Abstract
Parkinsonian symptoms arise due to over-activity of the indirect striatal output pathway, and under-activity of the direct striatal output pathway. l-DOPA-induced dyskinesia (LID) is caused when the opposite circuitry problems are established, with the indirect pathway becoming underactive, and the direct pathway becoming over-active. Here, we define synaptic plasticity abnormalities in these pathways associated with parkinsonism, symptomatic benefits of l-DOPA, and LID. We applied spike-timing dependent plasticity protocols to corticostriatal synapses in slices from 6-OHDA-lesioned mouse models of parkinsonism and LID, generated in BAC transgenic mice with eGFP targeting the direct or indirect output pathways, with and without l-DOPA present. In naïve mice, bidirectional synaptic plasticity, i.e. LTP and LTD, was induced, resulting in an EPSP amplitude change of approximately 50% in each direction in both striatal output pathways, as shown previously. In parkinsonism and dyskinesia, both pathways exhibited unidirectional plasticity, irrespective of stimulation paradigm. In parkinsonian animals, the indirect pathway only exhibited LTP (LTP protocol: 143.5 ± 14.6%; LTD protocol 177.7 ± 22.3% of baseline), whereas the direct pathway only showed LTD (LTP protocol: 74.3 ± 4.0% and LTD protocol: 63.3 ± 8.7%). A symptomatic dose of l-DOPA restored bidirectional plasticity on both pathways to levels comparable to naïve animals (Indirect pathway: LTP protocol: 124.4± 22.0% and LTD protocol: 52.1± 18.5% of baseline. Direct pathway: LTP protocol: 140.7 ± 7.3% and LTD protocol: 58.4 ± 6.0% of baseline). In dyskinesia, in the presence of l-DOPA, the indirect pathway exhibited only LTD (LTP protocol: 68.9 ± 21.3% and LTD protocol 52.0 ± 14.2% of baseline), whereas in the direct pathway, only LTP could be induced (LTP protocol: 156.6 ± 13.2% and LTD protocol 166.7 ± 15.8% of baseline). We conclude that normal motor control requires bidirectional plasticity of both striatal outputs, which underlies the symptomatic benefits of l-DOPA. Switching from bidirectional to unidirectional plasticity drives global changes in striatal pathway excitability, and underpins parkinsonism and dyskinesia.
Keywords: Parkinson’s disease, Dyskinesia, BAC transgenic mouse models, Slice electrophysiology, Striatum, Synaptic plasticity
Introduction
Symptoms of Parkinson’s disease (PD) are caused by loss of striatal dopamine (Hornykiewicz, 1962) and typically treated with l-DOPA (Utley and Carlsson, 1965). Over time, most PD patients develop involuntary movements, known as l-DOPA-induced dyskinesia (LID) (Anden et al., 1970) which impair even rudimentary motor tasks (Ahlskog and Muenter, 2001).
Parkinsonism and dyskinesia are caused by basal ganglia circuitry abnormalities originating in the striatum (Albin et al., 1989, 1995; Crossman, 1989, 1990; DeLong, 1990). Output neurons comprise 95% of the striatal neuronal population, with two distinct trajectories. The direct pathway is monosynaptic and terminates in the globus pallidus interna (GPi) and substantia nigra pars reticulata (SNr). The indirect pathway synapses in the globus pallidus externa (GPe) and subthalamic nucleus (STN) before terminating in the SNr and GPi. Each pathway expresses distinct receptor subtypes, impacting motor control differently. Within the striatum, the direct pathway expresses dopamine D1 receptors, whereas the striatal neurons associated with the indirect pathway express dopamine D2 and adenosine A2a receptors (Albin et al., 1989; Gerfen et al., 1990; Kravitz et al., 2010). Parkinsonism involves over-activity and under-activity of the indirect and direct pathways respectively. LID is caused by over-activity of the direct pathway with underactivity of the indirect pathway contributing to some if not all forms of LID (Pan et al., 1985; Crossman, 1989, 1990; Bezard et al., 2001; Brotchie, 2005; Jenner, 2008; Cenci and Konradi, 2010; Huot et al., 2013).
