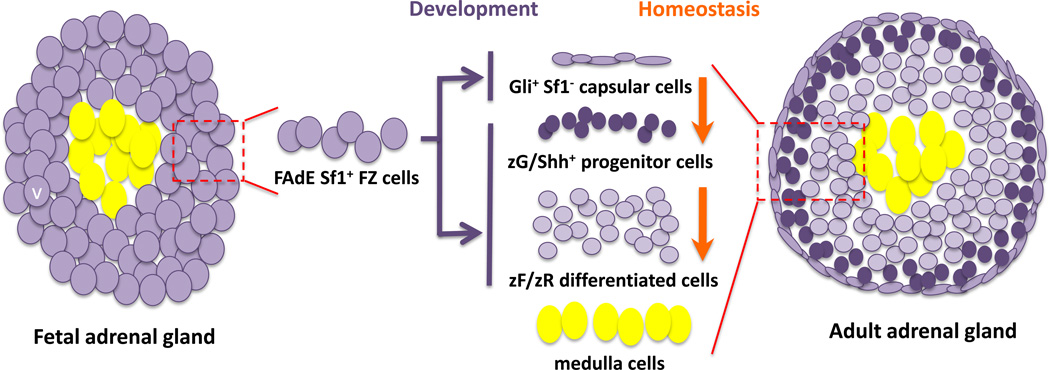
In contrast, studies focused on a downstream activator of the hedgehog pathway, Glioma-Associated Oncogene Homolog 1 (Zinc Finger Protein) (Gli1) provide evidence that the adrenal capsular cells also give rise to the DZ 62, 63. Gli1-expressing cells are specifically located in the adrenal capsule and do not express Sf1. As demonstrated by King et al, this subpopulation of cells is capable of giving rise to Sf1-expressing, differentiated adrenocortical cells during embryonic development63. However, these data are in conflict with the above-mentioned lineage tracing experiments using FAdE-LacZ reporter mouse that revealed that all DZ cells arise from FZ cells. While these apparently conflicting observations may reflect two temporally distinct lineages of the definitive cortex, in which both the Sf1-positive FZ and the Sf1-negative capsule cells can give rise to Sf1-positive DZ adrenocortical cells, recent studies support a single developmental and homeostatic mechanism of adrenal growth. Wood et al demonstrated that FZ cells that once expressed Sf1 under control of the FAdE enhancer gave rise to a subset of capsular cells64. These FZ cell descendants within the adrenal capsule express Gli1, suggesting some FZ cells can transition into Sf1-negative capsular cells which in turn can give rise to the underlying DZ /adult cortex (Figure 2).
Figure 2.
Stem cell theory of adrenal development and homeostasis
1.3 Postnatal adrenal development
The human adrenal gland continues to undergo significant remodeling during neonatal and pubertal periods. Immediately after birth, cells within the FZ undergo apoptosis, resulting in involution of the remaining FZ 65. On the other hand, under the influence of the trophic hormones angiotensin II (AngII) and ACTH, the adult zona glomerulosa (zG) and zona fasciculata (zF) mature. Later, at age 6–8 years old in females and 7–9 years old in males, the adrenal zona reticularis (zR) begins to form between the zF and the medulla. This process, known as adrenarche 66, is characterized by increased proliferation and production of adrenal androgens. Due to zR hormonal influence, secondary sexual characteristics appear, particularly development of pubic hair and altered sweat composition, which produces adult body odor. Additionally, increased oiliness of the skin and hair and mild acne may occur 67. In most males, these changes are indistinguishable from early testicular testosterone effects occurring at the beginning of gonadal puberty. In females, adrenal androgens from adrenarche drive most of the early androgenic changes of puberty. Adrenarche can occur prematurely in many children who are overweight and/or born small for gestational age, suggesting links between placental hormonal, intrauterine growth retardation, metabolic disease and zG function 68–71. However, the exact mechanisms for zR growth and the control factors for adrenarche remain elusive.
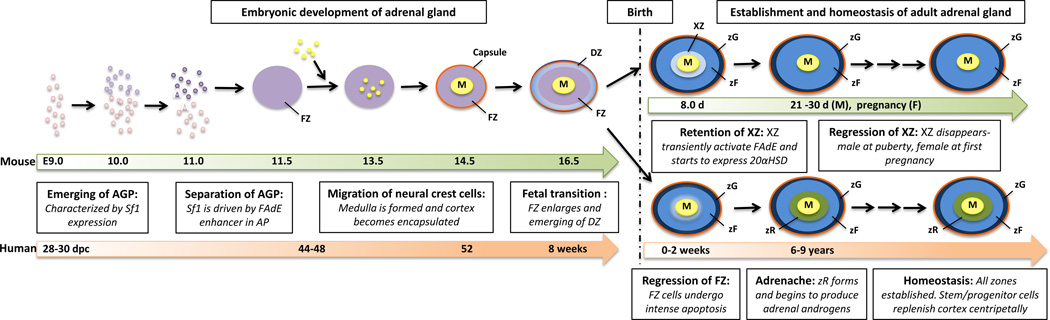
In rodents, the remaining remnants of the FZ (classically called X-Zone in mouse) ultimately regress at the onset of puberty in male and during the first pregnancy in females 72, 73. However, because FAdE activity is lost by E14.5, a definitive enhancer likely begins functioning before birth. Current efforts aim to identify and characterize a definitive/adult Sf1 enhancer in rodents. (Figure 3 summarizes the major milestones of human and mouse adrenal gland development)
Figure 3.
Development and zonation of the adrenal gland.
FZ: fetal zone. DZ: definitive zone. XZ: remnant of fetal zone (x-zone). zG: zona Glomerulosa. zF: zona Fasciculata. zR: zona Reticularis.
2. STRUCTURE AND FUNCTION OF THE ADULT ADRENAL CORTEX
2.1 Adrenal cortical zonation
2.1.1 Anatomy
In conjunction with FZ regression, the DZ forms discrete functional compartments within the adrenal cortex, termed the zG and the zF, respectively. The zG, composed of ovoid shaped cells, is the most outer layer . Although in kids with low salt diets, zG is normally presented as a full zone, due to high-salt consumption in western diet, zG cells in adults are actually scattered in clusters with a width of 200–1300 µm and a depth of 100–500 µm beneath the capsule74–76. These so-called aldosterone-producing cell clusters (APCCs) express CYP11B2 enzyme and is responsible for aldosterone production74. The zF constitutes the majority of the adrenal cortex, sitting directly under the zG. Importantly, zF cells are bigger cells organized into bundles, which led to its name, “fascicles”. Lastly, zR constitutes the innermost layer of the adrenal cortex, between the zF and the adrenal medulla. Here, ZR cells are arranged as cords that project in different directions, resulting in the net-like appearance that gives the zone its name (reticulum= net).
Extracellular matrix (ECM)
ECM, which is composed of glycoproteins and associated integrin receptors, has been shown to play an important role in both the fetal and adult adrenal77. Using second-trimester human fetal adrenal glands, in situ studies have demonstrated that collagen type IV is the major ECM glycoprotein present in the fetal adrenal and that it is evenly distributed throughout the gland. In contrast, other ECM glycoproteins, including laminin and fibronection, display more restricted expression patterns. Specifically, laminin is mainly expressed in the early expanding DZ while fibronectin is found with a gradient highest in the FZ and decreasing in the outer layers. Interestingly, the receptors for ECM components--integrin subunits also distribute in different zones of the human fetal adrenal. Integrin α1 chain is found primarily in the DZ, while α2-subunits are found abundantly at the interface of the FZ/DZ. The α3 chain (a low-affinity ligand for fibronectin), however, is observed in the DZ/FZ and FZ. Consistent with this pattern, in vitro studies using primary human fetal adrenal cells cultured on matrix-coated dishes found that the ECM not only contributes to maintenance of cell morphology of primary cultures, but also influences cellular behavior. For example, increased proliferation rates are observed on laminin- or collagen-coated dishes, while fibronectin supports higher levels of apoptosis78. Additionally, collagen IV strongly enhances ACTH-stimulated DHEA/DHEA-S as well as cortisol secretion 78, 79; fibronectin increases DHEA and DHEA/DHEA-S secretion through increased P450C17 gene expression79. These studies demonstrate that the unique ECM environment within each region of the fetal adrenal gland supports zone-specific cellular behavior.
Similar to its effects in the fetal gland, the ECM is also thought to influence behavior and function within the adult adrenal gland. Studies in adult rat adrenal glands have shown varying expression gradients for all four major glycoproteins. In adult rat adrenal glands, collagen I is the major glycoprotein in the outer capsule, with moderate levels of collagen IV, fibronectin, and laminin also present. Collagen IV, fibronectin, and laminin are found surrounding each glomerulosa cell in the zG. In the zG, collagen IV is present as thick fibrils while fibronectin and laminin transition to discontinuous and short fibrils, respectively. Interestingly, the expression of each of these glycoproteins progressively decreased in the inner cortex regions. In the zR, only collagen IV is observed. In addition to varying expression patterns, ECM components also have distinct effects on adult adrenal function in vitro80. Specifically, fibronectin, collagen I, and collagen IV increase 3β-HSD expression and subsequent aldosterone production in zG cells as well as corticosterone secretion in zF cells. In contrast, laminin decreased protein synthesis, 3β-HSD expression, and corticosteroid release80. Morphological changes are also detected on cells growing on different ECM, indicating potential changes in function as well. Similarly, induction of 11β-hydroxylase and 21-hydroxylase enzyme expression is observed in bovine adrenal cells cultured with ECMs81. These results suggest that complex interactions between different matrix gradients likely contribute to the precise control of cellular behaviors within each zone.
2.1.2 Adrenal blood supply and innervation
Three critical arteries supply each adrenal gland: the superior suprarenal artery provided by the inferior phrenic artery, the middle suprarenal artery provided by the abdominal aorta and the inferior suprarenal artery provided by the renal artery. Perhaps due to the energy intensive nature of steroidogenesis, the adrenal glands have one of the greatest blood supplies per gram of tissue of any organ, with up to 60 arterioles entering each adrenal gland. This may account for the high prevalence of cancer metastases found in the adrenal gland 82–84. Arterial blood first reaches the outer surface of adrenal cortex where the cortical arteries branch to form the subcapsular capillary plexus85. Most of the subcapsular plexus become fenestrated capillaries that follow the cord like structures of the ZF down into the ZR before dumping into the medullary veins, which then form the suprarenal veins. Some of the arterioles continue through the cortex and provide blood directly to parts of the medulla. The right suprarenal vein drains into the inferior vena cava and the left suprarenal vein drains into the left renal vein or the left inferior phrenic vein 86–89. This unique anatomy of blood supply in adrenal cortex provide an interesting spatial gradient of adrenal steroids in different zones and may be a contributing regulatory mechanism for the distinct characters of cells in each zone.
The adrenal gland is also richly innervated, with the majority of nerve plexuses residing in the capsular region. Their fibres mainly originate from the greater splanchnic nerve and associated abdominal plexuses of the sympathetic autonomic nervous system, together with some parasympathetic contributions from the phrenic and vagal nerves. The nerve bundles penetrate the cortex, mostly in association with blood vessels90. Similar to the vasculature structure, the majority of these postganglionic nerves terminate in the medulla. While controversial, it has been hypothesized that the innervation of the superficial blood vessels regulates blood flow in the cortical capillary bed. Moreover, in vitro studies have reported the regulation of cortical blood flow by neuropeptides released in response to splanchnic nerve stimulation. Specifically, vasoactive intestinal polypeptide and Met-enkephalin increase, while neuropeptide Y decreases, cortical flow rate91. Lastly, while of unclear pathophysiologic relevance, these same neuropeptides are present at lower levels in associated nerve fibers in hyperplastic cortical tissue92.
2.2 Function of adrenal cortical zones
The main function of the adrenal cortex is to produce a variety of steroids hormones. Although each zone uses cholesterol as the precursor molecule, distinct steroids result from differential enzyme expression.
