Abstract
Autophagy is a protective and life-sustaining process in which cytoplasmic components are packaged into double-membrane vesicles and targeted to lysosomes for degradation. This process of cellular self-digestion is an essential stress response and is cytoprotective by removing damaged organelles and proteins that threaten the cell’s survival. Key outcomes include energy generation and recycling of metabolic precursors. In the immune system, autophagy regulates processes such as antigen uptake and presentation, removal of pathogens, survival of short- and long-lived immune cells and cytokine-dependent inflammation. In all cases, a window of optimal autophagic activity appears critical to balance catabolic, reparative and inflammation-inducing processes. Dysregulation of autophagosome formation and autophagic flux can have deleterious consequences, ranging from a failure to “clean house” to the induction of autophagy-induced cell death. Abnormalities in the autophagic pathway have been implicated in numerous autoimmune diseases. Genome-wide association studies have linked polymorphisms in autophagy-related genes with predisposition for tissue-destructive inflammatory disease, specifically in inflammatory bowel disease and systemic lupus erythematosus. Although the precise mechanisms by which dysfunctional autophagy renders the host susceptible to continuous inflammation remain unclear, autophagy’s role in regulating the long-term survival of adaptive immune cells has recently surfaced as a defect in multiple sclerosis and rheumatoid arthritis. Efforts are underway to identify autophagy-inducing and autophagy-suppressing pharmacologic interventions that can be added to immunosuppressive therapy to improve outcomes of patients with autoimmune disease.
1. Introduction
Autophagy is a lysosome-mediated catabolic process that maintains cellular homeostasis through the degradation and recycling of cytoplasmic components and organelles (Figure 1) [1]. In general, autophagy is cytoprotective and allows the cell to adapt to internal and external stress conditions, such as nutrient starvation, oxidative stress, chronic stimulation and the intracellular accumulation of damaged proteins and organelles. By integrating with core cellular processes, such as the removal of waste product and the acquisition of energy and biosynthetic precursors, autophagy plays a critical role in the development and the functioning of the immune system. Within the immune system, autophagy participates in host protection by removing intracellular pathogens and by delivering antigens for presentation and immune recognition. Autophagy critically shapes the immune cell repertoire by interfering with negative and positive selection of developing lymphocytes in the thymus. The process of autophagy also provides nutrients and precursor molecules to mature peripheral lymphocytes, acting as a pro-survival mechanism. Central to innate immunity, autophagic activity promotes the clearance of dead cells and handling of intracellular waste and nucleic acids. More recent data have connected autophagy to the regulation of proinflammatory cytokines. Studies have identified genes in the autophagic cascade as potential risk factors for autoimmune disease. Consequently, understanding autophagy and misregulation of this catabolic process has become an important goal in conceptualizing what goes wrong in autoimmune and chronic inflammatory disease. In this review, we will briefly describe autophagy’s classic role in response to cellular stress, how it is involved in protective and pathogenic immunity and summarize current concepts on how autophagy confers risk to develop rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Crohn’s disease and multiple sclerosis (MS).
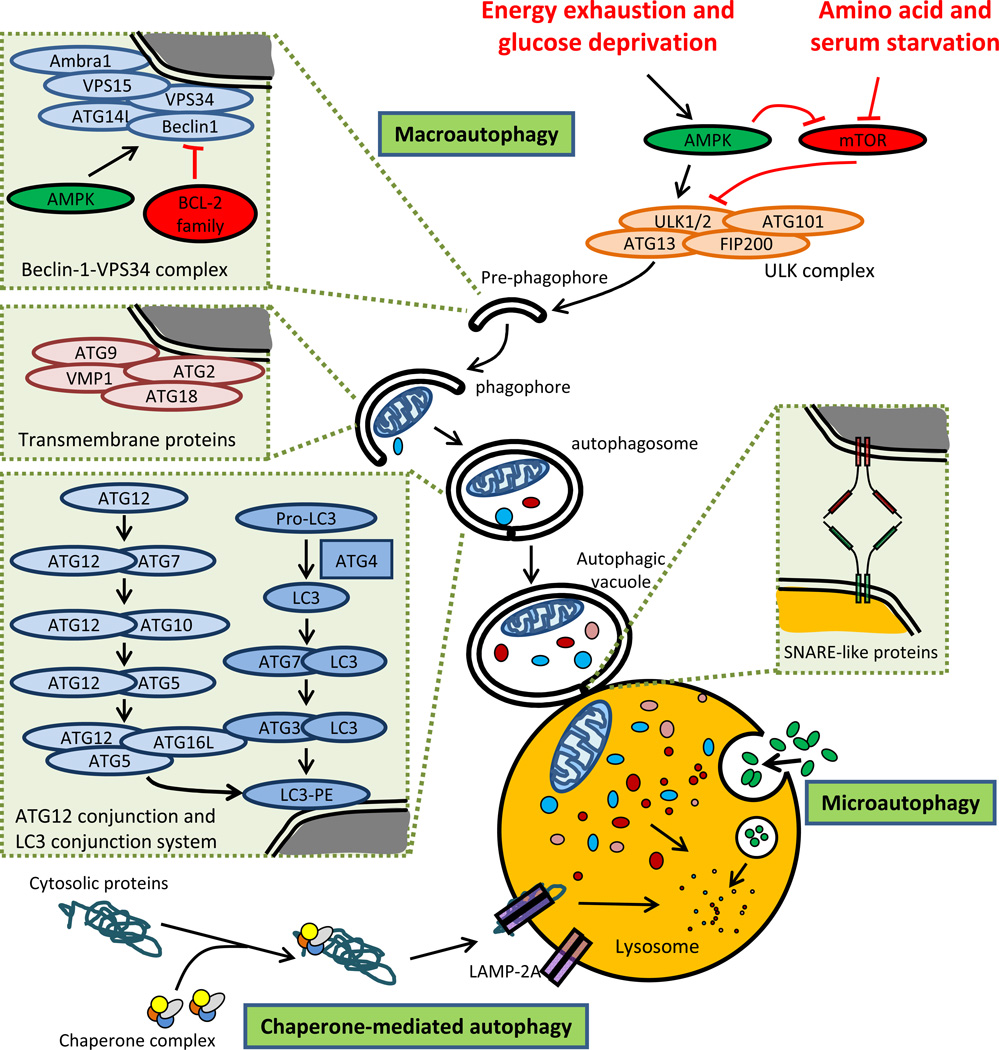
Figure 1. Schematic diagram of the main autophagic pathways.
Autophagy is modulated by environmental nutrient signals via a signaling pathway dependent on AMP-activated protein kinase (AMPK) and the mammalian (mechanistic) target of rapamycin (mTOR). In the absence of amino acids or in response to other stimuli, mTOR negatively regulates UNC-51–like kinase 1 (ULK1) through direct phosphorylation and destabilization of ULK1 protein, thus acting as a negative regulator of autophagy. Under energy depletion, AMPK negatively regulates mTOR and also directly phosphorylates ULK1, thereby acting as a positive regulator of autophagy. Macroautophagy is regulated by a set of autophagy-related proteins (ATG proteins). During macroautophagy, cytoplasmic constituents are engulfed in an isolation membrane that is elongated mainly through the action of two ubiquitin-like conjugation systems into a double-membraned autophagosome. Autophagosomes fuse with lysosomes to form autolysosomes, resulting in complete degradation of the sequestered cytoplasmic components by lysosomal hydrolases. In the case of microautophagy, cytosolic proteins are directly internalized in single membrane vesicles into lysosome by invaginating, protrusion and/or septation. In CMA, specific cytosolic proteins are transported into lysosomes via a molecular chaperone/receptor complex composed of HSPA8/HSC70 and LAMP-2A (the CMA receptor lysosome-associated membrane protein type-2A).