Physiologically, both the direct and indirect pathways display bidirectional synaptic plasticity (Kreitzer and Malenka, 2005; Shen et al., 2008). In models of parkinsonism, using juvenile mice with acute 6-OHDA-lesions, the direct and indirect pathways appear to exhibit only LTP and LTD respectively (Kreitzer and Malenka, 2005; Shen et al., 2008), while less is known about synaptic plasticity in LID. Studies by Picconi and colleagues suggested that synapses cannot be depotentiated, although l-DOPA was absent, so was modelling the OFF-treatment state of dyskinetic patients when dyskinesia is not observed (Picconi et al., 2003, 2008, 2011). Also the direct or indirect pathway was not defined. Restoration of depotentiation by a D1 receptor antagonist in this study suggests that this may originate in the direct pathway. With the development of eGFP D1, D2 and A2a BAC mice (Gong et al., 2003), studying plasticity in the indirect or direct pathway is possible. In the present study we used these mice to develop models of parkinsonism and LID (Gong et al., 2003; Thiele et al., 2011). We chose to use a stable 6-OHDA-lesion administered to adult mice, with the lesion developing for 14 days. At this point the amount of dopamine depletion has reached its full extent (Ungerstedt and Arbuthnott, 1970), and chronic l-DOPA-therapy can proceed in the absence of on-going dopamine depletion. Furthermore, while the pathogenesis of PD in patients is not replicated in this model, the downstream changes in basal ganglia circuitry do reflect those observed in patients with PD and LID. Thus, this is the most appropriate rodent model for studying the mechanisms underlying symptoms of PD and LID. Taking slices from these animals, we assessed synaptic plasticity in corticostriatal synapses. We modelled clinical states, both ON- and OFF-l-DOPA with respect to alleviation of parkinsonism, and with and without expression of LID, in the dyskinesiaprimed state (Table 1).
Table 1.
Animal model correlates of relevant Parkinson’s disease clinical states.
Healthy control patients are modelled using sham-operated animals. PD patients not treated with l-DOPA (untreated parkinsonism) are represented by 6-OHDA mice with >95% dopa-mine depletion, treated intra-peritoneally (i.p.) with vehicle for 21 days. Once slices were placed in the recording chamber aCSF was added to the bath. PD patients treated with a symp-tomatically beneficial dose of l-DOPA, absent of dyskinesia are represented using 6-OHDA mice, long-term treated (LTT) with vehicle, with aCSF supplemented with l-DOPA (250 nM) in the recording chamber. PD patients with a history of dyskinesia but not receiving l-DOPA at the time of observation (‘OFF‘ state dyskinesia) are modelled using 6-OHDA mice, followed by chronic treatment with l-DOPA that caused dyskinesia, and aCSF only in the recording chamber. Dyskinesia was modelled using 6-OHDA mice LTT with l-DOPA, with aCSF supplemented with l-DOPA (250 nM) in the recording chamber.
| Clinical correlate | Experimental design |
||
|---|---|---|---|
| Operation | Treatment (i.p. 2× daily for 21 days) | aCSF condition | |
| Control | Sham | Vehicle | aCSF |
| Untreated parkinsonian | 6-OHDA | Vehicle | aCSF |
| ON-state, treated parkinsonian, no dyskinesia | 6-OHDA | Vehicle | aCSF supplemented with l-DOPA (250 nM) |
| OFF state, dyskinesia | 6-OHDA | l-DOPA (4.5 mg/kg) | aCSF |
| On-state, dyskinesia | 6-OHDA | l-DOPA (4.5 mg/kg) | aCSF supplemented with l-DOPA (250 nM) |
Our findings confirm in adult mice with stable lesions, that in parkinsonism, bidirectional plasticity is lost. We extend these findings to show that a symptomatic dose of l-DOPA restores bidirectional plasticity, likely reflecting the symptomatic effects of l-DOPA, indicating that bidirectional synaptic plasticity is required for normal motor control. In a mouse dyskinesia model, repeated l-DOPA caused loss of bidirectional synaptic plasticity, in the opposite direction observed in parkinsonism. These alterations in striatal synaptic plasticity could underpin the changes in basal ganglia circuitry linked to the generation of parkinsonism and LID (Pan et al., 1985; Albin et al., 1989, 1995; Crossman, 1989, 1990; DeLong, 1990).
Methods
Animals
Bacterial artificial chromosome (BAC) mice on a Friend virus B-type (FVB) background in which the A2A receptor (indirect pathway) or D1 receptor (direct pathway) is reported by co-expression of eGFP were obtained from the Mutant Mouse Regional Resource Centre (Gong et al., 2003). Mice were maintained under 12:12 h lights on:dark cycle, with free access to food and water, according to the guidelines of the Canadian Council on Animal Care and the Animal Care Committees of the University of Toronto and University Health Network.