Mineralocorticoids
Angiotensinogen (synthesized in liver) is converted by renin (from the juxtaglomerular cells of the kidney) to Angiotensin I that is later converted to Angiotensin II by angiotensin converting enzyme (ACE) in the lung. Aldosterone is the primary mineralocorticoid produced by zG cells under the control of angiotensin II and extracellular potassium93–96. Aldosterone functions as the ligand for the nuclear receptor MR (mineralocorticoid receptor) in target tissues that include the colon, salivary gland and the renal distal convoluted tubule and collecting ducts, where it causes increased reabsorption of sodium and increased excretion of both potassium (by principal cells) and hydrogen ions (by intercalated cells of the collecting duct).
Glucocorticoids
Glucocorticoids are produced by zF cells at a basal level, but can be stimulated by ACTH secreted from the anterior pituitary under stress 97–99. In humans, cortisol is the main glucocorticoid produced by the adrenal cortex under normal conditions and its actions include mobilization of fats, proteins, and carbohydrates 100–102. In mice and rats, due to the lack of Cyp17 gene expression, corticosterone is the major glucocorticoid produced and utilized 103–105. Once produced and released into the bloodstream, glucocorticoids facilitate the release of energy stores for utilization during stress. Integral to the feedback control of the activated HPA axis, glucocorticoids inhibits production and secretion of ACTH from the HPA axis 106, 107.
Adrenal androgens
In primates, cells in the zona reticularis produce precursor androgens including DHEA and androstenedione108–110. DHEA is further converted to DHEA-sulfate by the action of dehydroepiandrosterone sulfotransferase (SULT2A1). These precursors are weak androgens and get released into the bloodstream and are taken up in peripheral tissues that include hair follicles, genital skin, and prostate for production of more potent androgens including testosterone111–113. Recent work using human adrenal vein sampling has detected testosterone and other downstream androgen metabolites from the adrenal cortex, suggesting the presence of a wider variety of adrenal androgens than previously appreciated 114. The regulatory mechanism of adrenal androgen production has not been fully elucidated. However, LH/hCG receptors have been shown to be present in zR115 and hCG can stimulate DHEA-sulfate synthesis in cultured human fetal adrenals116, 117. This suggested that LH/hCG might regulate androgen synthesis in an ACTH-independent manner. Moreover, the illicit action of LH has been implicated in excess cortisol production in primary adrenal macronodular hyperplasia (PMAH) patient118.
2.3 Homeostasis of adult cortex
2.3.1 Homeostatic Maintenance of the DZ/adult cortex
After the functional adult adrenocortical zones are established, they are maintained by stem or progenitor cells. Beginning in the 1930s, numerous studies have suggested that cells from the outer region of the adrenal cortex proliferate and migrate to repopulate the adrenal cortex119–121 Several studies show that most proliferating cells are located in the outer layer of the mature adrenal gland, while most cell death occurs near the boundary of cortex and medulla. These historic data are consistent with the presence of a stem/progenitor cell population in or directly under the capsule of the cortex122–125.
Shh pathway
In 2009, three laboratories independently utilized lineage-tracing to provide evidence that the Shh signaling pathway was essential for mouse adrenal gland development and maintenance 63, 126, 127. In these studies, Shh was found in the adrenal gland at E11.5, primarily in the subcapsular zG where stem/progenitor cells reside. In adult adrenal glands, Shh colocalizes with Sf1 in non-steroidogenic cortical cells of the zG region but not in differentiated zG or zF cells, which express both Sf1 and markers of fully differentiated steroidogenic cells (Cyp11b2 and Cyp11b1, respectively). However, descendants of Shh expressing cells express adrenocortical differentiation markers, suggesting Shh-positive cells serve as progenitor cells for the adrenal cortex63. Mice in which Shh is ablated specifically in Sf1-expressing cells reveal marked adrenal hypoplasia, decreased proliferation, and a thin capsule126. However, despite a decrease in size, the adrenal glands in these mice maintain proper zonation, suggesting Shh does not have a role in the initiation of differentiation. Together, these data implicate the Shh pathway in actively promoting maintenance of the adrenal cortex.
Wnt pathway
The Wnt/β-catenin signaling pathway is also critical for adrenocortical homeostasis. Studies performed in the mouse adrenal revealed that β-catenin expression and activity is present early in the fetal cortex. By E18.5, with the emergence of the DZ, β-catenin is restricted to the subcapsular zG128. Employing a cre-lox technology to ablate β-catenin specifically in Sf1-expressing cells of the adrenal cortex129, Kim et al observed adrenal aplasia at birth in mice expressing a highly penetrant Sf1-cre transgene. Careful examination of these mice showed normal adrenal development until E12.5, after which adrenal failure became evident precisely as DZ cells emerged between the coalescing capsule and the FZ. Intriguingly, in mice that bear a weakly penetrant Sf1-cre transgene (express β -catenin in about half of the adrenocortical cells), adrenal development progresses normally. However, as the mice age (i.e. 30 weeks) a progressive cortical thinning and a decreased steroidogenic capacity is observed. This progressive failure of the cortex is hypothesized to be a result of the loss of adrenocortical progenitor cells.
Additional support for a role of Wnt/β-catenin signaling pathway in the maintenance of adrenocortical stem/progenitor cells comes from studies in which the pathway has been genetically “over-activated” in the mouse adrenal cortex. In these studies, both an expansion of undifferentiated progenitor-like cells and ultimate tumor formation are observed. In one model of elevated Wnt signaling, hyperaldosteronism due to zG-specfic expansion is characterized129, 130. Moreover, activation of the Wnt pathway is frequently observed in adrenocortical neoplasms131–133. Although the exact function of Wnt/ β-catenin signaling in the adrenal cortex remains unknown, the fact that β-catenin and Sf1 can directly activate Dax1 suggests that Dax1 may be a critical mediator of Wnt action in the adrenal cortex134. Recent data indicates that Wnt/ β -catenin signaling may additionally regulate adrenal homeostasis by inhibiting fasciculata differentiation, promoting the undifferentiated state of progenitor cells and priming the progenitor cell for zG fate135, 136.
2.3.2 Homeostasis of cortical zones
Homeostasis of the adrenal cortex also entails the maintenance of zonal structure and function in the context of continual centripetal cellular repopulation of the gland. Because stem/progenitor cells reside primarily in the outer/subcapsular zG, investigators have asked whether differentiated cells first adopt a zG fate and later transition to zF and zR, or alternatively whether the stem/progenitor cells differentiate directly into each of the three cell types?
As previously mentioned, lineage-tracing studies using an inducible GFP marker to track the fate of Shh-descendants revealed that GFP expression was more prevalent in zG cells initially following induction of Shh-GFP. At later times however, an increasing number of GFP-expressing zF cells were observed63. These results suggest that the majority of progenitor cells become zG cells first and then transition to zF cells.
More direct evidence for this model comes from recently published work using a zG-specific Cyp11b2-promoter-driven GFP knock-in mouse, wherein zG cell descendants are labeled by GFP 137. In this model, GFP-positive cells are rare in the adrenal gland of newborn mice. However, within three months, the entire cortex becomes GFP-positive, suggesting all cells within the zF and x-zone originated from the zG cells. Furthermore, treatment with dexamethasone, a cortisol analog used to repress ACTH, can prevent zG cells from transitioning to zF cells, manifesting in atrophy of the zF. These results implicate ACTH as an important mediator of zF homeostasis. Surprisingly, when Sf1is ablated in zG cells, the mice are able to maintain a normal functional zF zone, despite the complete absence of zG-derived GPF cells within the zF, suggesting perhaps that progenitor cells can (although they usually do not) directly adopt a zF phenotype without first becoming a Cyp11b2-expressing zG cell. (Figure 2)
3. DEFECTS IN CORTICAL ZONAL DEVELOPMENT
3.1 Adrenal hypoplasia
The term “adrenal hypoplasia” refers to distinct clinical conditions characterized by the underdevelopment or hypotrophism of the adrenal cortex. Patients with complete adrenal hypoplasia or aplasia characteristically develop adrenal insufficiency soon after birth, or less commonly, in a more insidious manner throughout childhood or even in young adulthood. Adrenal insufficiency is potentially life-threatening if not promptly recognized and treated.
Based on its physiopathology, adrenal hypoplasia can be divided into two broad categories: primary and secondary. Primary adrenal hypoplasia is characterized by a hypotrophic (and hypofunctional) adrenal cortex in the presence of normal hypothalamic/pituitary function. The causes of primary adrenal hypoplasia can be further categorized into 1) defects of adrenal cortex formation or differentiation – a condition known as congenital adrenal hypoplasia (AHC) and 2) “ACTH resistance” and related conditions (also known as familial glucocorticoid deficiency). AHC is usually associated with a severe phenotype, which might also include disorders of sexual development caused by gonadal failure, severe adrenal insufficiency associated with salt-wasting and other developmental disorders138–141. The most common form of AHC is the X-linked congenital adrenal hypoplasia due to disruption of the nuclear receptor DAX1 (OMIM 300200). Mutations of SF1 are one contributing cause of the autosomal form of AHC139–141. Rare autosomal forms of AHC include the IMAGE syndrome (OMIM 600856) and the SERKAL syndrome (OMIM 603490), caused by mutations of CDKN1C and WNT4, respectively138, 141. Familial isolated glucocorticoid deficiency syndromes (broadly known as “ACTH resistance” syndromes) are autosomal recessive diseases caused by mutations in genes that participate in the ACTH signaling. These include mutations in MC2R and MRAP (a gene that codes for an accessory protein of the ACTH receptor, essential for its targeting and function)142, 143. More recently, mutations in the NNT and MCM4 genes were also associated with familial glucocorticoid deficiency 143–145. Patients with ACTH resistance usually do not present with the salt-wasting phenotype because aldosterone-producing cells are not affected by these mutations.
“Secondary adrenal hypoplasia” is caused by a pituitary or hypothalamic dysfunction that results in the disruption of ACTH synthesis, processing and/or release by the pituitary gland. Thus the adrenal zF becomes hypotrophic and hypofunctioning due to the lack of adequate stimulus. Causes of secondary adrenal hypoplasia include 1) developmental abnormalities of the CNS such as anencephaly, septo-optic dysplasia (OMIM 182230) and holoprosencephaly/Pallister-Hall syndrome 2 spectrum (OMIM 615849), the last two caused by mutations in HESX1 and GLI2; 2) diseases of pituitary development such as pituitary combined hormone deficiency syndromes (caused by mutations in PROP1, POU1F1, OTX2, LHX3 and LHX4); 3) isolated ACTH deficiency (OMIM 201400) (caused by TBX19 mutations)); 4) defects in POMC and neuroendocrine convertase 1 (PCSK1), which leads to deficiencies in all POMC-related peptides146, 147; and 5) miscellaneous non-genetic causes that lead to hypothalamic or pituitary dysfunction such as idiopathic, intracranial tumors, infections or birth-related traumatic injuries143, 148–151.
Considerable progress has been made in the understanding of the genetic basis of many of these forms of adrenal hypoplasia in the past 20 years. These breakthroughs have been very important to allow for counseling families and screening of other family members at risk of adrenal failure, as well to provide insight into the molecular mechanisms of adrenal development in humans. However, for a significant percentage of cases of primary adrenal hypoplasia, the underlying cause remains unknown.