2. Autophagy – Basic Principles
Types of autophagy
Mammalian cells use three basic autophagic pathways for “self-eating”: macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA) (Figure 1), with macroautophagy being the best understood [2, 3]. As a general rule, autophagosomes form at points of contact between the endoplasmic reticulum and mitochondria. Two ubiquitin-like conjugation systems (Atg12 and Atg8/LC3) extend double-membrane autophagosomes to sequester a portion of cytoplasm, which then use microtubular tracks to encounter and fuse with lysosomes. Once fused with a lysosome, luminal hydrolases degrade any cargo [4–8]. Autophagy-related genes (Atg) sequentially participate in the macroautophagic process to assemble a phagotrophic complex which can be recognized by receptors anchored in a double membrane. This ultimately leads to the sequestration from the cytosol. So far, 36 Atg proteins have been identified in yeast and are hierarchically organized in functional complexes to regulate autophagic vacuole (autophagosome) initiation, formation and maturation. In mammals, many of these proteins are represented by multiple isoforms, underlining a high degree of complexity [7]. Macroautophagy can happen ‘in bulk’ – when cargo is sequestered in a random manner, or selectively – when the cargo is identified through interactions between cargo-receptors and structural components of the autophagosome [9]. Examples of selective macroautophagy include mitophagy (mitochondria), lipophagy (lipid droplets), ribophagy (ribosomes), aggregophagy (aggregosomes) or xenophagy (extracellular pathogens), which deal with special types of cargo.
Cargo sequestration in vesicles (lysosomes or late endosomes) is also the first step in microautophagy, but in this case, vesicles form from the invagination of the membrane of the autophagic vacuole. These cargo-containing vesicles then pinch off into the lumen of the lysosome for degradation (Figure 1) [10].
In CMA, target proteins are selectively identified by a cytosolic chaperone that delivers them to the lysosomal membrane (Figure 1). Once bound to the integral lysosomal membrane protein (LAMP-2A), the protein unfolds and reaches the lumen through a LAMP-2A–enriched translocation complex, followed by degradation in the autolysosome [11].
Autophagy and metabolism
An important interface between autophagy and immunity lies in the role of autophagy in providing energy resources, which are needed for immune cells to fulfill their effector functions. The first descriptions of autophagy in mammalian cells date back more than 40 years and demonstrate the intimate connection between autophagy and cellular energetics [12]. In a more refined paradigm, autophagy is now recognized as a critical mechanism in the replenishment of intracellular amino acids through protein degradation and in the regeneration of lipids from cellular macromolecules [13]. Genetic deletion of autophagic genes, including Atg3, Atg5, Atg7, Atg9 and Atg16L, significantly lower intracellular amino acid levels under starvation conditions, suggesting a key role of autophagy in modulating amino acids homeostasis. Lipids are stored in the cell in the form of lipid droplets that consist of cholesterol and triglycerides and function to supply free fatty acids needed to sustain mitochondrial β-oxidation and ATP generation [14]. Chemical or genetic disruption of autophagy proteins promotes the storage of triglycerides into lipid droplets in the liver, suggesting that autophagy can also regulate lipid metabolism and storage [15].
In immune cells, the interface between the cell’s energy production and the need for self-digestion is particularly relevant. Immune responses, due to the urgency of massive cellular expansion, are bioenergetically expansive. Activated T cells undergo a rapid switch from oxidation of pyruvate and fatty acids via the TCA cycle in mitochondria to aerobic glycolysis in the cytoplasm [16, 17]. They use enhanced anaplerotic glutaminolysis to replenish the oxidative phosphorylation cascade to be able to promote synthesis of lipids, polyamines and amino acids, all needed as biosynthetic precursors during clonal growth. During this process, autophagy is induced as an alternative means to access energy and building blocks for biomolecules [18]. Functionally distinct T cell subsets have different metabolic needs and rely on autophagy as a supply mechanism in a differential way. Unlike effector T cells, regulatory T cells depend on oxidizing fatty acids for fuel. Not only are the metabolic demands consistently hard-wired into the immune response, both the innate and adaptive immunity fundamentally affect metabolic regulation as suggested by a recent clinical trial where neutralizing interleukin 1β (IL-β) led to improved glycemic control [19].
3. Autophagy and the Immune system
A number of immunological processes are highly dependent on cellular autophagy, including pathogen recognition and destruction, antigen presentation, lymphocyte development and effector function, and inflammatory regulation (Table 1) [20, 21]. Selective autophagy (in particular xenophagy) plays a pivotal role in the processing of antigen. Conventionally, MHC class I molecules present intracellular antigens to CD8+ T cells, and MHC class II molecules present extracellular antigens to CD4+ T cells. In MHC class II presentation to CD4+ T cells, autophagy can mediate processing of both extracellular as well as nonconventional intracellular antigen [22]. Extracellular antigens are phagocytosed by antigen-presenting cells, then processed in endolysosomal compartments and loaded onto MHC class II molecules. MHC class II can also be loaded with intracellular antigen through the fusion of autophagosomes with the MHC class II compartment instead of the lysosome. Indeed, in primary monocyte-derived dendritic cells, autophagosomes frequently fuse with MHC class II compartments [23]. Together, these data suggest that autophagy may comprise a significant source of MHC class II antigens derived from intracellular sources through the delivery of material to the lysosome. Dysregulation of autophagosome fusion with the appropriate compartment (i.e. MHC class II versus lysosomal compartments) could plausibly play a role in the development of autoimmune disease.
Table 1.
Autophagy and the immune system
| Pathway | Role of Autophagy |
|---|---|
| Thymic selection | Elimination of autoreactive T cells in the thymus; involved in both negative and positive selection |
| Programmed cell death | Mediates growth-factor withdrawal induced cell death; anti-apoptosis in CD4 T cell upon TCR stimulation |
| Lymphocyte homeostasis | Survival signaling and homeostasis of T and B cells |
| T cell activation | Autophagy-dependent T cell expansion upon TCR stimulation; altered IL-2 and IFN-γ production in helper T cells |
| Apoptotic corpse clearance | Removal of apoptotic bodies and damaged organelles |
| Oxidative stress | Clearance of dysfunctional mitochondria; increases ROS production and modulates Ca2+ signaling |
| Antigen processing and presentation | Cross-presentation in dendritic cells; response to pathogen associated molecular patterns |
| Pathogen degradation | Xenophagy and phagolysosomal maturation |
| Cytokines production | Biogenesis and secretion of proinflammatory cytokines (IL-1β, IL-18), adipocytokines, type I IFN, TNF, IL-7Rα chain, etc. |
| Inflammasome activation | Limits inflammasome activity |
| Antimicrobial peptides | Mediates CD40-induced antimicrobial activity in macrophages |
| Cell differentiation | Involved in colony-stimulating factor 1-driven monocyte differentiation into macrophages; participates in B cell development |
Autophagy in T cell biology
In T cells, autophagy is constitutively on-going despite limited cytoplasmic space and is upregulated following T cell receptor stimulation [24]. Also, during HIV infection, T cell autophagy has been reported to be activated [25]. A number of genetic model systems have been developed to investigate autophagy in T cells in vivo. Atg5−/−, Atg7−/− and Vps34−/− T cells fail to proliferate effectively following activation [24, 26, 27]. Due to inefficient organelle quality control, autophagy-deficient T cells show an increase in mitochondrial load. As a consequence, such autophagy-deficient T cells have enhanced levels of reactive oxygen species and are prone to die [26]. Increased cell death and decreased cell proliferation also stem from insufficient supply in energy and precursors since depressed autophagy prevents the rise in intracellular ATP as T cell undergo activation. More recent data link autophagy to T cell differentiation and lineage commitment, a process central in protective and pathogenic immune responses. In a model system of chronic virus infection, T cell autophagy was dynamically regulated over the course of anti-viral immunity. Whereas deletion of Atg5 or Atg7 did not affect proliferation or function of CD8 effector cells, such autophagy-deficient effector cells had survival defects, leaving the host with compromised T cell memory [28].