Unilateral 6-OHDA lesions
Surgeries were performed as previously described (Thiele et al., 2011, 2012). In brief, 30 min prior to surgery, a mixture of desipramine hydrochloride (25 mg/kg, Sigma Aldrich) and pargyline hydrochloride (5 mg/kg, Sigma Aldrich) in 0.9% sterile saline (pH 7.4) was systemically administered intra-peritoneally (i.p.). Mice (P35, 24–28 g) were anaesthetised (isoflurane (Abbott), 2–3%) and placed in a stereotaxic frame (David Kopf Instruments, USA). 6-Hydroxydopamine (6-OHDA) (15 µg/µl, 0.02% ascorbic acid, w/v in 0.9% saline) or vehicle was unilaterally injected into the medial forebrain bundle (MFB) at a rate of 0.1 µl/min (total delivery of 3 µg total, as a 0.2 µl bolus) at the following coordinates: AP: −1.2 mm, ML: −1.1 mm, and DV: −5.0 mm (Franklin, 2007). This protocol results in a N95% dopamine depletion of the SNc (Thiele et al., 2011, 2012).
Behavioural assessment of the 6-OHDA-induced dopamine depletion in mice
Spontaneous rotational behaviour was performed 14 days following 6-OHDA-lesion surgery, when nigro-striatal degeneration has reached a maximum and is stable (Warre et al., 2011). For assessment of spontaneous rotational behaviour, mice were placed in glass cylinders (11 × 9.5 cm), and their activity was immediately recorded for 40 min using a video camera. The number of complete 360° rotations made in the direction ipsiversive and contraversive to the side of the lesion was quantified post-hoc, over the 40 min period (Ungerstedt and Arbuthnott, 1970). Our previous studies have shown that in the 6-OHDA-lesioned mouse, animals exhibiting a net ipsiversive rotational bias of > 70% measurement of spontaneous rotational behaviour or more is the most reliable behavioural test as compared to forelimb use asymmetry and amphetamine-induced rotations for selecting animals with >95% lesion (Thiele et al., 2011).
Generation and assessment of a mouse model of l-DOPA-induced dyskinesia
Commencing 15 days post-6-OHDA lesion surgery, l-DOPA methyl ester plus benserazide was administered systemically in a 4:1 ratio, twice daily for 21 days (4.5 mg/kg and 1.125 mg/kg respectively i.p., bid) (both Sigma-Aldrich). In an attempt to mimic l-DOPA levels in patients with dyskinesia, we used a dose of l-DOPA (4.5 mg/kg) that we found alleviated parkinsonism initially, without causing any dyskinesia. Then, as observed in patients, this dose of l-DOPA began to cause abnormal involuntary movements (AIMs) in the mice around day 5 (Thiele et al., 2012). AIMs gradually intensified then plateaued with treatment continuation. In rodent models of LID, AIMs are considered the behavioural and mechanistic equivalent of LID in patients with PD (Picconi et al., 2003; Lundblad et al., 2004, 2005; Cenci and Lundblad, 2007; Valastro et al., 2007; Ahmed et al., 2010). To assess the motor abnormalities in our mouse model of dyskinesia, a modified form of the AIMs was used (Cenci et al., 1998) which resembles scales used to evaluate human dyskinesia (Park et al., 2010). Following administration of the morning dose of l-DOPA, mice were immediately recorded with a video camera and scored post-hoc for 1 min every 20 min over a 2 h period. Dosing of animals was staggered so that videotaping could begin immediately after l-DOPA administration. Post-hoc analysis was performed by a person that was blinded to the groups. The development of AIMs did not begin until 15 to 20 min following l-DOPA, thus the initial time point (t = 0) led to a null score. l-DOPA-induced changes in behaviour usually lasted no more than 90 min following l-DOPA administration. This procedure was repeated on days 1, 5, 9,13,17, and 21. Movements were identified as dyskinetic only when they were repetitive, affected the side of the body contralateral to the lesion, and could not be described as ‘normal’ behaviour, such as grooming or gnawing that generally involves both sides of the body. AIMs were classified based on their topographical distribution of presentation, being divided into axial, orolingual or forelimb abnormalities. Forelimb AIMs involve choreiform, repetitive, rhythmic jerky movements with minimal to no dystonic posturing of the forelimb on the unaffected side. Axial AIMs include lateral flexion and axial rotation of the neck and trunk towards the side contralateral to the lesioned hemisphere. Orolingual AIMs are characterized as repetitive and vacuous chewing movements of the jaw with or without tongue protrusion. Each subtype was scored on a scale from 0 to 4 where: 0 = absence of AIMs, 1 = occasional occurrence, less than half the observation time, 2 = frequent occurrence, present between 50 and 75% of the observation period, 3 = continuous AIMs which can be interrupted by sensory distraction, and 4 = continuous AIMs, not interrupted by sensory distraction. AIMs were measured every 20 min from time 0 to 120 min, i.e. a total of 7 times. Since 4 different types of AIMs were measured, each with a maximum score of 4, the maximum possible score was 84 for the entire session. Net 360° rotational behaviour was also quantified, but displayed separately to other AIMs in contrast to previous groups that have used similar rodent models of LID (Cenci et al., 1998; Konradi et al., 2004; Lundblad et al., 2004, 2005; Dekundy et al., 2007).