3.2 Disorders of excessive growth
3.2.1 Adrenal hyperplasia
Disorders of the adrenal cortex characterized by excessive growth include hyperplasias and tumors. Hyperplasias are invariably polyclonal disorders defined by bilateral enlargement of the adrenal cortex. Adrenal hyperplasia can be secondary to ACTH overstimulation (also referred as ACTH-dependent hyperplasia), such as in Cushing's disease or congenital adrenal hyperplasia. (CAH). The latter is a common denomination of a heterogeneous group of autosomal recessive disorders that affects the function of key enzymes of the steroidogenic cascade, resulting in deficient cortisol production. As a consequence, the pituitary produces higher amounts of ACTH, which leads to 1.accumulation of steroid precursors before the enzymatic defect and 2.increase in adrenal size, ultimately leading to nodules formation. The symptoms will depend on the level of the enzymatic blockage (which in turn will determine which type of steroid precursors will accumulate) and the severity of the defect (how much does it impair cortisol production). The most common form of CAH is the 21-hydroxylase deficiency (CYP21A2), accounting for more than 95% of the cases152. More than 100 mutations have been described in this disease and a strong genotype-phenotype correlation is observed153. The classical form is characterized by the presence of virilization at birth with genital ambiguity on the female fetuses (due do androgen precursor accumulation) and severe adrenal insufficiency, that might be aggravated by mineralocorticoid deficiency in the salt-wasting forms154. The non-classical form is usually characterized by mild signs of hyperandrogenism that frequently goes unnoticed until adulthood154. Other types of CAH (each of these with distinct clinical manifestations) may result from mutations in other genes that code for enzymes of the steroidogenesis cascade, including STAR, CYP11A1, HSD3B2, CYP17A1, CYP11B1 and POR155–157. Primary causes of adrenal hyperplasias are by definition ACTH-independent. However, intra-adrenal ACTH production has recently been described in some cases of primary adrenal hyperplasia, suggesting that some of the growth might be mediated by paracrine/autocrine ACTH stimulation from the hyperplastic tissue itself158. Primary adrenal hyperplasias are often associated with a genetic syndrome. Some of these syndromes, such as Multiple Endocrine Neoplasia type 1 (MEN1), Gardner syndrome, McCune-Albright syndrome and Carney complex, are characterized by multiple organ involvement. In other syndromes, such as primary adrenal macronodular hyperplasia (PMAH) and isolated primary pigmented micronodular adrenal hyperplasia (PPNAD), the adrenals are the only organs affected159, 160. Both PMAH and PPNAD have been described in familial and isolated forms. Recently, germline mutations in ARMC5 have been described in about half of the PMAH cases, being the most frequent genetic alteration associated with this disease. Remarkably, the prevalence of germline ARMC5 mutations is high even among patients with apparent sporadic disease, suggesting that familial forms are actually much more prevalent than previously appreciated. Regarding the endocrine manifestations, the adrenal hyperplasia can either functioning or non-functioning. The most common endocrine manifestation is Cushing syndrome, but rare cases of mineralocorticoid and androgen production have been reported161, 162. Abnormal activation of the protein kinase A (PKA) pathway by different molecular mechanisms, such as ectopic expression of G-protein-coupled receptors by adrenal cells, activating somatic mutations in GNAS, inactivating mutations of PRKAR1A (the gene that codes for one of the regulatory subunits of the PKA complex), inactivating mutations of PDE11A and PDE8B and gene amplification of a catalytic subunit of the PKA complex (PRKACA), is usually present in the hormonally-active group163, 164. (Table 1)
TABLE 1.
Genetic Syndromes Associated with Adrenal Hyperplasia/Neoplasia
| Syndrome | Heritage | Locus | Gene | Clinical features | Adrenal manifestations |
Comments |
|---|---|---|---|---|---|---|
| Multiple endocrine neoplasia type 1 |
Autosoma l dominant |
11q13 | MEN1 | Primary hyperparathyroidism, gastric, pancreatic, and duodenal neuroendocrine tumors, pituitary adenomas, thymic carcinoid tumors |
Non-functioning macronodular hyperplasia in up to 40% of patients. ACCs rarely described |
Somatic MEN1 mutations have been frequently described in sporadic ACC; 11q LOH is a frequent finding |
| Carney's complex |
Autosoma l dominant |
17q22–24 | PRKAR1A | Cutaneous lentigens, pituitary adenomas, cardiac myxomas, pancreatic, and cutaneous tumors |
Micronodular pigmented adrenal hyperplasia |
Somatic PRKAR1A have been described in functioning ACAs; ACCs have been described in patients previouly diagnosed with CC; 17q LOH frequently described in ACTs |
| McCune– Albright syndrome |
Sporadic (post- zygotic somatic mosaicis m) |
20q13.3 | GNAS1 | Polyostotic bone dysplasia, gonadotropin- independent precocious puberty, café-au-lait spots, pituitary adenomas |
Cortisol-producing bilateral nodular hyperplasia |
Activating GNAS1 mutations have been described in cortisol- producing ACAs and PMAH |
| Gardner's syndrome |
Autosoma l dominant |
5q21–q22 | APC | Familial adenomatosis polyposis, increased risk for colon cancer, thyroid tumors, osteomas of the skull |
Bilateral adrenocortical hyperplasia in 7– 13% |
Somatic APC mutations have not been described in sporadic ACTs. Abnormal nuclear β-catenin staining has been described in one- third of ACCs and ACAs |
| Primary adrenalmacro nodular hyperplasia (PMAH) |
Sporadic/ autosoma l dominant |
16p11.2 | ARMC5/ | Intracranial meningiomas have been described in some patients, suggesting that these tumors are also a manifestation of the disease. |
Bilateral macronodular enlargement of adrenal glands associated with Cushing's syndrome |
Overexpression of GPCRs are virtually omnipresent; intraadrenal ACTH production with paracrine/autocrine stimulation of cortisol production have been described in some cases |
| Li–Fraumeni syndrome |
Autosoma l dominant |
17p13 | TP53 | Increased risk for sarcomas, hematologic malignancies, lung tumors, breast tumors |
ACCs in 5% | Germline inactivating TP53 mutations are very frequent in pediatric ACCs but rarely seen in adults. Somatic inactivating TP53 mutations are present in 30% of samples |
| Beckwith– Wiedemann syndrome |
Autosoma l dominant /sporadic |
11p15 | IGF2 | Organomegalia, omphalocele, microcephalia, mental retardation, fetal neoplasms (Wilm's tumor, hepatoblastoma, ACC) |
ACT in 1.5% | IGF2 overexpression and structural abnormalities of 11p15 are present in up to 90% of sporadic ACCs. |
3.2.2 Adrenal tumors
Adrenocortical tumors (ACTs) are common neoplasms especially in older adults, in whom the prevalence can reach up to 6% of the population165. Unlike the hyperplasias, ACTs are usually unilateral, although bilateral tumors are not an infrequent finding165, 166. The majority of them are benign and non-functioning adenomas (ACA) and incidentally found during abdominal imaging for unrelated reasons (being also designated “incidentaloma” in this setting). ACAs can also be hormonally active, being able to secrete cortisol (Cushing syndrome), aldosterone (Conn syndrome) or androgens165, 166. Recently, the molecular mechanisms that lead to the formation of both aldosterone-producing adenomas (APA) and cortisol-producing adenomas (CPA) have been elucidated by next-generation sequencing approaches. APAs are the second most common cause of idiopathic (non-familial) primary aldosteronism, accounting for ∼30% of the cases (hyperplasias are the leading cause, accounting for 70%) 167,154. Molecularly, APAs are characterized by somatic mutations in several genes involved in the regulation of the intracellular calcium concentration, leading to abnormal activation of the calcium-calmodulin-dependent protein kinase 1/II (CAMK1/2), the main regulators of angiotensin-II and potassium-stimulated CYP11B2 transcription154, 155. Somatic mutations in KCNJ5 have been identified in approximately 40% of the APAs168. Interestingly, heterozygous germline KCNJ5 mutations have also been described as the cause of familial primary aldosteronism type III169. This gene encodes an inward-rectifying potassium channel (GIRK4) localized at the plasma membrane, which is responsible for the maintenance of the resting membrane potential, by regulating the potassium efflux. KCNJ5 is highly expressed in normal adrenals, specifically at ZG and outer ZF with its activity stimulated by AngII. Mutations in KCNJ5 increase its permeability to potassium, favoring membrane depolarization (which in turn lead to the opening of membrane voltage-gated calcium channels, increasing the intracellular calcium concentrations resulting in activation of the CAMK1/2)170, 171. Subsequently, mutations in other genes that regulate intracellular calcium concentrations have been described in APAs, including mutations of the sodium/potassium-transporting ATPase subunit alpha-1 (ATP1A1), the plasma membrane calcium-transporting ATPase 3 (ATP2B3) and the voltage-dependent L-type calcium channel subunit alpha-1D (CACNA1D). Taken together, somatic mutations in these genes are present in ∼15% of APAs. Importantly, mutations in these genes are mutually exclusive, reinforcing their causative role in intracellular calcium homeostasis172. Cortisol-producing adenomas are characterized by abnormally high levels of PKA activation163. Recently, three different groups have described the recurrent somatic activating mutations of a catalytic subunit of the PKA (PRKACA) in a large subset of cortisol-producing adenomas173–175. This mutation leads to a defective protein-protein interaction with the regulatory subunit (encoded by the gene PRKAR1A), causing its constitutive activation.
The malignant counterparts of ACAs, adrenocortical carcinoma (ACC), however, are rare tumors with an incidence of one case per 0.5–2 million per year176, 177. A bimodal age distribution has been described, with a first peak occurring early in childhood and a second one around the fourth of fifth decades. Clinically, around 60% of ACCs are hormonally active and unlike ACAs, they can secrete more than one class of steroids (e.g., cortisol + androgens)178, 179. ACC is a highly malignant tumor with few therapeutic options once surgical cure cannot be achieved. At the time of diagnosis, about 50% of the patients will present with a stage III (locally advanced) and IV (metastatic) disease. Even after a complete surgical resection in early stage disease, the recurrence rates are remarkably high178–180. In comparison to other types of tumors, little is known about the molecular pathogenesis of ACTs. Much of the current knowledge comes from the understanding of the molecular basis of rare cancer syndromes of which ACC is one manifestation, although the great majority of ACCs are sporadic163.
3.2.3 Molecular pathways involved in adrenal tumorigenesis
The few studies focusing on the clonality of human ACTs have concluded that, while adenomas are either monoclonal or polyclonal, carcinomas are primarily monoclonal, indicating that they are derived from a single cell that undergoes transformation and clonal expansion after a set of mutational events181, 182. Although there are no clear data to identify the origin of this initiating cell, whether it is a stem, progenitor or even differentiated cell, it is reasonable to expect that mutations in genes involved in maintenance of stem cell properties are critical for the above transformation process. As mentioned in previous sections, the IGF system and the Wnt pathway are of key importance for the maintenance and homeostasis of adrenal stem cell and progenitor cell populations. In fact, abnormalities of the IGF system and of the Wnt pathway are present in a large subset of ACCs, as will be discussed below.
Wnt/β-Catenin
Adrenal hyperplasia and tumors are manifestations of the Gardner syndrome183, 184. This syndrome is caused by germline mutations of the APC gene, which is part of a multiprotein complex (destruction complex) that ubiquitinates cytoplasmatic β-catenin, targeting it to proteosomal degradation185. Without the proper function of the destruction complex, β-catenin accumulates in the cytoplasm and is translocated to the nucleus where it activates Wnt pathway target genes. Thus, nuclear accumulation of β-catenin, as observed by immunohistochemistry, is an indicator of pathway activation. Studies have shown that about a third of both ACAs and ACCs exhibit nuclear β-catenin immunostaining186. Accordingly, somatic activating CTNNB1 mutations are also observed in both ACAs and ACCs, suggesting that some ACAs may be precursor lesions of ACCs 186, 187. Animal models further corroborate these observations. A study using a transgenic mouse model with constitutively active β-catenin specifically expressed in adrenocortical cells shows elevated Axin2 levels and β-catenin scattered throughout the cortex and in clusters of cells at the cortical/medullary boundary130. Although differing in degree, these mutant mice all exhibit adrenal hyperplasia and dysplasia, and disruption of normal adrenal zonation, with zG cells expanding into the zF. By 10 months of age, Sf1 expression can be found in the medulla region, suggesting that these abnormal cells are resistant to apoptosis. More importantly, all the mice between 5–10 months of age show increased Vegf expression, and by 17 months, some females develop large masses with malignant characteristics, such as high proliferation rate and decreased Sf1 expression130.
These results suggest that abnormal activation of canonical Wnt signaling pathway can lead to an expansion of the stem/progenitor cell compartment. Alternatively, autonomous Wnt signaling in a differentiated cell results in the acquisition of stem/progenitor cell characteristics and coincident cellular expansion. Ultimately, imbalances in proliferation, differentiation and apoptosis of these cells favor tumor formation. Further extending the importance of the Wnt pathway in adrenocortical tumorigenesis, biallelic inactivation of ZNRF3 (an ubiquitin ligase that is a negative regulator of the Frizzled receptor), have been described as the most common changes in a large cohort of adrenocortical carcinomas188. Interestingly, these mutations are mutually exclusive with those of the CTNNB1 gene, further suggesting that either mutation resulting in elevated Wnt signaling can have a causative role in adrenocortical tumorigenesis.