4. Autophagy in Autoimmune disease
Insufficient removal of pathogens and dead cells inevitably leads to tissue inflammation. The process of autophagy removes pathogens and dead cells in a noninflammatory way. Thus, defects in autophagy may cause inflammation as well as provoke or exacerbate autoimmune disease. One central role of autophagy is sensing cytoplasmic danger signals, including the sensing of nucleic acids, which are known inducers of inflammatory responses and are frequently targeted by autoimmune responses. Other defects in autophagy potentially related to autoimmunity include: (1) lapses in controlling cytokine release that could prolong inflammatory responses, (2) depleting immune cells of energy that could deprive the immune system of needed effectors and hamper immune resolution, and (3) excessive death of energy-deprived immune cells that should result in enhanced compensatory proliferation and favor the outgrowth of autoreactive cells. Here, we summarize what is currently known about autophagy as a pathogenic component in human autoimmune diseases.
Autophagy and SLE
SLE is a systemic autoimmune disease associated with production of autoantibodies, formation of immune complexes and persistent inflammation that can target essentially every organ system. Life-threatening disease manifestations in SLE result from inflammatory tissue damage in the kidneys, the lung, the vascular system and the central nervous system. Autophagy is now considered a core pathogenic contributor to the abnormal immunity in SLE, affecting both innate and adaptive immunity [29, 30]. Autophagy has been implicated in multiple malfunctions relevant to SLE patients; including removal of dead cells, scavenging of intracellular DNA and RNA, regulation of type I interferon (IFN) responses and control of the long-term survival of B cells and T cells (Figure 2).
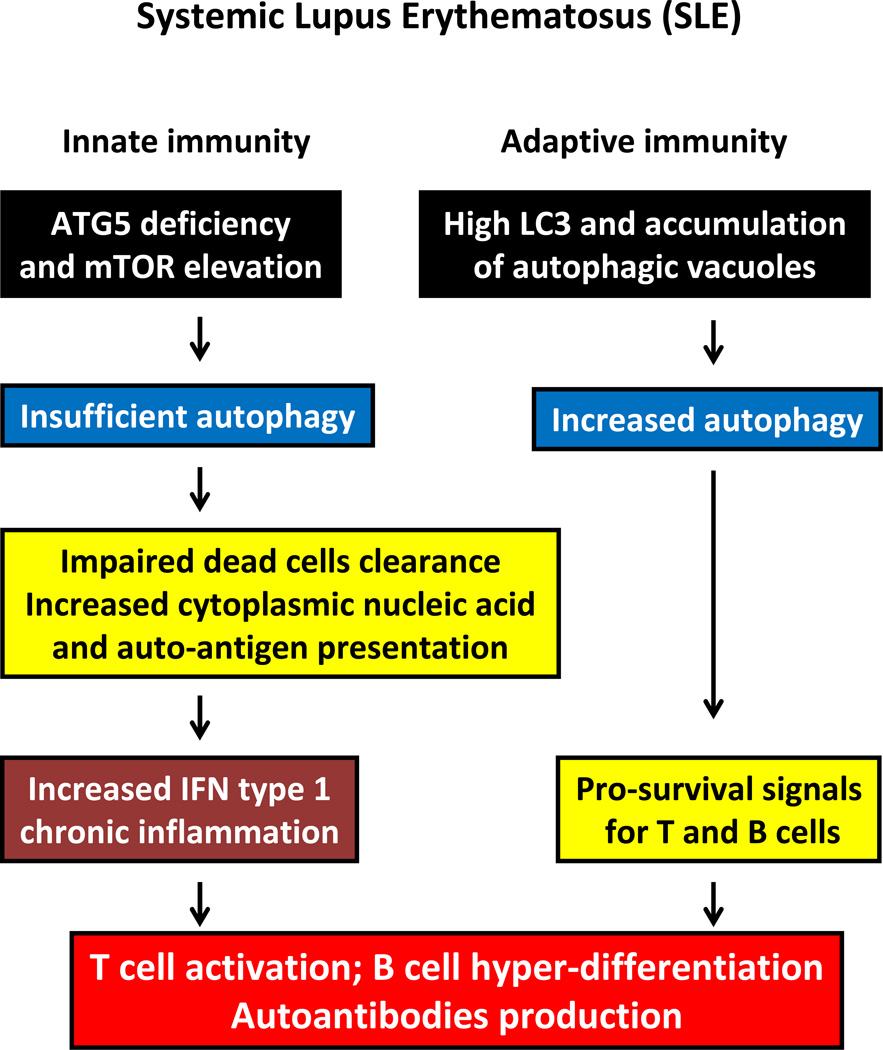
Figure 2. Autophagy in SLE.
In SLE, maladaptive autophagy is present in both innate and adaptive immunity. Insufficient autophagy due to the potential involvement of autophagic genes (e.g., Atg5) results in impaired dead cell clearance, increased autoantigen presentation and excessive type I IFN production. Conversely, increased autophagic activity when present in lymphocytes promotes T and B cell survival. Thus, defective clearance of apoptotic cells, increased antigenic load in APCs, excess T cell help, B cell hyperdifferentiation, and increased autoantibody production are all important contributors to the development of SLE.
Genome-wide association studies have linked nucleotide polymorphisms in autophagy-related genes to susceptibility for SLE [29–31]. At least five single nucleotide polymorphisms (SNPs) near to and in the Atg5 locus are associated with SLE initiation and/or development. Specifically, SNPs in the PRDM1-Atg5 intergenic region (rs548234, rs6937876 and rs6568431), in the Atg5 intron 1 (rs573775) and in the Atg5 intron 6 (rs2245214) have all surfaced as potential disease risk elements. According to Zhou et.al., SNPs within the intragenic region of PRDM1-Atg5 are also associated with SLE in the Chinese population [31]. It is currently unknown how SNPs within intragenic regions regulate gene expression of Atg5 and an integrated mechanism for Atg5 in the development of SLE has not been defined. Atg7 and IRGM have also been implicated as possible SLE susceptibility genes [32]. Positive gene to gene interactions occur among Atg5, Atg7 and IRGM, suggesting abnormalities within an integrated pathway [33]. These data identify Atg5 as a candidate therapeutic target in SLE [34] and hold the promise for the identification of an essential disease mechanism in SLE.
Prototypic abnormalities in SLE patients include autoimmune responses against DNA and RNA, excessive production of type I IFN, and difficulties dealing with immune complexes and dead cells, which may serve as a perpetual source of autoantigen. Defects in autophagic processes could be involved in all of these pathologies. It has been speculated that ineffective autophagy results in accumulation of damaged mitochondria and dead cells [35, 36]. Excessive dead cell-derived DNA/RNA protein complexes may activate the innate immune system leading to inflammatory cytokine production, either directly or following the formation of antigen-antibody complexes. Specifically, immune complexes containing autoantibodies against DNA or RNA activate Toll-like receptors (TLR)7 or 9, leading to chronic cellular activation [37, 38]. Dendritic cells produce large amounts of IFN-α via activation of TLR7 or TLR9 once they phagocytose DNA-immune complexes [39, 40]. Accordingly, autophagy-deficient dendritic cells have decreased TLR7 and TLR9 activation and reduced IFN-α production. Precise molecular mechanisms through which Atg5 or other autophagy-related molecules interfere with induction of IFN-α responses have not been reported, but it appears plausible that mishandling of intracellular DNA-antibody complexes and failures in the clearance of apoptotic bodies are key steps in the breakdown of self-tolerance and the initiation of an autoimmune response.