Slice preparation for electrophysiology recordings
Thirty-seven days post 6-OHDA surgery, animals (P72) were anaesthetised with ketamine (150 mg/kg) and xylazine (10 mg/kg) (CDMV, Canada), and transcardially perfused with modified artificial cerebrospinal fluid (mCSF) (4 °C) that had been partially frozen and blended containing high sucrose, low calcium, and high magnesium to minimise excitotoxicity (in mM: 2.5 KCl,1.25 NaH2PO4, 25 NaHCO3, 7 MgSO4, 0.5 CaCl, 25 glucose (Bishop, Canada), aerated with 95% O2/ 5% CO2, 310 mOsm, pH 7.4). Brains were removed, left and right hemispheres dissected separately, then mounted on a 10° incline. Sagittal slices were sectioned (230 µm) using a vibratome (VT1000S Leica Microsystems, Germany) set at slow speed (0.125 mm/s, setting 2.5), and high sectioning frequency of 80–90 Hz (setting 8–9), while submerged in mCSF (4 °C) aerated with 95% O2, 5% CO2 (Beurrier et al., 2006). This method generates slices that preserve the thalamic and cortical inputs onto striatal output neurons, and also contains basal ganglia nuclei of the direct and indirect pathways (Beurrier et al., 2006). The striatum of one slice from each hemisphere was snap frozen and retained for high-pressure liquid chromatography (HPLC) analysis of dopamine content. Slices were transferred to aCSF, comprising more physiological levels of calcium and magnesium (36 °C) (in mM: 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 MgSO4, 2.5 CaCl, 25 glucose (Bishop, Canada), aerated with 95% O2/5% CO2, 310 mOsm, pH 7.4) for 15 min and then equilibrated for a further 15 min at room temperature (29–30 °C). Electrophysiological recordings were performed 30 min to 5 h after slice preparation.
Striatal output neurons were identified visually using infrared-differential interference microscopy (IR-DIC, Zeiss) and eGFP expression was confirmed using epifluorescence (Axioscope 2 with HBO-50 illuminator, Zeiss). Patch clamp recordings were made in aCSF using borosilicate glass pipettes (6–10 MΩ) (Clark, Warner Instruments, USA) filled with internal recording solution (in mM: 120 K-gluconate, 20 KCl, 10 HEPES, 4 NaCl, 4 ATP-Mg, 0.3 GTP-NaCl, 10 phosphocreatine, 280 mOsm,pH 7.2 (Sigma Aldrich, Canada)). The liquid junction potential was −12 mV and not corrected. Recordings were rejected if the membrane potential was <−80 mV at the beginning of the experiment, if the input resistance changed by >30%, or if the amount of dopamine depletion was<95%. Data were acquired, sampled at 20 kHz and filtered at 5 kHz (Axopatch 200B amplifier and 1322A digidata board, Molecular Devices, USA). All analysis was conducted in Clampfit versions 10 (Axon Instruments, USA).
To prevent feedback inhibition from neighbouring striatal output neurons, which influences back-propagation of action potentials (bAP), and also to inhibit any modulation by GABAergic interneurons, picrotoxin (50 µM, Tocris Biosciences, UK) was included in the aCSF. To improve the condition of the slices pyruvate (1 mM, Sigma Aldrich, Canada) was added to the slice holding chamber. The NMDA receptor co-agonist, glycine (10 µM, Bioshop, Canada), was added to the aCSF during electrophysiological recordings. When indicated, the A2a antagonist (SCH 58261, 100 nM), A2a agonist (CGS 21680, 100 nM), D2 antagonist ((S)-(–)-Sulpiride, 10 µM), and D2 agonist (Quinpirole, 10 µM) were added to the aCSF (all Tocris Biosciences, UK).