IGF2
As discussed before, the IGF system has an important role in adrenal organogenesis and homeostasis. The link between abnormal activation of the IGF system and adrenocortical tumorigenesis came from observations of patients with the Beckwith-Wiedemann syndrome (BWS). BWS is an overgrowth disorder characterized by organomegalia, congenital malformations and a predisposition to embryonic tumors and adrenocortical cancer 189. The cause of this disorder is an imprinting defect at the 11p15 region, which leads to high postnatal levels of IGF2189. Interestingly, very high expression levels of IGF2 are observed in ∼90% of adult ACC190–192. Somatic parental isodissomy of the 11p15 locus have been described in these tumors193. Mutant mice overexpressing Igf2 exhibit a BWS-like phenotype, including adrenal hyperplasia and cytomegaly. However, these mice do not develop ACTs194. In a recent study, Igf2 overexpressing mice are crossed with mutant mice that have stabilized β-catenin (Apc mutant). The offsprings of these mice that exhibit both abnormalities develop bigger tumors than those mice with stabilized β-catenin alone. Additionally, malignant transformation occurred at an earlier age in mice harboring both elevated Igf2 and stabilized β-catenin than in the single mutant mice195. Taken together, the data support the role of IGF2 in tumorigenesis and provide a rationale for molecular targeted therapy. Unfortunately, although preclinical studies indicate that blocking the IGF1R, (one of the most important mediators of the IGF2 mitogenic effects) had anti-growth effects in-vitro and in-vivo, phase I and phase II clinical studies combining different agents displayed low therapeutic efficacy196–200. However, long-term anti-tumor activity was indeed observed in a small cohort of patients. Molecular markers that can predict which patients would respond to IGF1R blockage would be extremely useful for personalized medicine36, 198, 199.
TP53
The TP53 tumor suppressor gene is a critical component of the DNA repair and the senescence pathways. The clinical association between TP53 and ACC was identified in patients with the Li-Fraumeni syndrome (LFS). This syndrome is caused by germline inactivating mutations of the TP53 and is characterized by various types of early-onset malignant tumors, such as breast cancer, brain tumors and sarcomas201. ACC is also a manifestation of the syndrome, occurring in ∼10% of the patients202. In sporadic ACC, somatic inactivating mutations of TP53 are present in a significant proportion of cases (∼25%-30%), usually associated with a more aggressive phenotype203, 204. Among sporadic ACC patients, germline TP53 mutations are estimated to account for ∼5%205, 206. However, unlike in adults, the prevalence of germline TP53 mutations in the pediatric population is high, ranging from 50 to 90%207–209.
Telomere maintenance machinery
One of the fundamental properties of the stem/progenitor cells is self-renewal, the ability to give rise to two daughter cells, at least one of which is an exact copy of the stem/progenitor cell itself. With each cell division and coincident DNA replication, the telomeres at the end of each chromosome shorten. Unabated, the chromosomes become too short to engage in further DNA replication. Moreover, the protective cap on the end of the chromosome is disassembled, leaving the chromosome short and unprotected. Such DNA is recognized as damaged DNA and can initiate the DNA damage response with the cell ultimately undergoing apoptosis or senescence. Therefore to perpetually engage in cell division /self renewal, stem/progenitor cells must maintain telomere length and protect the naked telomere end210. This task is accomplished by increased telomerase activity and by maintaining the integrity of the shelterin complex. The shelterin complex is a set of proteins that caps and protects the ends of the chromosomes and regulates the activity of the repair mechanisms and telomerase211. Telomere dysfunction is a universal feature in cancer. In order to expand its replicative potential, many cancers overexpress telomerase212. A recent study has demonstrated that TERT, a gene that codes for the catalytic subunit of the telomerase enzyme, is frequently amplified in ACC188. The importance of the telomere maintenance mechanisms for both normal stem cell function and cancer can be exemplified by a mouse model. The adrenocortical dysplasia mouse (acd) bears mutations in the ACD/TPP1 gene, which codes for one component of the shelterin complex213. Since the shelterin complex is critical for protecting the ends of chromosomes and ensuing complete replication of coding sequences, loss of function promotes non-proliferative states and may explain some of the phenotypes seen in the acd mouse. These mice display early onset adrenal failure, with dysplasia and cytomegaly of the cortex. Interestingly, crossing the acd mutant mice with Tp53 null mice results in rescue of adrenal senescence and overall dysplasia, highlighting the role of the DNA repair mechanisms in removing cells with dysfunctional telomeres from the pool214. Both the acd/acd mice and the Tp53 −/− mice develop a spectrum of cancers during their lifespan. Interestingly, the cross between the two strains results in offsprings with earlier onset of a larger spectrum of tumors including adrenocortical carcinoma, suggesting that mutations that affect genes involved in telomere maintenance together with cell cycle checkpoint genes may act by synergistic mechanisms214.
3.2.4 Development and stem cell implications for adrenal cancers
Genetic profiling indicates that most cancers are clonal (derived from a single cell) but cells within a given cancer are heterogeneous in terms of both differentiation state and proliferation potential. The significant heterogeneity within a given cancer may be a result of secondary mutations in daughter cells. Two models have been proposed to explain cancer cell proliferation potentials. The “stochastic model” predicts that all cells in a tumor are biologically equivalent and any heterogeneity is caused by extrinsic/environmental factors; every cancer cell has the ability to proliferate and metastasize215. In contrast, the “cancer stem cell” model posits that only a distinct, generally rare subpopulation of tumor cells possess the so-called stem cell potential and the ability to differentiate into multiple cell types in a cancer and originate new metastatic lesions215. The properties of such cells theoretically have a profound impact on therapeutics, since they are able to repopulate the entire tumor if they are not targeted by a given treatment. Indeed, subsets of cancer cells with these unique properties have been identified in a variety of cancers, including hematologic malignancies, breast, brain, colon and pancreatic cancers215. However, the discovery of cancer stem cell does not rule out the existence of the stochastic model, which may be more appropriate for some tumor types. Alternatively, both models may also coexist in the same tumor, depending on the context (i.e., tumor cells may undergo a phenomenon called stem cell plasticity, in which they may fluctuate in a range of different degrees of differentiation and “stemness” when challenged)216, 217.
Studies have been directed at determining whether ACC expand through the stochastic or cancer stem cell model. Lichtenauer et.al took advantage of the multidrug resistant properties of progenitor cells to study the “side population” of H295R human adrenocortical carcinoma cell line218. Cells that are able to efflux the Hoescht 33342 vital dye by increased activity of members of the ATP-binding cassette family have been demonstrated to exhibit stem cell properties in a wide variety of tissues and tumor samples. Based on these properties, these cells can be sorted by FACS after being treated with Hoescht 33342. These cells, which constitute a small fraction of the total cell pool, are consequently called side populations. Side populations derived from the H295R cells exhibited a less differentiated phenotype in terms of steroidogenic enzyme expression. However, in contrast to other cancer stem cells, these cells do not show a difference in growth potential or resistance to cytotoxic drugs compared to the predominant population218. Furthermore, after the side population cells are maintained for some time in culture, they give rise to a population of cells similar to the original H295R culture in terms of differentiation and the proportion of cells with properties of side populations. Although these observations argue against the existence of cancer stem cells in the H295R cell line, it does not completely rule out this possibility. Additionally, since the study used a cell line rather than cells directly isolated from ACC patients, it remains unclear if the side population with multidrug resistance properties is a major contributor to ACC initiation and/or maintenance.
As detailed in this review, the most common mutated genes in both adrenocortical hypoplasia and adrenocortical tumors are also stem/ progenitor cell factors critical for proper adrenal development and homeostasis. It is reasonable to hypothesize that defects in the adrenocortical stem cells serve as drivers for a spectrum of adrenal disorders of growth and differentiation. Future studies utilizing novel genomic approaches in tumor samples and model systems should shed insight into the genetic and cellular defects underlying these diseases of the adrenal cortex.
KEY POINTS.
The human adult adrenal cortex is composed of three different zones: zona glomerulosa (zG), zona fasciculata (zF) and zona reticularis (zR), responsible for production of mineralocorticoids, glucocorticoids, and adrenal androgens, respectively.
The establishment of the adrenal zG and zF occurs late in fetal development with a transition from the fetal to adult cortex; however, the final completion of cortical zonation in humans does not occur until puberty with the establishment of the zR and its production of adrenal androgens, a process called adrenarche.