Beside abnormalities in innate immunity, inappropriate activation and survival of T cells and B cells are critical elements in driving multiple organ inflammation and sustaining the production of autoantibodies in SLE. A possible role of Atg7 in modulating SLE pathology has been examined in a murine model of SLE. Atg7−/− B cells have reduced survival in culture and fail to efficiently develop into plasma cells, suggesting that Atg7 promotes B cell survival and contributes to their persistence in autoimmune conditions [41]. In addition, mitochondrial density in freshly isolated Atg7−/− B cells was significantly higher than in controls, supporting the notion that mitochondrial clearance regulates B cell survival and B cell–dependent autoreactivity (Figure 2). While these data are compatible with insufficient autophagy in SLE-prone mice, SLE patients have been reported to have increased B cell autophagy [41], possible leading to increased mobilization of plasmablasts. Both B cells and T cells had more autophagic vacuoles, whereas monocytes appeared unaffected and similar to those in controls. The amount of B cell autophagic vacuoles, measured as LC3-positive punctae, was positively correlated with the SELENA-SLEDAI disease activity score [41].
T cells from SLE patients have multiple abnormalities, including aberrant T cell activation and prolonged T cell survival. A recent study analyzed LC3 conversion as an indicator for autophagic activity in T cells from both lupus-prone mouse models and human lupus patients [42]. Currently, the consensus is that autophagy is higher in SLE. This does not seem to be a result of inhibited autophagic flux, but rather a consequence of stimulation of the pathway [42]. In T cells from lupus mice, PMA/ionomycin stimulation was sufficient to reveal higher autophagic activity than in controls, suggesting that at least one of the triggers for this “hyperautophagic” phenotype relates to T cell activation [43].
Although the precise mechanisms leading to increased autophagic activity in lupus are still not understood, dysregulation of the pathway has been established and has been implicated in promoting survival of autoimmune T and B cells. An intriguing aspect of the connection between autophagy and SLE derives from the observation that drugs modulating autophagy, including hydroxychloroquine, rapamycin and the P140 peptide, provide beneficial effects in lupus-prone mouse models as well as in patients with SLE, emphasizing that resetting autophagic flux may be an important therapeutic goal in this autoimmune disease [44–46].
Autophagy and Crohn’s disease
Crohn’s disease, previously categorized as an autoimmune disease, is now recognized as an autoinflammatory syndrome of the gastrointestinal tract. Autoimmune and autoinflammatory diseases have several common features, including the inability of the host to properly regulate inflammation mediated by pathways within the innate immune system. Autoantigen recognition is considered a pinnacle event in autoimmune, but not autoinflammatory, diseases. Environmental factors and genetic predisposition are believed to contribute to Crohn’s disease, a scenario that holds true for most chronic inflammatory syndromes.
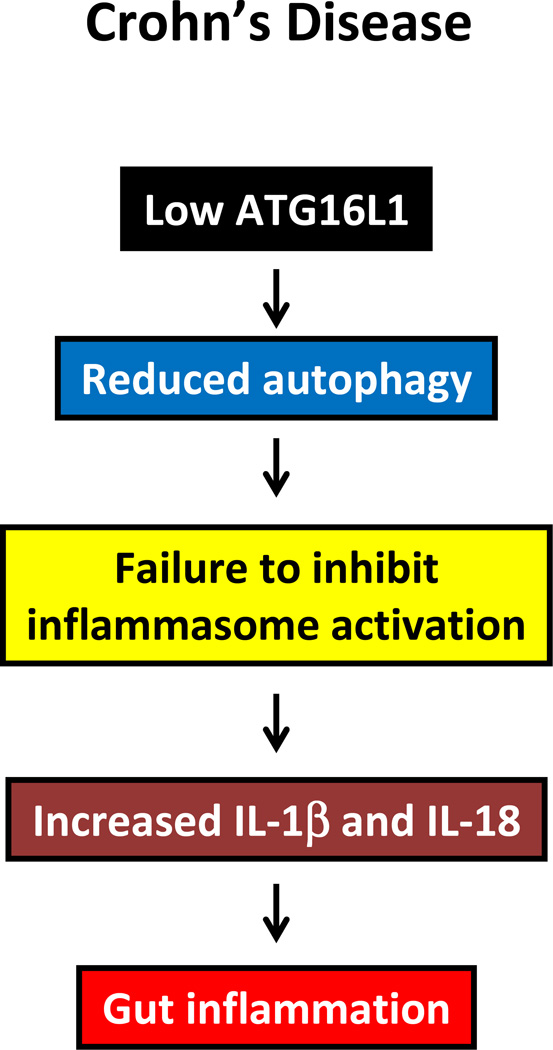
Genetic polymorphisms in the genes coding for Atg16L1 and IRGM confer a strong risk to develop Crohn’s disease in Caucasians, identifying the process of autophagy as a critical pathogenic mechanism in this inflammatory bowel disease (Figure 3) [47, 48]. Intriguingly, the associations are not observed in Asian populations. Despite the association of Atg16L1 gene polymorphisms with disease susceptibility, the underlying mechanisms have remained incompletely understood, mostly due to lack of knowledge how the Atg16L1 protein functions within the autophagy cascade. In lipopolysaccharide-stimulated macrophages, Atg16L1 deficiency stimulates the Toll/IL-1 receptor domain-containing adaptor to induce IFN-β–dependent activation of caspase-1, eventually leading to increased production of IL-1b [49]. Also, high amounts of the proinflammatory cytokine IL-18 were released from Atg16L1-deficient macrophage, strengthening the mechanistic link between Atg16L1 and inflammasome activation [49]. Mice lacking Atg16L1 are highly susceptible to dextran sulphate sodium-induced acute colitis, which can be alleviated by injection of anti-IL-1β and IL-18 antibodies, indicating the importance of Atg16L1 in the suppression of intestinal inflammation [49, 50]. These studies emphasize that an intact Atg16L1 gene is protective against inflammatory bowel disease and suggest that autophagy has a prime role in maintaining a healthy host-microbe relationship in the gut.
Figure 3. Autophagy in Crohn’s disease.
In Crohn’s disease, SNPs in the autophagic gene ATG16L1 are suspected to cause an autophagy defect that leads to failed inhibition of inflammasome activation and, thereafter, increasing cytokine production and chronic gut inflammation.
More recent studies have reported that a haplotype tagging SNP (rs12303764) in the UNC-51–like kinase 1 (ULK1) gene increases susceptibility to Crohn’s disease [51], confirming the relationship between autophagic regulation and gut inflammtion. ULK1 is a homolog of yeast Atg1, and, like the other Atg genes, is highly conserved among eukaryotes. The ULK1 protein is ubiquitously expressed in mammalian tissue. ULK1 complex activation has been shown to be the most upstream step of autophagosome formation. Thus, multiple lines of evidence support the model that autophagy has a critical role in the regulation of immune homeostasis within the intestinal tract.