The maintenance of the adrenal cortex involves the centripetal displacement and differentiation of peripheral Sonic hedgehog (Shh) positive progenitors cells into zG that later transition to zF cells and subsequently zR cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting sources:
AML is supported by CAPES - grant number BEX 8726/13-2
Contributor Information
Yewei Xing, Research Fellow, Internal Medicine, Medical School University of Michigan Ann Arbor, MI
Antonio Lerario, Visiting Scholar, Internal Medicine, Medical School University of Michigan Ann Arbor, MI
William Rainey, Research Departments of Molecular & Integrative Physiology and Internal Medicine University of Michigan Ann Arbor, MI
Gary D. Hammer, Millie Schembechler Professor of Adrenal Cancer University of Michigan Director - Endocrine Oncology Program Director - Center for Organogenesis University of Michigan 109 Zina Pitcher Place, 1528 BSRB Ann Arbor, MI 48109-2200 ghammer@umich.edu Phone: 734-615-2421
Reference
- 1.Gruenwald P. Embryonic and postnatal development of the adrenal cortex, particularly the zona glomerulosa and accessory nodules. Anat Rec. 1946;95:391–421. doi: 10.1002/ar.1090950404. [DOI] [PubMed] [Google Scholar]
- 2.Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1(7):663–671. doi: 10.1046/j.1365-2443.1996.00254.x. [DOI] [PubMed] [Google Scholar]
- 3.X L, Y I, KL P. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 4.Morohashi K. The ontogenesis of the steroidogenic tissues. Genes Cells. 1997;2(2):95–106. doi: 10.1046/j.1365-2443.1997.1060304.x. [DOI] [PubMed] [Google Scholar]
- 5.Le Douarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974;41(1):162–184. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- 6.Doupe AJ, Landis SC, Patterson PH. Environmental influences in the development of neural crest derivatives: glucocorticoids, growth factors, and chromaffin cell plasticity. J Neurosci. 1985;5(8):2119–2142. doi: 10.1523/JNEUROSCI.05-08-02119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillarp NAH B. Evidence of adrenaline and noradrenaline in separate adrenal medullary cells. Acta Physiol Scand. 1953;30(1):55–68. doi: 10.1111/j.1748-1716.1954.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 8.Ce K, Hammer GD. Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol Metab. 2002;13(5):200–208. doi: 10.1016/s1043-2760(02)00602-1. [DOI] [PubMed] [Google Scholar]
- 9.Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19(2):101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- 10.Seidl K, Unsicker K. The determination of the adrenal medullary cell fate during embryogenesis. Dev Biol. 1989;136(2):481–490. doi: 10.1016/0012-1606(89)90273-x. [DOI] [PubMed] [Google Scholar]
- 11.Finotto S, Krieglstein K, Schober A, Deimling F, Lindner K, Bruhl B, et al. Analysis of mice carrying targeted mutations of the glucocorticoid receptor gene argues against an essential role of glucocorticoid signalling for generating adrenal chromaffin cells. Development. 1999;126(13):2935–2944. doi: 10.1242/dev.126.13.2935. [DOI] [PubMed] [Google Scholar]
- 12.Gut P, Huber K, Lohr J, Bruhl B, Oberle S, Treier M, et al. Lack of an adrenal cortex in Sf1 mutant mice is compatible with the generation and differentiation of chromaffin cells. Development. 2005;132(20):4611–4619. doi: 10.1242/dev.02052. [DOI] [PubMed] [Google Scholar]
- 13.Bland M, Fowkes RC, Ingraham HA. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol. 2004;18(4):941–952. doi: 10.1210/me.2003-0333. [DOI] [PubMed] [Google Scholar]
- 14.Huber K, Combs S, Ernsberger U, Kalcheim C, Unsicker K. Generation of neuroendocrine chromaffin cells from sympathoadrenal progenitors: beyond the glucocorticoid hypothesis. Ann N Y Acad Sci. 2002;971:554–559. doi: 10.1111/j.1749-6632.2002.tb04526.x. [DOI] [PubMed] [Google Scholar]
- 15.Unsicker K, Huber K, Schutz G, Kalcheim C. The chromaffin cell and its development. Neurochem Res. 2005;30(6–7):921–925. doi: 10.1007/s11064-005-6966-5. [DOI] [PubMed] [Google Scholar]
- 16.E J. The foetal adrenal cortex in the human. Its ultrastructure at different stages of development and in different functional states. Acta Endocrinol. 1968;58(130) [PubMed] [Google Scholar]
- 17.M G, Piper Hanley K, Marcos J, Wood PJ, Wright S, Postle AD, et al. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest. 2006;116(4):953–960. doi: 10.1172/JCI25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NA H, Rainey WE, Wilson DI, Ball SG, Parker KL. Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Mol Endocrinol. 2001;15(1):57–68. doi: 10.1210/mend.15.1.0585. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 20.Achermann JC, Meeks JJ, Jameson JL. Phenotypic spectrum of mutations in DAX-1 and SF-1. Mol Cell Endocrinol. 2001;185(1–2):17–25. doi: 10.1016/s0303-7207(01)00619-0. [DOI] [PubMed] [Google Scholar]
- 21.El-Khairi R, Martinez-Aguayo A, Ferraz-de-Souza B, Lin L, Achermann JC. Role of DAX-1 (NR0B1) and steroidogenic factor-1 (NR5A1) in human adrenal function. Endocr Dev. 2011;20:38–46. doi: 10.1159/000321213. [DOI] [PubMed] [Google Scholar]
- 22.Wong M, Ikeda Y, Luo X, Caron KM, Weber TJ, Swain A, et al. Steroidogenic factor 1 plays multiple roles in endocrine development and function. Recent Prog Horm Res. 1997;52:167–182. [PubMed] [Google Scholar]
- 23.Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007;21(12):2968–2987. doi: 10.1210/me.2007-0120. [DOI] [PubMed] [Google Scholar]
- 24.P V, Martinez-Barbera J-P, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134(12):2349–2358. doi: 10.1242/dev.004390. [DOI] [PubMed] [Google Scholar]
- 25.R B, Vidal VPI, Motamedi FJ, Clarkson M, Sahut-Barnola I, von Gise A, et al. WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland. Dev Cell. 2013;27(1):5–18. doi: 10.1016/j.devcel.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K-i. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26(11):4111–4121. doi: 10.1128/MCB.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zubair M, Parker KL, Morohashi K-i. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol. 2008;28(23):7030–7040. doi: 10.1128/MCB.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad I, Paterson WF, Lin L, Adlard P, Duncan P, Tolmie J, et al. A novel missense mutation in DAX-1 with an unusual presentation of X-linked adrenal hypoplasia congenita. Horm Res. 2007;68(1):32–37. doi: 10.1159/000099835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani G, De Menis E, Borretta G, Radetti G, Bondioni S, Spada A, et al. DAX1 and X-linked adrenal hypoplasia congenita: clinical and molecular analysis in five patients. Eur J Endocrinol. 2006;154(5):685–689. doi: 10.1530/eje.1.02132. [DOI] [PubMed] [Google Scholar]
- 30.Wood M, Hammer GD. Adrenocortical stem and progenitor cells: unifying model of two proposed origins. Mol Cell Endocrinol. 2011;336(1–2):206–212. doi: 10.1016/j.mce.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheys JO, Heaton JH, Hammer GD. Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology. 2011;152(9):3430–3439. doi: 10.1210/en.2010-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gummow BM, Scheys JO, Cancelli VR, Hammer GD. Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Mol Endocrinol. 2006;20(11):2711–2723. doi: 10.1210/me.2005-0461. [DOI] [PubMed] [Google Scholar]
- 33.Kelly VR, Hammer GD. LRH-1 and Nanog regulate Dax1 transcription in mouse embryonic stem cells. Mol Cell Endocrinol. 2011;332(1–2):116–124. doi: 10.1016/j.mce.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khalfallah O, Rouleau M, Barbry P, Bardoni B, Lalli E. Dax-1 knockdown in mouse embryonic stem cells induces loss of pluripotency and multilineage differentiation. Stem Cells. 2009;27(7):1529–1537. doi: 10.1002/stem.78. [DOI] [PubMed] [Google Scholar]
- 35.Feek CM, Marante DJ, Edwards CR. The hypothalamic-pituitary-adrenal axis. Clin Endocrinol Metab. 1983;12(3):597–618. doi: 10.1016/s0300-595x(83)80057-7. [DOI] [PubMed] [Google Scholar]
- 36.Janes M, Chu KME, Clark AJL, King PJ. Mechanisms of adrenocorticotropin-induced activation of extracellularly regulated kinase 1/2 mitogen-activated protein kinase in the human H295R adrenal cell line. Endocrinology. 2008;149(4):1898–1905. doi: 10.1210/en.2007-0949. [DOI] [PubMed] [Google Scholar]
- 37.Pepe GJ, Albrecht ED. Regulation of the primate fetal adrenal cortex. Endocr Rev. 1990;11(1):151–176. doi: 10.1210/edrv-11-1-151. [DOI] [PubMed] [Google Scholar]
- 38.Rainey W, Rehman KS, Carr BR. Fetal and maternal adrenals in human pregnancy. Obstet Gynecol Clin North Am. 2004;31(4):817–835. doi: 10.1016/j.ogc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Beshay V, Carr BR, Rainey WE. The human fetal adrenal gland, corticotropin-releasing hormone, and parturition. Semin Reprod Med. 2007;25(1):14–20. doi: 10.1055/s-2006-956772. [DOI] [PubMed] [Google Scholar]
- 40.Hornsby PJ. Regulation of adrenocortical cell proliferation in culture. Endocr Res. 1984;10(3–4):259–281. doi: 10.1080/07435808409036501. [DOI] [PubMed] [Google Scholar]
- 41.Simpson ER, Waterman MR. Regulation by ACTH of steroid hormone biosynthesis in the adrenal cortex. Can J Biochem Cell Biol. 1983;61(7):692–707. doi: 10.1139/o83-088. [DOI] [PubMed] [Google Scholar]
- 42.LeRoith D, Roberts CT., Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003;195(2):127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 43.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 44.Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A. 1981;78(10):6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukuda T, Takahashi K, Suzuki T, Saruta M, Watanabe M, Nakata T, et al. Urocortin 1, urocortin 3/stresscopin, and corticotropin-releasing factor receptors in human adrenal and its disorders. J Clin Endocrinol Metab. 2005;90(8):4671–4678. doi: 10.1210/jc.2005-0090. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi K, Totsune K, Saruta M, Fukuda T, Suzuki T, Hirose T, et al. Expression of urocortin 3/stresscopin in human adrenal glands and adrenal tumors. Peptides. 2006;27(1):178–182. doi: 10.1016/j.peptides.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Karteris E, Randeva HS, Grammatopoulos DK, Jaffe RB, Hillhouse EW. Expression and coupling characteristics of the CRH and orexin type 2 receptors in human fetal adrenals. J Clin Endocrinol Metab. 2001;86(9):4512–4519. doi: 10.1210/jcem.86.9.7849. [DOI] [PubMed] [Google Scholar]
- 48.Dermitzaki E, Tsatsanis C, Minas V, Chatzaki E, Charalampopoulos I, Venihaki M, et al. Corticotropin-releasing factor (CRF) and the urocortins differentially regulate catecholamine secretion in human and rat adrenals, in a CRF receptor type-specific manner. Endocrinology. 2007;148(4):1524–1538. doi: 10.1210/en.2006-0967. [DOI] [PubMed] [Google Scholar]
- 49.R S, Rehman KS, Carr BR, Parker CR, Jr., Rainey WE. Corticotropin-releasing hormone directly stimulates cortisol and the cortisol biosynthetic pathway in human fetal adrenal cells. J Clin Endocrinol Metab. 2005;90(1):279–285. doi: 10.1210/jc.2004-0865. [DOI] [PubMed] [Google Scholar]
- 50.Andreis PG, Neri G, Nussdorfer GG. Corticotropin-releasing hormone (CRH) directly stimulates corticosterone secretion by the rat adrenal gland. Endocrinology. 1991;128(2):1198–1200. doi: 10.1210/endo-128-2-1198. [DOI] [PubMed] [Google Scholar]
- 51.Willenberg HS, Bornstein SR, Hiroi N, Path G, Goretzki PE, Scherbaum WA, et al. Effects of a novel corticotropin-releasing-hormone receptor type I antagonist on human adrenal function. Mol Psychiatry. 2000;5(2):137–141. doi: 10.1038/sj.mp.4000720. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz J, Huo JS, Piwien-Pilipuk G. Growth hormone regulated gene expression. Minerva Endocrinol. 2002;27(4):231–241. [PubMed] [Google Scholar]
- 53.Backlin C, Rastad J, Skogseid B, Hellman P, Akerstrom G, Juhlin C. Immunohistochemical expression of insulin-like growth factor 1 and its receptor in normal and neoplastic human adrenal cortex. Anticancer Res. 1995;15(6B):2453–2459. [PubMed] [Google Scholar]
- 54.Brice AL, Cheetham JE, Bolton VN, Hill NC, Schofield PN. Temporal changes in the expression of the insulin-like growth factor II gene associated with tissue maturation in the human fetus. Development. 1989;106(3):543–554. doi: 10.1242/dev.106.3.543. [DOI] [PubMed] [Google Scholar]
- 55.Coulter CL, Goldsmith PC, Mesiano S, Voytek CC, Martin MC, Han VK, et al. Functional maturation of the primate fetal adrenal in vivo: I. Role of insulin-like growth factors (IGFs), IGF-I receptor, and IGF binding proteins in growth regulation. Endocrinology. 1996;137(10):4487–4498. doi: 10.1210/endo.137.10.8828511. [DOI] [PubMed] [Google Scholar]
- 56.Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448(7157):1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 57.Jiang F, Frederick TJ, Wood TL. IGF-I synergizes with FGF-2 to stimulate oligodendrocyte progenitor entry into the cell cycle. Dev Biol. 2001;232(2):414–423. doi: 10.1006/dbio.2001.0208. [DOI] [PubMed] [Google Scholar]
- 58.JL P, Calvel P, Romero Y, Conne B, Truong V, Papaioannou MD, et al. Insulin and IGF1 receptors are essential for XX and XY gonadal differentiation and adrenal development in mice. PLoS Genet. 2013;9(1):3. doi: 10.1371/journal.pgen.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.K M, Zubair M. The fetal and adult adrenal cortex. Mol Cell Endocrinol. 2011;336(1–2):193–197. doi: 10.1016/j.mce.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 60.Zubair M, Oka S, Parker KL, Morohashi K-I. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol Endocrinol. 2009;23(10):1657–1667. doi: 10.1210/me.2009-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulte D, Shapiro I, Reincke M, Beuschlein F. Expression and spatio-temporal distribution of differentiation and proliferation markers during mouse adrenal development. Gene Expr Patterns. 2007;7(1–2):72–81. doi: 10.1016/j.modgep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Guasti L, Paul A, Laufer E, King P. Localization of Sonic hedgehog secreting and receiving cells in the developing and adult rat adrenal cortex. Mol Cell Endocrinol. 2011;336(1–2):117–122. doi: 10.1016/j.mce.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106(50):21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wood MA, Acharya A, Finco I, Swonger JM, Elston MJ, Tallquist MD, et al. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development. 2013;140(22):4522–4532. doi: 10.1242/dev.092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ishimoto H, Jaffe RB. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr Rev. 2011;32(3):317–355. doi: 10.1210/er.2010-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Havelock J, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22(4):337–347. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- 67.Rosenfield RL, Lucky AW. Acne, hirsutism, and alopecia in adolescent girls. Clinical expressions of androgen excess. Endocrinol Metab Clin North Am. 1993;22(3):507–532. [PubMed] [Google Scholar]
- 68.Corvalan C, Uauy R, Mericq V. Obesity is positively associated with dehydroepiandrosterone sulfate concentrations at 7 y in Chilean children of normal birth weight. Am J Clin Nutr. 2013;97(2):318–325. doi: 10.3945/ajcn.112.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.l'Allemand D, Schmidt S, Rousson V, Brabant G, Gasser T, Gruters A. Associations between body mass, leptin, IGF-I and circulating adrenal androgens in children with obesity and premature adrenarche. Eur J Endocrinol. 2002;146(4):537–543. doi: 10.1530/eje.0.1460537. [DOI] [PubMed] [Google Scholar]
- 70.P S, Dimartino-Nardi J. Premature adrenarche. J Endocrinol Invest. 2001;24(9):724–733. doi: 10.1007/BF03343917. [DOI] [PubMed] [Google Scholar]
- 71.Pintor C, Loche S, Faedda A, Fanni V, Nurchi AM, Corda R. Adrenal androgens in obese boys before and after weight loss. Horm Metab Res. 1984;16(10):544–548. doi: 10.1055/s-2007-1014845. [DOI] [PubMed] [Google Scholar]
- 72.Howard E. The effect of dietary factors on the adrenal X zone. Fed Proc. 1947;6(1 Pt 2):133. [PubMed] [Google Scholar]
- 73.Tanaka S, Matsuzawa A. [What mouse contributed the first representation of the adrenal cortex X zone?] Jikken Dobutsu. 1993;42(3):305–316. doi: 10.1538/expanim1978.42.3_305. [DOI] [PubMed] [Google Scholar]
- 74.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95(5):2296–2305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- 75.Nakamura Y, Maekawa T, Felizola SJA, Satoh F, Qi X, Velarde-Miranda C, et al. Adrenal CYP11B1/2 expression in primary aldosteronism: immunohistochemical analysis using novel monoclonal antibodies. Mol Cell Endocrinol. 2014;392(1–2):73–79. doi: 10.1016/j.mce.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1–2):111–117. doi: 10.1016/j.mce.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chamoux E, Otis M, Gallo-Payet N. A connection between extracellular matrix and hormonal signals during the development of the human fetal adrenal gland. Braz J Med Biol Res. 2005;38(10):1495–1503. doi: 10.1590/s0100-879x2005001000006. [DOI] [PubMed] [Google Scholar]
- 78.Chamoux E, Narcy A, Lehoux J-G, Gallo-Payet N. Fibronectin, laminin, and collagen IV as modulators of cell behavior during adrenal gland development in the human fetus. J Clin Endocrinol Metab. 2002;87(4):1819–1828. doi: 10.1210/jcem.87.4.8359. [DOI] [PubMed] [Google Scholar]
- 79.Chamoux E, Bolduc L, Lehoux JG, Gallo-Payet N. Identification of extracellular matrix components and their integrin receptors in the human fetal adrenal gland. J Clin Endocrinol Metab. 2001;86(5):2090–2098. doi: 10.1210/jcem.86.5.7462. [DOI] [PubMed] [Google Scholar]
- 80.Otis M, Campbell S, Payet MD, Gallo-Payet N. Expression of extracellular matrix proteins and integrins in rat adrenal gland: importance for ACTH-associated functions. J Endocrinol. 2007;193(3):331–347. doi: 10.1677/JOE-07-0055. [DOI] [PubMed] [Google Scholar]
- 81.Cheng CY, Hornsby PJ. Expression of 11 beta-hydroxylase and 21-hydroxylase in long-term cultures of bovine adrenocortical cells requires extracellular matrix factors. Endocrinology. 1992;130(5):2883–2889. doi: 10.1210/endo.130.5.1572301. [DOI] [PubMed] [Google Scholar]
- 82.Filippi L, Sardella B, Ciorra A, Scopinaro F, Bagni O. Tumor thrombus in the renal vein from an adrenal metastasis of lung cancer: 18FDG PET/CT findings. Cancer Biother Radiopharm. 2014;29(5):189–192. doi: 10.1089/cbr.2014.1612. [DOI] [PubMed] [Google Scholar]
- 83.Puech A, Pages A, Comelade P. [Cutaneous metastases as the first manifestation of a cancer of the lungs; adrenal metastasis; terminal acute adrenal insufficiency] Montp Med. 1955;47(6):565–566. [PubMed] [Google Scholar]
- 84.Wi O. Lung Cancer Metastasis to Adrenal Cortical Adenomas. J Pathol Bacteriol. 1963;86:541–543. doi: 10.1002/path.1700860232. [DOI] [PubMed] [Google Scholar]
- 85.Jd L. Observations on the adrenal blood vessels in the rat. J Anat. 1952;86(4):459–467. [PMC free article] [PubMed] [Google Scholar]
- 86.Anson BJ, Cauldwell EW, et al. The blood supply of the kidney, suprarenal gland, and associated structures. Surg Gynecol Obstet. 1947;84(3):313–320. [PubMed] [Google Scholar]
- 87.Johnstone FR. The suprarenal veins. Am J Surg. 1957;94(4):615–620. doi: 10.1016/0002-9610(57)90590-1. [DOI] [PubMed] [Google Scholar]
- 88.Monkhouse WS, Khalique A. The adrenal and renal veins of man and their connections with azygos and lumbar veins. J Anat. 1986;146:105–115. [PMC free article] [PubMed] [Google Scholar]
- 89.RG The venous drainage of the human adrenal gland. Rev Can Biol. 1956;14(4):350–359. [PubMed] [Google Scholar]
- 90.WC E. Functional innervation of the adrenal cortex by the splanchnic nerve. Horm Metab Res. 1998;30(6–7):311–314. doi: 10.1055/s-2007-978890. [DOI] [PubMed] [Google Scholar]
- 91.Hinson JP, Cameron LA, Purbrick A, Kapas S. The role of neuropeptides in the regulation of adrenal vascular tone: effects of vasoactive intestinal polypeptide, substance P, neuropeptide Y, neurotensin, Met-enkephalin, and Leu-enkephalin on perfusion medium flow rate in the intact perfused rat adrenal. Regul Pept. 1994;51(1):55–61. doi: 10.1016/0167-0115(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 92.Li Q, Johansson H, Kjellman M, Grimelius L. Neuroendocrine differentiation and nerves in human adrenal cortex and cortical lesions. Apmis. 1998;106(8):807–817. doi: 10.1111/j.1699-0463.1998.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 93.Burnay MM, Python CP, Vallotton MB, Capponi AM, Rossier MF. Role of the capacitative calcium influx in the activation of steroidogenesis by angiotensin-II in adrenal glomerulosa cells. Endocrinology. 1994;135(2):751–758. doi: 10.1210/endo.135.2.8033823. [DOI] [PubMed] [Google Scholar]
- 94.Johnson Bb, Fau - Lieberman AH, Lieberman Ah, Fau - Mulrow PJ, Pj M. Aldosterone excretion in normal subjects depleted of sodium and potassium. J Clin Invest. 1957;36(6 Part 1):757–766. doi: 10.1172/JCI103480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laragh JH, Stoerk HC. A study of the mechanism of secretion of the sodium-retaining hormone (aldosterone) J Clin Invest. 1957;36(3):383–392. doi: 10.1172/JCI103434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCaa RE, Gillespie JB. Effects of captopril and enalapril on sodium excretion and blood pressure in sodium-deficient dogs. Fed Proc. 1984;43(5):1336–1341. [PubMed] [Google Scholar]
- 97.Deane HW, Bergner GE. Chemical and cytochemical studies of the rat's adrenal cortex following the administration of pituitary adrenocorticotropic hormone (ACTH) J Clin Endocrinol Metab. 1947;7(6):457. [PubMed] [Google Scholar]
- 98.Hills AG, Thorn GW. An estimation of the quantity of 11–17-oxysteroid excretion by the human adrenal stimulated by ACTH. J Clin Endocrinol Metab. 1948;8(7):606. [PubMed] [Google Scholar]
- 99.Mason HL, Power MH, et al. Results of administration of anterior pituitary adrenocorticotropic hormone to a normal human being. J Biol Chem. 1947;169(1):223. [PubMed] [Google Scholar]
- 100.Ingle DJ, Prestrud MC, Li CH. A further study of the essentiality of the adrenal cortex in mediating the metabolic effects of adrenocorticotrophic hormone. Endocrinology. 1948;43(4):202–207. doi: 10.1210/endo-43-4-202. [DOI] [PubMed] [Google Scholar]
- 101.Bellamy D, Leonard RA. The effect of cortisol on the activity of glutamate-pyruvate transaminase and the formation of glycogen and urea in starved rats. Biochem J. 1964;93(2):331–336. doi: 10.1042/bj0930331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sie HG, Hablanian A, Fishman WH. Solubilization of Mouse Liver Glycogen Synthetase and Phosphorylase during Starvation Glycogenolysis and Its Reversal by Cortisol. Nature. 1964;201:393–394. doi: 10.1038/201393a0. [DOI] [PubMed] [Google Scholar]
- 103.Bandy HE, Darrach M, Newsom SE, Southcott CM. Metabolism of adrenal steroids in the mouse. I. Observations on 20alpha-dihydrocorticosterone and corticosterone in the plasma of mice treated with corticotropin. Can J Biochem Physiol. 1956;34(5):913–918. [PubMed] [Google Scholar]
- 104.Halberg F, Albrecht PG, Bittner JJ. Corticosterone rhythm of mouse adrenal in relation to serum corticosterone and sampling. Am J Physiol. 1959;197:1083–1085. doi: 10.1152/ajplegacy.1959.197.5.1083. [DOI] [PubMed] [Google Scholar]
- 105.Halberg F, Haus E. Corticosterone in mouse adrenal in relation to sex and heterotopic pituitary isografting. Am J Physiol. 1960;199:859–862. doi: 10.1152/ajplegacy.1960.199.5.859. [DOI] [PubMed] [Google Scholar]
- 106.Ortega E, Rodriguez C, Strand LJ, Segre E. Effects of cloprednol and other corticosteroids on hypothalamic-pituitary-adrenal axis function. J Int Med Res. 1976;4(5):326–337. doi: 10.1177/030006057600400506. [DOI] [PubMed] [Google Scholar]
- 107.Storrs FJ. Use and abuse of systemic corticosteroid therapy. J Am Acad Dermatol. 1979;1(2):95–106. doi: 10.1016/s0190-9622(79)80029-8. [DOI] [PubMed] [Google Scholar]
- 108.Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22(4):311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- 109.Davison B, Large DM, Anderson DC, Robertson WR. Basal steroid production by the zona reticularis of the guinea-pig adrenal cortex. J Steroid Biochem. 1983;18(3):285–290. doi: 10.1016/0022-4731(83)90104-8. [DOI] [PubMed] [Google Scholar]