Autophagy and multiple sclerosis
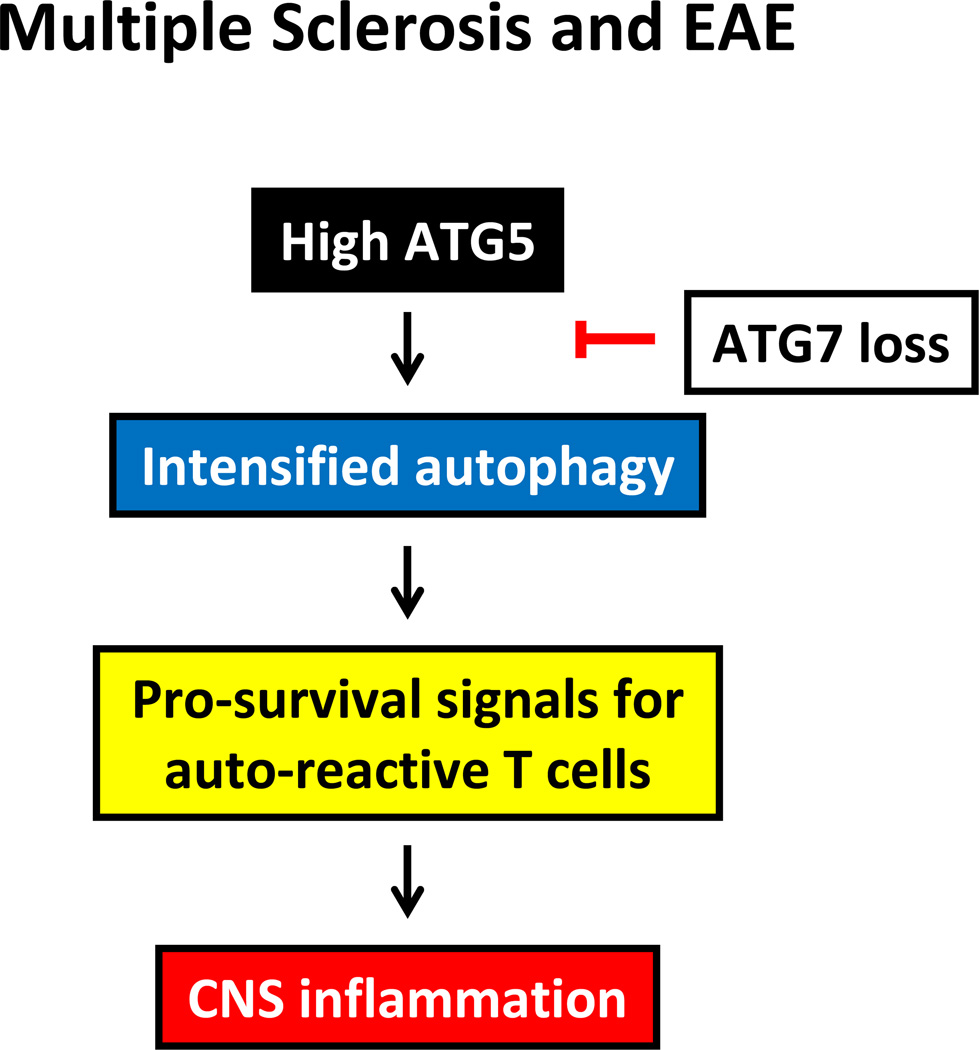
Studies linking autophagy to risk for central nervous system (CNS) inflammatory disease, such as multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE), have mostly focused on autophagic processes regulating adaptive immunity, including lymphocyte survival and antigen presentation. T cell activation in both the periphery and in the CNS is required for the induction of MS, and defects in culling out autoreactive T cells would inevitably render individuals susceptible to prolonged autoreactivity (Figure 4). In patients suffering from MS, upregulation of the ATG5 protein has been described in T cells infiltrating inflammatory sites [52]. In EAE, an animal model that partially mimics MS [53], autophagic flux degrades procaspases in T cells, antagonizing apoptosis and implicating autophagy in the survival of autoreactive T cells. Similar to MS patients, increased expression of the autophagic gene Atg5 was correlated with immune-mediated myelin injury in EAE [52]. In MS, prolonged T cell survival and increased T cell proliferation is believed to promote disease relapses and progression, suggesting that hyperexpression of Atg5 interferes with abnormalities in the adaptive immune response causing the disease [52].
Figure 4. Autophagy in CNS inflammation.
In multiple sclerosis/EAE, intensified autophagy supports the survival of autoreactive T cells and results in CNS inflammation.
Dendritic cells infiltrate the CNS during inflammation and have been identified as the key antigen-presenting cells in EAE. Atg7−/− dendritic cells significantly reduced the incidence and onset of EAE by impairing in vivo priming of T cells [54]. Treatment with chloroquine, an autophagy-lysosomal inhibitor, had a similar immunosuppressive effect. Atg7 depletion did not affect the function of CD8 T cells or NK cells; the severity of hapten-induced contact hypersensitivity remained unchanged [54]. This data suggests a second important pathway through which autophagy can alter host innate immune responses and impacts CNS inflammation.
Autophagy and rheumatoid arthritis (RA)
RA is a progressive polyarthritis of diarthrodial joints [55], which results in pain, deformities and disability. An important pathological element is autoimmune responses to citrullinated proteins. Antibodies reactive to citrullinated peptides are highly specific biomarkers for RA patients, yet their pathogenic role in the disease process remains a matter of debate. Rheumatoid joint inflammation is characterized by the infiltration of the synovial membrane with macrophages, T cells and B cells. As a maladaptive response to this inflammation, synovial fibroblasts proliferate and display tissue-invasive behavior and the excessive activation of osteoclast leads to destruction of the bone. Systemic manifestations of RA can occur in essentially any organ system, but acceleration of cardiovascular disease has attracted attention because of the shortening of life expectancy associated with premature atherosclerotic disease. Both innate and adaptive immunity have been implicated in initiating and sustaining these disease processes. Indeed, the loss of self-tolerance precedes frank joint disease by at least one decade. Moreover, immune abnormalities in RA patients have been related to premature immune aging, which has mostly been described in T cells and is easily detected by the age-inappropriate loss of telomeric ends and the expansion of clonal T cell populations that have lost expression of the CD28 molecule [56–58].
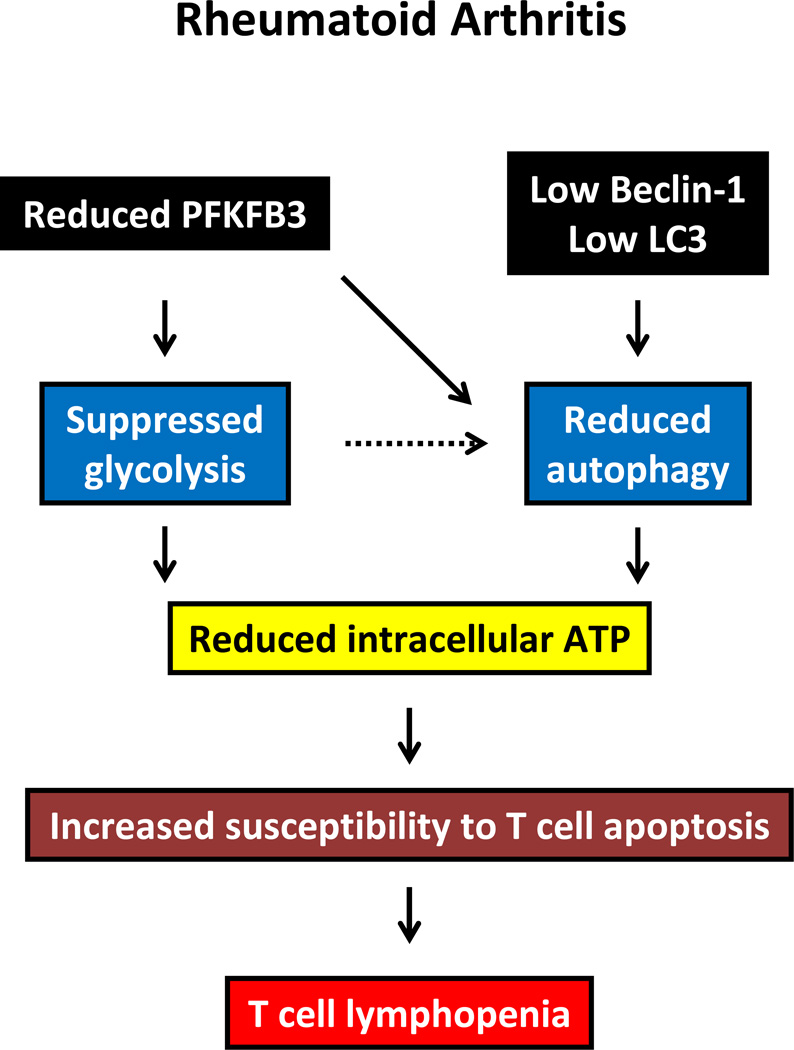
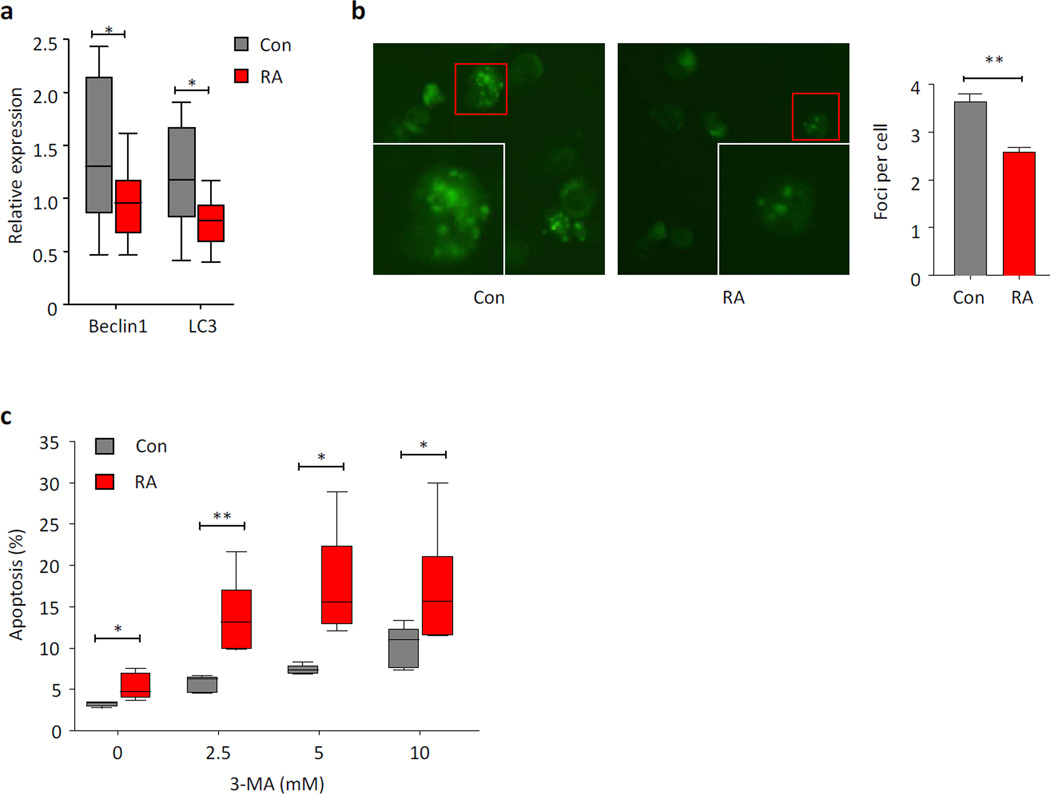
Two recent publications have fueled the concept that autophagy has a place in regulating immune abnormalities; one study in a mouse model of polyarthritis and the other in patients with RA [16, 59]. In a model system of hen egg-white lysozyme immunized mice, antigen-presenting cells, such as dendritic cells and macrophages, constitutively present citrullinated peptides. The autophagic inhibitor of 3-methyladenine (3-MA) reduced presentation of such modified peptides. Similarly, genetic depletion of Atg5 interfered with presentation of citrullinated peptides, demonstrating an important role for autophagy in breaking tolerance to modified self-antigens [59]. The second study has raised the possibility of insufficient autophagic fluxing contributing to immune abnormalities in RA (Figure 5) [16]. Specifically, a metabolic analysis of naïve CD4 T cells from RA patients revealed that these cells have switched to lower metabolic activity. The study was prompted by the premature aging phenotype that RA T cells have. Usually, activation of T cells is associated with a massive increase in aerobic glycolysis, resembling the Warburg effect in cancer cells. While inefficient, aerobic glycolysis provides fast access to ATP and, in parallel, secures formation of biosynthetic precursor molecules needed for clonal expansion. RA T cells are metabolically reprogrammed and fail to utilize glucose to a similar degree as healthy control T cells. ATP levels and lactate production are both reduced in RA T cells. The underlying molecular defect in these cells lies with PFKFB3, a regulatory enzyme in the glycolytic cascade. RA T cells produce only 50% of the PFKFB3 compared with control T cells. Interestingly, PFKFB3 also regulates autophagy and the PFKFB3-deficient cells from RA patients have significantly lower levels of the autophagy-related genes Beclin-1 and LC3 (Figure 6A). LC3 conversion assays and microscopic studies for autophagic punctae confirmed that RA CD4 T cells deviate from healthy T cells in that they utilize autophagy to a much lower degree (Figure 6B). The combined abnormality in glucose breakdown and autophagic energy generation leaves the cells metabolically stressed [18]. Functional consequences include increased susceptibility to spontaneous apoptosis and even higher death rates when the cells are treated with the autophagic inhibitor 3-MA (Figure 6C). Knockdown of PFKFB3 in healthy T cells and overexpression of PFKFB3 in RA T cells confirmed the mechanistic link between glycolytic regulation and autophagy in that PFK loss consistently impaired autophagic flux. These data question the simplified concept that autophagy serves as a default process to generate energy in starving cells. Rather, in T cells, it seems that energy generation is a coordinated process in which several pathways function in parallel.
Figure 5. Autophagy in RA.
In RA, low expression of autophagic genes, as well as insufficiency of the glycolytic enzyme PFKFB3, leave T cells with low glycolytic flux and ATP deficiency. This metabolic stress confers susceptibility for apoptotic death and eventually results in a state of lymphopenia.
Figure 6. Repressed autophagic activity in RA T cells.
CD4 T cells from RA patients fail to upregulate autophagic genes (a) and generate low numbers of GFP-LC3 foci (b) when stimulated through the T cell receptor. Defective autophagosome formation and autophagic flux renders the T cells highly sensitive to the autophagy inhibitor 3-MA (c). The frequency of apoptotic cells is shown in relationship to increasing doses of 3-MA. Cells from RA patients and age-matched controls are compared.