- 110.Hyatt PJ, Bhatt K, Tait JF. Steroid biosynthesis by zona fasciculata and zona reticularis cells purified from the mammalian adrenal cortex. J Steroid Biochem. 1983;19(1C):953–959. doi: 10.1016/0022-4731(83)90039-0. [DOI] [PubMed] [Google Scholar]
- 111.Kaufman FR, Stanczyk FZ, Matteri RK, Gentzschein E, Delgado C, Lobo RA. Dehydroepiandrosterone and dehydroepiandrosterone sulfate metabolism in human genital skin. Fertil Steril. 1990;54(2):251–254. [PubMed] [Google Scholar]
- 112.Rosenfield RL. Hirsutism and the variable response of the pilosebaceous unit to androgen. J Investig Dermatol Symp Proc. 2005;10(3):205–208. doi: 10.1111/j.1087-0024.2005.10106.x. [DOI] [PubMed] [Google Scholar]
- 113.Pelletier G. Expression of steroidogenic enzymes and sex-steroid receptors in human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22(2):223–228. doi: 10.1016/j.beem.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 114.Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98(3):1182–1188. doi: 10.1210/jc.2012-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pabon JE, Li X, Lei ZM, Sanfilippo JS, Yussman MA, Rao CV. Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J Clin Endocrinol Metab. 1996;81(6):2397–2400. doi: 10.1210/jcem.81.6.8964884. [DOI] [PubMed] [Google Scholar]
- 116.Jaffe RB, Seron-Ferre M, Crickard K, Koritnik D, Mitchell BF, Huhtaniemi IT. Regulation and function of the primate fetal adrenal gland and gonad. Recent Prog Horm Res. 1981;37:41–103. doi: 10.1016/b978-0-12-571137-1.50007-4. [DOI] [PubMed] [Google Scholar]
- 117.Vuorenoja S, Rivero-Muller A, Kiiveri S, Bielinska M, Heikinheimo M, Wilson DB, et al. Adrenocortical tumorigenesis, luteinizing hormone receptor and transcription factors GATA-4 and GATA-6. Mol Cell Endocrinol. 2007;269(1–2):38–45. doi: 10.1016/j.mce.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 118.Lacroix A, Hamet P, Boutin JM. Leuprolide acetate therapy in luteinizing hormone--dependent Cushing's syndrome. N Engl J Med. 1999;341(21):1577–1581. doi: 10.1056/NEJM199911183412104. [DOI] [PubMed] [Google Scholar]
- 119.BL B. A comparison of the histologica changes induced by experimental hyperadrenocorticalism and inanition. Recent Prog Hormone Res. 1952;(7):331. [Google Scholar]
- 120.ZRWRN MG. A study of corticoadrenal cells. Anat Rec. 1938;72(2):249–263. [Google Scholar]
- 121.Salmon TNZ RL. A study of the life history of cortico-adrenal gland cells of the rat by means of trypan blue injections. Anat Rec. 1941;80(4):421–429. [Google Scholar]
- 122.Malendowicz LK, Dembinska M. Proliferation and distribution of adrenocortical cells in ACTH treated female hamsters. Folia Histochem Cytobiol. 1990;28(1–2):51–59. [PubMed] [Google Scholar]
- 123.Stachowiak A, Nussdorfer GG, Malendowicz LK. Proliferation and distribution of adrenocortical cells in the gland of ACTH- or dexamethasone-treated rats. Histol Histopathol. 1990;5(1):25–29. [PubMed] [Google Scholar]
- 124.Sasano H, Imatani A, Shizawa S, Suzuki T, Nagura H. Cell proliferation and apoptosis in normal and pathologic human adrenal. Mod Pathol. 1995;8(1):11–17. [PubMed] [Google Scholar]
- 125.Morley SD, Viard I, Chung BC, Ikeda Y, Parker KL, Mullins JJ. Variegated expression of a mouse steroid 21-hydroxylase/beta- galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol Endocrinol. 1996;10(5):585–598. doi: 10.1210/mend.10.5.8732689. [DOI] [PubMed] [Google Scholar]
- 126.Ching S, Vilain E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis. 2009;47(9):628–637. doi: 10.1002/dvg.20532. [DOI] [PubMed] [Google Scholar]
- 127.Huang C, Miyagawa S, Matsumaru D, Parker K, Yao PHH-C. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151(3):1119–1128. doi: 10.1210/en.2009-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alex K, Barlaskar FM, Heaton JH, Else T, Kelly VR, Krill KT, et al. In search of adrenocortical stem and progenitor cells. Endocr Rev. 2009;30(3):241–263. doi: 10.1210/er.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, et al. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135(15):2593–2602. doi: 10.1242/dev.021493. [DOI] [PubMed] [Google Scholar]
- 130.Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, Louiset E, et al. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Human molecular genetics. 2010;19:1561–1576. doi: 10.1093/hmg/ddq029. [DOI] [PubMed] [Google Scholar]
- 131.Assie G, Guillaud-Bataille M, Ragazzon B, Bertagna X, Bertherat J, Clauser E. The pathophysiology, diagnosis and prognosis of adrenocortical tumors revisited by transcriptome analyses. Trends Endocrinol Metab. 2010;21(5):325–334. doi: 10.1016/j.tem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 132.Schteingart DE, Doherty GM, Gauger PG, Giordano TJ, Hammer GD, Korobkin M, et al. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer. 2005;12(3):667–680. doi: 10.1677/erc.1.01029. [DOI] [PubMed] [Google Scholar]
- 133.A B, Drelon C, Ragazzon B, Boulkroun S, Tissier F, Amar L, et al. WNT/beta-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum Mol Genet. 2014;23(4):889–905. doi: 10.1093/hmg/ddt484. [DOI] [PubMed] [Google Scholar]
- 134.Mizusaki H, Kawabe K, Mukai T, Ariyoshi E, Kasahara M, Yoshioka H, et al. Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) gene transcription is regulated by wnt4 in the female developing gonad. Mol Endocrinol. 2003;17(4):507–519. doi: 10.1210/me.2002-0362. [DOI] [PubMed] [Google Scholar]
- 135.Walczak EM, Kuick R, Finco I, Bohin N, Hrycaj SM, Wellik DM, et al. Wnt signaling inhibits adrenal steroidogenesis by cell-autonomous and non-cell-autonomous mechanisms. Mol Endocrinol. 2014;28(9):1471–1486. doi: 10.1210/me.2014-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Berthon A, Drelon C, Ragazzon B, Boulkroun S, Tissier F, Amar L, et al. WNT/beta-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum Mol Genet. 2014;23(4):889–905. doi: 10.1093/hmg/ddt484. [DOI] [PubMed] [Google Scholar]
- 137.BD F, Kempna PB, Carlone DL, Shah MS, Guagliardo NA, Barrett PQ, et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013;26(6):666–673. doi: 10.1016/j.devcel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arboleda VA, Lee H, Parnaik R, Fleming A, Banerjee A, Ferraz-de-Souza B, et al. Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome. Nature genetics. 2012;44:788–792. doi: 10.1038/ng.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Achermann JC, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22(2):125–126. doi: 10.1038/9629. [DOI] [PubMed] [Google Scholar]
- 140.Habiby RL, Boepple P, Nachtigall L, Sluss PM, Crowley WF, Jr., Jameson JL. Adrenal hypoplasia congenita with hypogonadotropic hypogonadism: evidence that DAX-1 mutations lead to combined hypothalmic and pituitary defects in gonadotropin production. J Clin Invest. 1996;98(4):1055–1062. doi: 10.1172/JCI118866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mandel H, Shemer R, Borochowitz ZU, Okopnik M, Knopf C, Indelman M, et al. SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am J Hum Genet. 2008;82(1):39–47. doi: 10.1016/j.ajhg.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clark AJ, McLoughlin L, Grossman A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet. 1993;341(8843):461–462. doi: 10.1016/0140-6736(93)90208-x. [DOI] [PubMed] [Google Scholar]
- 143.Gineau L, Cognet C, Kara N, Lach FP, Dunne J, Veturi U, et al. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest. 2012;122(3):821–832. doi: 10.1172/JCI61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hughes CR, Guasti L, Meimaridou E, Chuang C-H, Schimenti JC, King PJ, et al. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest. 2012;122(3):814–820. doi: 10.1172/JCI60224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meimaridou E, Kowalczyk J, Guasti L, Hughes CR, Wagner F, Frommolt P, et al. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nature Genetics. 2012:740–742. doi: 10.1038/ng.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jackson RS, Creemers JWM, Farooqi IS, Raffin-Sanson M-L, Varro A, Dockray GJ, et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest. 2003;112(10):1550–1560. doi: 10.1172/JCI18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 148.Dattani MT, Martinez-Barbera JP, Thomas PQ, Brickman JM, Gupta R, Mårtensson IL, et al. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nature genetics. 1998;19:125–133. doi: 10.1038/477. [DOI] [PubMed] [Google Scholar]
- 149.Wu W, Cogan JD, Pfäffle RW, Dasen JS, Frisch H, O'Connell SM, et al. Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nature genetics. 1998;18:147–149. doi: 10.1038/ng0298-147. [DOI] [PubMed] [Google Scholar]
- 150.Wettstein M, Diez LF, Twohig M, Abourizk NN. The role of birth injury and the consequences of inadequately treated hypogonadism in longstanding panhypopituitarism. Conn Med. 1996;60(10):583–586. [PubMed] [Google Scholar]
- 151.Cohen LE. Genetic disorders of the pituitary. Curr Opin Endocrinol Diabetes Obes. 2012;19(1):33–39. doi: 10.1097/MED.0b013e32834ed639. [DOI] [PubMed] [Google Scholar]
- 152.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(9):4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nimkarn S, New MI. Prenatal diagnosis and treatment of congenital adrenal hyperplasia. Horm Res. 2007;67(2):53–60. doi: 10.1159/000096353. [DOI] [PubMed] [Google Scholar]
- 154.S N, Lin-Su K, New MI. Steroid 21 hydroxylase deficiency congenital adrenal hyperplasia. Pediatr Clin North Am. 2011;58(5):1281–1300. doi: 10.1016/j.pcl.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 155.Hauffa B, Hiort O. P450 side-chain cleavage deficiency--a rare cause of congenital adrenal hyperplasia. Endocr Dev. 2011;20:54–62. doi: 10.1159/000321215. [DOI] [PubMed] [Google Scholar]
- 156.Krone N, Arlt W. Genetics of congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23(2):181–192. doi: 10.1016/j.beem.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lekarev O, Mallet D, Yuen T, Morel Y, New MI. Congenital lipoid adrenal hyperplasia (a rare form of adrenal insufficiency and ambiguous genitalia) caused by a novel mutation of the steroidogenic acute regulatory protein gene. Eur J Pediatr. 2012;171(5):787–793. doi: 10.1007/s00431-011-1620-5. [DOI] [PubMed] [Google Scholar]
- 158.Louiset E, Duparc C, Young J, Renouf S, Tetsi Nomigni M, Boutelet I, et al. Intraadrenal corticotropin in bilateral macronodular adrenal hyperplasia. N Engl J Med. 2013;369(22):2115–2125. doi: 10.1056/NEJMoa1215245. [DOI] [PubMed] [Google Scholar]
- 159.Groussin L, Jullian E, Perlemoine K, Louvel A, Leheup B, Luton JP, et al. Mutations of the PRKAR1A gene in Cushing's syndrome due to sporadic primary pigmented nodular adrenocortical disease. The Journal of clinical endocrinology and metabolism. 2002;87:4324–4329. doi: 10.1210/jc.2002-020592. [DOI] [PubMed] [Google Scholar]
- 160.Lacroix A. ACTH-independent macronodular adrenal hyperplasia. Best practice & research Clinical endocrinology & metabolism. 2009;23:245–259. doi: 10.1016/j.beem.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 161.Ghayee HK, Rege J, Watumull LM, Nwariaku FE, Carrick KS, Rainey WE, et al. Clinical, biochemical, and molecular characterization of macronodular adrenocortical hyperplasia of the zona reticularis: a new syndrome. The Journal of clinical endocrinology and metabolism. 2011;96:E243–E250. doi: 10.1210/jc.2010-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hayashi Y, Takeda Y, Kaneko K, Koyama H, Aiba M, Ikeda U, et al. A case of Cushing's syndrome due to ACTH-independent bilateral macronodular hyperplasia associated with excessive secretion of mineralocorticoids. Endocrine journal. 1998;45:485–491. doi: 10.1507/endocrj.45.485. [DOI] [PubMed] [Google Scholar]
- 163.Lerario AM, Moraitis A, Hammer GD. Genetics and epigenetics of adrenocortical tumors. Molecular and cellular endocrinology. 2014;386:67–84. doi: 10.1016/j.mce.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Espiard S, Ragazzon B, Bertherat J. Protein Kinase A Alterations in Adrenocortical Tumors. Horm Metab Res. 2014;8:8. doi: 10.1055/s-0034-1385908. [DOI] [PubMed] [Google Scholar]