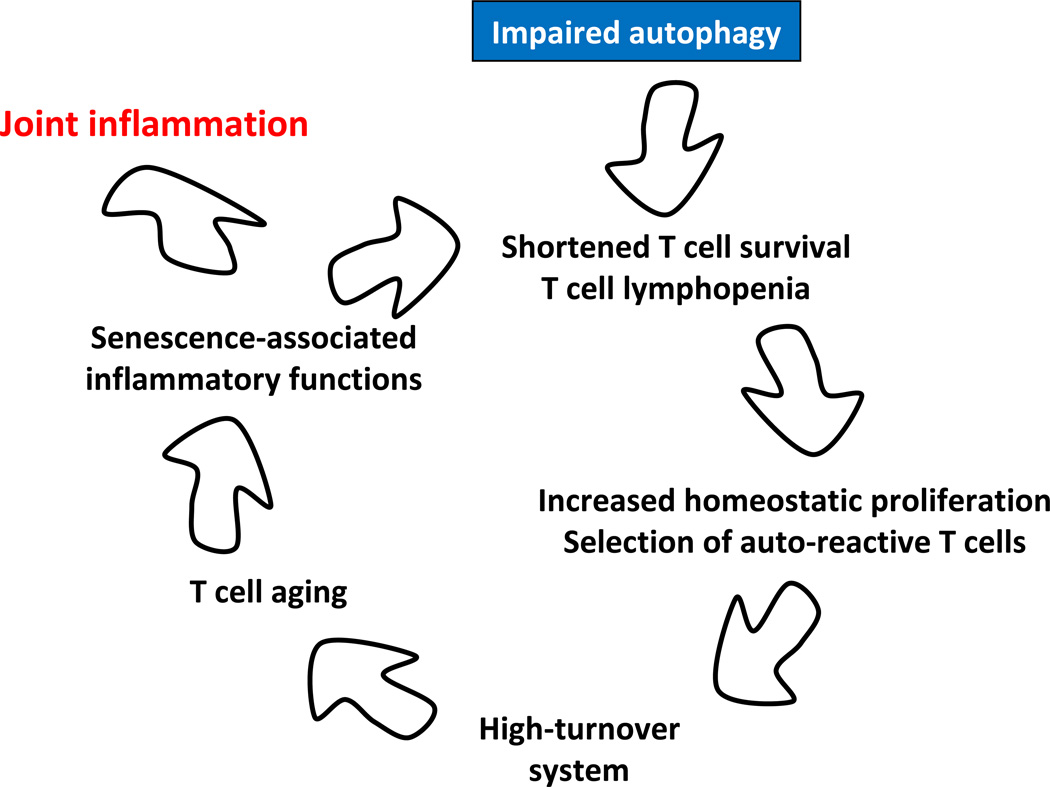
Although a link between autophagy and the development of RA has become more defined, the exact mechanisms that lead to specific immune abnormalities in RA patients remain unclear. It is possible that the lack of “self-eating” leaves T cells with damaged organelles and eventually causes altered differentiation of these T cells. An alternative model proposes that metabolic and autophagic deficiencies lead to slow, but persistent, loss of naïve CD4 T cells. In aging individuals, such chronic T cell loss would not be compensated for with increased thymic T cell production and thus would eventually lead to lymphopenia-induced homeostatic proliferation within the periphery. Because peripheral T cells require recognition of self HLA to proliferate, enforced homeostatic proliferation would bias the T cell receptor repertoire towards self-reactive T cells. Both of these models could foster autoreactivity and thus have the potential to render a patient susceptible to autoimmune disease (Figure 7). An important consideration is whether deficiency of autophagic flux is a general feature of aged T cells or whether the process of T cell aging in healthy individuals and RA patients follows different molecular routes.
Figure 7. Defective autophagy as a risk factor for autoimmunity – a hypothetical model.
In response to antigen and danger signals, lymphocytes mobilize autophagy as a basal mechanism to alleviate stress and fulfill the energy demands associated with clonal expansion. T cells from RA patients fail to upregulate autophagic activity, rendering them susceptible to apoptosis. Evolving lymphopenia triggers compensatory homeostatic proliferation to make up for the T cell loss. Proliferating T cells are selected on autoantigens, biasing the T cell repertoire towards recognition of self. Chronic T cell loss sustains a high-turnover system, eventually causing T cell aging. Prematurely aged T cells acquire the so-called senescence-associated secretory phenotype (SASP) and release massive amounts of inflammatory mediators. The end result is a host predisposed to tissue inflammation. Synovia-specific antigens may direct hyperinflammatory T cells towards the joint.
5. Conclusion
Biological implications of insufficient and excessive autophagic activity are a rapidly growing area of study. Previous views of autophagy as a strictly catabolic recycling pathway are now being expanded. A new understanding of autophagy recognizes that this process is a critical component in metabolic adaptations, intracellular and extracellular quality control mechanisms and the regulation of inflammatory pathways. The healthy immune system relies on autophagy to provide access to antigens, remove damaged and dead cells and fine-tune the longevity of functional immune effector cells. Driven by the observation that polymorphisms in autophagy-related genes are disease susceptibility factors, multiple studies have implicated dysregulation of autophagy in promoting tissue injury and persistent inflammation. Associations of autophagy genes with Crohn’s disease and SLE have prompted a fresh look at the role of cell death in the pathogenesis of these autoimmune syndromes. Patients with SLE typically produce autoantibodies against nuclear antigens. Malfunctioning autophagy has been suspected to interfere with such processes as sensing of DNA by cytoplasmic sensors, induction of antibody production reactive to DNA and the tissue-damaging effects of deposited immune complexes. In MS and RA, autophagy interferes with survival of antigen-presenting and antigen-responsive cells. While prolonging survival of autoreactive lymphocytes is a straight-forward disease-promoting abnormality, a different paradigm is emerging for RA. Here, naïve T cells have a defect in glucose utilization and in autophagy, leaving them more prone to apoptosis. Thus, in RA, it is predicted that lymphopenia sways the host to generate an autoreactive immune repertoire by replenishing the shrinking T cell pool with autoreactive cells.
As studies connecting autophagic abnormalities to autoimmune disease move forward, efforts should be made to standardize the methods used to measure autophagosome formation and autophagic flux. Similarly, reliable means of quantifying autophagic activity in vivo will be needed to bring cutting-edge mechanistic insights to clinical application. Great potential lies in expanding the toolbox of pharmacologic interventions targeting autophagy to rebalance its cytoprotective effects against deleterious consequences of excessive or insufficient “self-eating”.
Acknowledgments
This work was supported by the National Institutes of Health (R01 AR042547, R01 AI044142, HL 117913, R01 AI108906 and P01 HL058000 to CMW and R01 AI108891, R01 AG045779 and I01 BX001669 to JJG). ZY received fellowship support from the Govenar Discovery Fund.
Footnotes
Conflict of interest The authors declare no competing financial interests.
References
- 1.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 2.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geng J, Klionsky DJ. The Atg8 and Atg12 ubiquitin-like conjugation systems in macroautophagy. 'Protein modifications: beyond the usual suspects' review series. EMBO Rep. 2008;9:859–864. doi: 10.1038/embor.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092–2100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 8.Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- 9.Hamasaki M, Shibutani ST, Yoshimori T. Up-to-date membrane biogenesis in the autophagosome formation. Curr Opin Cell Biol. 2013;25:455–460. doi: 10.1016/j.ceb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Sakai Y, Koller A, Rangell LK, Keller GA, Subramani S. Peroxisome degradation by microautophagy in Pichia pastoris: identification of specific steps and morphological intermediates. J Cell Biol. 1998;141:625–636. doi: 10.1083/jcb.141.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22:407–417. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deter RL, De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967;33:437–449. doi: 10.1083/jcb.33.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu K, Czaja MJ. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013;20:3–11. doi: 10.1038/cdd.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013;210:2119–2134. doi: 10.1084/jem.20130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Matteson EL, Goronzy JJ, Weyand CM. T-cell metabolism in autoimmune disease. Arthritis Res Ther. 2015;17:29. doi: 10.1186/s13075-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, Goronzy JJ, Weyand CM. The glycolytic enzyme PFKFB3/phosphofructokinase regulates autophagy. Autophagy. 2014;10:382–383. doi: 10.4161/auto.27345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon A. Cholesterol metabolism and immunity. N Engl J Med. 2014;371:1933–1935. doi: 10.1056/NEJMcibr1412016. [DOI] [PubMed] [Google Scholar]
- 20.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Munz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 21.Munz C. Enhancing immunity through autophagy. Annu Rev Immunol. 2009;27:423–449. doi: 10.1146/annurev.immunol.021908.132537. [DOI] [PubMed] [Google Scholar]
- 22.Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci U S A. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Gao Y, Tan J, Devadas K, Ragupathy V, Takeda K, Zhao J, Hewlett I. HIV-1 and HIV-2 infections induce autophagy in Jurkat and CD4+ T cells. Cell Signal. 2012;24:1414–1419. doi: 10.1016/j.cellsig.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 27.Jia W, He YW. Temporal regulation of intracellular organelle homeostasis in T lymphocytes by autophagy. J Immunol. 2011;186:5313–5322. doi: 10.4049/jimmunol.1002404. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Araki K, Li S, Han JH, Ye L, Tan WG, Konieczny BT, Bruinsma MW, Martinez J, Pearce EL, et al. Autophagy is essential for effector CD8(+) T cell survival and memory formation. Nat Immunol. 2014;15:1152–1161. doi: 10.1038/ni.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.International Consortium for Systemic Lupus Erythematosus G. Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, Xu JH, Cai ZM, Huang W, Zhao GP, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. 2009;41:1234–1237. doi: 10.1038/ng.472. [DOI] [PubMed] [Google Scholar]
- 31.Zhou XJ, Zhang H. Autophagy in immunity: implications in etiology of autoimmune/autoinflammatory diseases. Autophagy. 2012;8:1286–1299. doi: 10.4161/auto.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou XJ, Lu XL, Lv JC, Yang HZ, Qin LX, Zhao MH, Su Y, Li ZG, Zhang H. Genetic association of PRDM1-ATG5 intergenic region and autophagy with systemic lupus erythematosus in a Chinese population. Ann Rheum Dis. 2011;70:1330–1337. doi: 10.1136/ard.2010.140111. [DOI] [PubMed] [Google Scholar]
- 33.Ramos PS, Criswell LA, Moser KL, Comeau ME, Williams AH, Pajewski NM, Chung SA, Graham RR, Zidovetzki R, Kelly JA, et al. A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet. 2011;7:e1002406. doi: 10.1371/journal.pgen.1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou XJ, Lu XL, Nath SK, Lv JC, Zhu SN, Yang HZ, Qin LX, Zhao MH, Su Y, Shen N, et al. Gene-gene interaction of BLK, TNFSF4, TRAF1, TNFAIP3, and REL in systemic lupus erythematosus. Arthritis Rheum. 2012;64:222–231. doi: 10.1002/art.33318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN, Gilpin C, Levine B. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 36.Rogers NJ, Lees MJ, Gabriel L, Maniati E, Rose SJ, Potter PK, Morley BJ. A defect in Marco expression contributes to systemic lupus erythematosus development via failure to clear apoptotic cells. J Immunol. 2009;182:1982–1990. doi: 10.4049/jimmunol.0801320. [DOI] [PubMed] [Google Scholar]
- 37.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savarese E, Chae OW, Trowitzsch S, Weber G, Kastner B, Akira S, Wagner H, Schmid RM, Bauer S, Krug A. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood. 2006;107:3229–3234. doi: 10.1182/blood-2005-07-2650. [DOI] [PubMed] [Google Scholar]
- 39.Lichtman EI, Helfgott SM, Kriegel MA. Emerging therapies for systemic lupus erythematosus--focus on targeting interferon-alpha. Clin Immunol. 2012;143:210–221. doi: 10.1016/j.clim.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 41.Clarke AJ, Ellinghaus U, Cortini A, Stranks A, Simon AK, Botto M, Vyse TJ. Autophagy is activated in systemic lupus erythematosus and required for plasmablast development. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gros F, Arnold J, Page N, Decossas M, Korganow AS, Martin T, Muller S. Macroautophagy is deregulated in murine and human lupus T lymphocytes. Autophagy. 2012;8:1113–1123. doi: 10.4161/auto.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nambiar MP, Juang YT, Krishnan S, Tsokos GC. Dissecting the molecular mechanisms of TCR zeta chain downregulation and T cell signaling abnormalities in human systemic lupus erythematosus. Int Rev Immunol. 2004;23:245–263. doi: 10.1080/08830180490452602. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69:20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 45.Perl A. Emerging new pathways of pathogenesis and targets for treatment in systemic lupus erythematosus and Sjogren's syndrome. Curr Opin Rheumatol. 2009;21:443–447. doi: 10.1097/BOR.0b013e32832efe6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Page N, Gros F, Schall N, Decossas M, Bagnard D, Briand JP, Muller S. HSC70 blockade by the therapeutic peptide P140 affects autophagic processes and endogenous MHCII presentation in murine lupus. Ann Rheum Dis. 2011;70:837–843. doi: 10.1136/ard.2010.139832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scharl M, Wojtal KA, Becker HM, Fischbeck A, Frei P, Arikkat J, Pesch T, Kellermeier S, Boone DL, Weber A, et al. Protein tyrosine phosphatase nonreceptor type 2 regulates autophagosome formation in human intestinal cells. Inflamm Bowel Dis. 2012;18:1287–1302. doi: 10.1002/ibd.21891. [DOI] [PubMed] [Google Scholar]
- 48.Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 49.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 50.Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henckaerts L, Cleynen I, Brinar M, John JM, Van Steen K, Rutgeerts P, Vermeire S. Genetic variation in the autophagy gene ULK1 and risk of Crohn's disease. Inflamm Bowel Dis. 2011;17:1392–1397. doi: 10.1002/ibd.21486. [DOI] [PubMed] [Google Scholar]
- 52.Alirezaei M, Fox HS, Flynn CT, Moore CS, Hebb AL, Frausto RF, Bhan V, Kiosses WB, Whitton JL, Robertson GS, et al. Elevated ATG5 expression in autoimmune demyelination and multiple sclerosis. Autophagy. 2009;5:152–158. doi: 10.4161/auto.5.2.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kovacs JR, Li C, Yang Q, Li G, Garcia IG, Ju S, Roodman DG, Windle JJ, Zhang X, Lu B. Autophagy promotes T-cell survival through degradation of proteins of the cell death machinery. Cell Death Differ. 2012;19:144–152. doi: 10.1038/cdd.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhattacharya A, Parillon X, Zeng S, Han S, Eissa NT. Deficiency of autophagy in dendritic cells protects against experimental autoimmune encephalomyelitis. J Biol Chem. 2014;289:26525–26532. doi: 10.1074/jbc.M114.575860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weyand CM, Yang Z, Goronzy JJ. T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol. 2014;26:93–100. doi: 10.1097/BOR.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7− CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goronzy JJ, Li G, Yang Z, Weyand CM. The janus head of T cell aging - autoimmunity and immunodeficiency. Front Immunol. 2013;4:131. doi: 10.3389/fimmu.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ireland JM, Unanue ER. Autophagy in antigen-presenting cells results in presentation of citrullinated peptides to CD4 T cells. J Exp Med. 2011;208:2625–2632. doi: 10.1084/jem.20110640. [DOI] [PMC free article] [PubMed] [Google Scholar]