- 165.Grumbach MM, Biller BMK, Braunstein GD, Campbell KK, Carney JA, Godley PA, et al. Management of the clinically inapparent adrenal mass ("incidentaloma") Annals of internal medicine. 2003;138:424–429. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 166.Arnaldi G, Boscaro M. Adrenal incidentaloma. Best practice & research Clinical endocrinology & metabolism. 2012;26:405–419. doi: 10.1016/j.beem.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 167.Mulatero P, Stowasser M, Loh K-C, Fardella CE, Gordon RD, Mosso L, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89(3):1045–1050. doi: 10.1210/jc.2003-031337. [DOI] [PubMed] [Google Scholar]
- 168.Fernandes-Rosa FL, TA W, Riester A, Steichen O, Beuschlein F, Boulkroun S, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64(2):354–361. doi: 10.1161/HYPERTENSIONAHA.114.03419. [DOI] [PubMed] [Google Scholar]
- 169.Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995;374(6518):135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 171.Monticone S, Hattangady NG, Nishimoto K, Mantero F, Rubin B, Cicala MV, et al. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab. 2012;97(8):2011–3132. doi: 10.1210/jc.2011-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Monticone S, Else T, Mulatero P, Williams TA, Rainey WE. Understanding primary aldosteronism: Impact of next generation sequencing and expression profiling. Mol Cell Endocrinol. 2014;18(14):00295–00300. doi: 10.1016/j.mce.2014.09.015. LID - S0303-7207(14)00295-0 [pii] LID - 10.1016/j.mce.2014.09.015 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cao Y, He M, Gao Z, Peng Y, Li Y, Li L, et al. Science. Vol. 344. New York, NY: 2014. Activating hotspot L205R mutation in PRKACA and adrenal Cushing's syndrome; pp. 913–917. [DOI] [PubMed] [Google Scholar]
- 174.Goh G, Scholl UI, Healy JM, Choi M, Prasad ML, Nelson-Williams C, et al. Recurrent activating mutation in PRKACA in cortisol-producing adrenal tumors. Nature genetics. 2014;46:613–617. doi: 10.1038/ng.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sato Y, Maekawa S, Ishii R, Sanada M, Morikawa T, Shiraishi Y, et al. Science. Vol. 344. New York, NY: 2014. Recurrent somatic mutations underlie corticotropin-independent Cushing's syndrome; pp. 917–920. [DOI] [PubMed] [Google Scholar]
- 176.Kebebew E, Reiff E, Duh Q-Y, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30(5):872–878. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 177.Kerkhofs TMA, Verhoeven RHA, Van der Zwan JM, Dieleman J, Kerstens MN, Links TP, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer. 2013;49(11):2579–2586. doi: 10.1016/j.ejca.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 178.Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. The Journal of clinical endocrinology and metabolism. 2006;91:2650–2655. doi: 10.1210/jc.2005-2730. [DOI] [PubMed] [Google Scholar]
- 179.Wajchenberg BL, Albergaria Pereira MA, Medonca BB, Latronico AC, Campos Carneiro P, Alves VA, et al. Adrenocortical carcinoma: clinical and laboratory observations. Cancer. 2000;88:711–736. [PubMed] [Google Scholar]
- 180.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best practice & research Clinical endocrinology & metabolism. 2009;23:273–289. doi: 10.1016/j.beem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 181.Beuschlein F, Reincke M, Karl M, Travis WD, Jaursch-Hancke C, Abdelhamid S, et al. Clonal composition of human adrenocortical neoplasms. Cancer research. 1994;54:4927–4932. [PubMed] [Google Scholar]
- 182.Gicquel C, Leblond-Francillard M, Bertagna X, Louvel A, Chapuis Y, Luton JP, et al. Clonal analysis of human adrenocortical carcinomas and secreting adenomas. Clinical endocrinology. 1994;40:465–477. doi: 10.1111/j.1365-2265.1994.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 183.Kartheuser A, Walon C, West S, Breukel C, Detry R, Gribomont AC, et al. Familial adenomatous polyposis associated with multiple adrenal adenomas in a patient with a rare 3' APC mutation. Journal of medical genetics. 1999;36:65–67. [PMC free article] [PubMed] [Google Scholar]
- 184.Marshall WH, Martin FI, Mackay IR. Gardner's syndrome with adrenal carcinoma. Australasian annals of medicine. 1967;16:242–244. doi: 10.1111/imj.1967.16.3.242. [DOI] [PubMed] [Google Scholar]
- 185.Kim W, Kim M, Jho E-h. Wnt/β-catenin signalling: from plasma membrane to nucleus. The Biochemical journal. 2013;450:9–21. doi: 10.1042/BJ20121284. [DOI] [PubMed] [Google Scholar]
- 186.Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagneré A-M, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer research. 2005;65:7622–7627. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 187.Tadjine M, Lampron A, Ouadi L, Bourdeau I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clinical endocrinology. 2008;68:264–270. doi: 10.1111/j.1365-2265.2007.03033.x. [DOI] [PubMed] [Google Scholar]
- 188.Assié G, Letouzé E, Fassnacht M, Jouinot A, Luscap W, Barreau O, et al. Integrated genomic characterization of adrenocortical carcinoma. Nature genetics. 2014;46:607–612. doi: 10.1038/ng.2953. [DOI] [PubMed] [Google Scholar]
- 189.Choufani S, Shuman C, Weksberg R. Beckwith-Wiedemann syndrome. American journal of medical genetics Part C, Seminars in medical genetics. 2010;154C:343–354. doi: 10.1002/ajmg.c.30267. [DOI] [PubMed] [Google Scholar]
- 190.Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E, et al. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer research. 2001;61:6762–6767. [PubMed] [Google Scholar]
- 191.Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, et al. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:668–676. doi: 10.1158/1078-0432.CCR-08-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Giordano TJ, Thomas DG, Kuick R, Lizyness M, Misek DE, Smith AL, et al. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. The American journal of pathology. 2003;162:521–531. doi: 10.1016/S0002-9440(10)63846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gicquel C, Raffin-Sanson ML, Gaston V, Bertagna X, Plouin PF, Schlumberger M, et al. Structural and functional abnormalities at 11p15 are associated with the malignant phenotype in sporadic adrenocortical tumors: study on a series of 82 tumors. The Journal of clinical endocrinology and metabolism. 1997;82:2559–2565. doi: 10.1210/jcem.82.8.4170. [DOI] [PubMed] [Google Scholar]
- 194.Weber MM, Fottner C, Schmidt P, Brodowski KM, Gittner K, Lahm H, et al. Postnatal overexpression of insulin-like growth factor II in transgenic mice is associated with adrenocortical hyperplasia and enhanced steroidogenesis. Endocrinology. 1999;140:1537–1543. doi: 10.1210/endo.140.4.6660. [DOI] [PubMed] [Google Scholar]
- 195.Heaton JH, Wood MA, Kim AC, Lima LO, Barlaskar FM, Almeida MQ, et al. Progression to adrenocortical tumorigenesis in mice and humans through insulinlike growth factor 2 and beta-catenin. Am J Pathol. 2012;181(3):1017–1033. doi: 10.1016/j.ajpath.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Almeida MQ, Fragoso MCBV, Lotfi CFP, Santos MG, Nishi MY, Costa MHS, et al. Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. The Journal of clinical endocrinology and metabolism. 2008;93:3524–3531. doi: 10.1210/jc.2008-0065. [DOI] [PubMed] [Google Scholar]
- 197.Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, et al. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. The Journal of clinical endocrinology and metabolism. 2009;94:204–212. doi: 10.1210/jc.2008-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, et al. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer chemotherapy and pharmacology. 2010;65:765–773. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lerario AM, Worden FP, Ramm CA, Hasseltine EA, Stadler WM, Else T, et al. The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: a multi-institutional NCI-sponsored trial. Hormones & cancer. 2014;5:232–239. doi: 10.1007/s12672-014-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Naing A, Lorusso P, Fu S, Hong D, Chen HX, Doyle LA, et al. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. British journal of cancer. 2013;108:826–830. doi: 10.1038/bjc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li FP, Fraumeni JF, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, et al. A cancer family syndrome in twenty-four kindreds. Cancer Research. 1988;48:5358–5362. [PubMed] [Google Scholar]
- 202.Bougeard G, Sesboüé R, Baert-Desurmont S, Vasseur S, Martin C, Tinat J, et al. Molecular basis of the Li–Fraumeni syndrome: an update from the French LFS families. Journal of Medical Genetics. doi: 10.1136/jmg.2008.057570. [DOI] [PubMed] [Google Scholar]
- 203.Libè R, Groussin L, Tissier F, Elie C, René-Corail F, Fratticci A, et al. Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:844–850. doi: 10.1158/1078-0432.CCR-06-2085. [DOI] [PubMed] [Google Scholar]
- 204.Waldmann J, Patsalis N, Fendrich V, Langer P, Saeger W, Chaloupka B, et al. Clinical impact of TP53 alterations in adrenocortical carcinomas. Langenbeck's archives of surgery / Deutsche Gesellschaft für Chirurgie. 2012;397:209–216. doi: 10.1007/s00423-011-0868-6. [DOI] [PubMed] [Google Scholar]
- 205.Herrmann LJM, Heinze B, Fassnacht M, Willenberg HS, Quinkler M, Reisch N, et al. TP53 germline mutations in adult patients with adrenocortical carcinoma. The Journal of clinical endocrinology and metabolism. 2012;97:E476–E485. doi: 10.1210/jc.2011-1982. [DOI] [PubMed] [Google Scholar]
- 206.Raymond VM, Else T, Everett JN, Long JM, Gruber SB, Hammer GD. Prevalence of germline TP53 mutations in a prospective series of unselected patients with adrenocortical carcinoma. The Journal of clinical endocrinology and metabolism. 2013;98:E119–E125. doi: 10.1210/jc.2012-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ribeiro RC, Sandrini F, Figueiredo B, Zambetti GP, Michalkiewicz E, Lafferty AR, et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9330–9335. doi: 10.1073/pnas.161479898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Varley JM, McGown G, Thorncroft M, James LA, Margison GP, Forster G, et al. Are there low-penetrance TP53 Alleles? evidence from childhood adrenocortical tumors. American journal of human genetics. 1999;65:995–1006. doi: 10.1086/302575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wagner J, Portwine C, Rabin K, Leclerc JM, Narod SA, Malkin D. High frequency of germline p53 mutations in childhood adrenocortical cancer. Journal of the National Cancer Institute. 1994;86:1707–1710. doi: 10.1093/jnci/86.22.1707. [DOI] [PubMed] [Google Scholar]
- 210.Choudhary B, Karande AA, Raghavan SC. Telomere and telomerase in stem cells: relevance in ageing and disease. Front Biosci. 2012;4:16–30. doi: 10.2741/248. [DOI] [PubMed] [Google Scholar]
- 211.Ju Z, Rudolph KL. Telomeres and telomerase in stem cells during aging and disease. Genome dynamics. 2006;1:84–103. doi: 10.1159/000092502. [DOI] [PubMed] [Google Scholar]
- 212.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Science. Vol. 266. New York, NY: 1994. Specific association of human telomerase activity with immortal cells and cancer; pp. 2011–2015. [DOI] [PubMed] [Google Scholar]
- 213.Keegan CE, Hutz JE, Else T, Adamska M, Shah SP, Kent AE, et al. Urogenital and caudal dysgenesis in adrenocortical dysplasia (acd) mice is caused by a splicing mutation in a novel telomeric regulator. Human molecular genetics. 2005;14:113–123. doi: 10.1093/hmg/ddi011. [DOI] [PubMed] [Google Scholar]
- 214.Else T, Trovato A, Kim AC, Wu Y, Ferguson DO, Kuick RD, et al. Genetic p53 deficiency partially rescues the adrenocortical dysplasia phenotype at the expense of increased tumorigenesis. Cancer Cell. 2009;15(6):465–476. doi: 10.1016/j.ccr.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 216.Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 217.Welte Y, Adjaye J, Lehrach HR, Regenbrecht CR. Cancer stem cells in solid tumors: elusive or illusive? Cell communication and signaling : CCS. 2010;8:6. doi: 10.1186/1478-811X-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lichtenauer UD, Shapiro I, Geiger K, Quinkler M, Fassnacht M, Nitschke R, et al. Side population does not define stem cell-like cancer cells in the adrenocortical carcinoma cell line NCI h295R. Endocrinology. 2008;149(3):1314–1322. doi: 10.1210/en.2007-1001. [DOI] [PubMed] [Google Scholar]