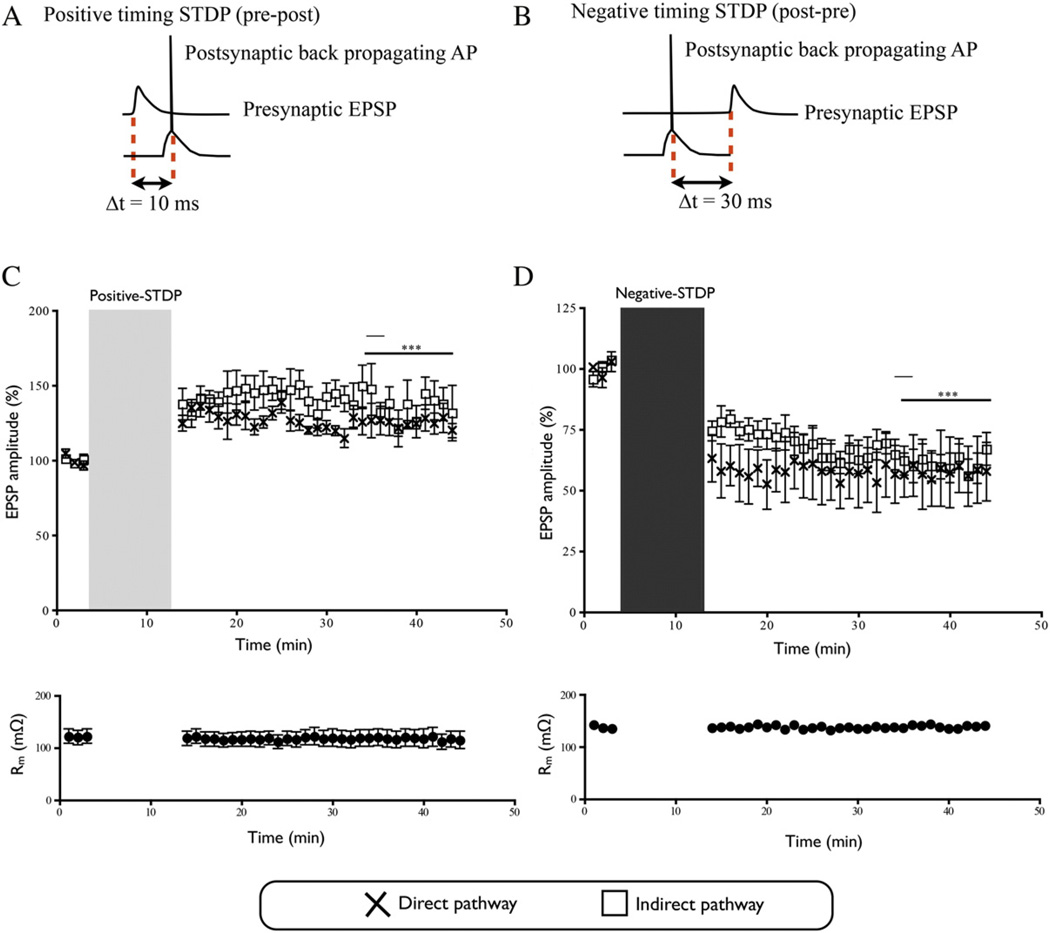
Spike-timing dependent protocols (Pawlak and Kerr, 2008), were used to induce LTP and LTD in cortico-striatal synapses of striatal output neurons from both the direct and indirect pathways. Excitatory postsynaptic potentials (EPSPs) were evoked in striatal output neurons by extracellular stimulation (0.02 ms, 0.1 Hz) with bipolar platinum/iridium electrodes (FHC, USA) placed in cortical layer 5, approximately 0.5 mm away from the recording electrode. One clearly defined EPSP (5.5 ± 0.15 mV) using low intensity (207 ± 44 µA) was generated, with no intermittent EPSP failures. The EPSPs were comparable amongst all groups (4.41–6.63 mV). The series resistance was 19– 32 MΩ, and input resistance was 90–250 MΩ, as estimated from the response to a hyperpolarizing voltage step (−5 mV). For baseline recordings, EPSP amplitudes were recorded over 3 min (baselines were comparable amongst all groups). EPSPs were then paired with a bAP elicited with brief current injections through the recording electrode (968 ± 68 pA, 2 ms) for 60 pairings at a low frequency (0.1 Hz) for 10 min. LTP was most consistently induced using a short delay (10 ms) in which the EPSP preceded the bAP. LTD was reliably induced using a longer delay (30 ms) period, and with the bAP preceding the EPSP. Spike-timing was defined as the time between the EPSP onset and bAP peak. After the pairing period, EPSPs were recorded for a further 30 min. For every recording the extent of plasticity was measured as the percentage change of the average EPSP amplitude 20–30 min after pairing when compared to the EPSP at baseline.
Groups utilised to study the effect of l-DOPA in the parkinsonian and dyskinetic striatum
The objectives of this study were to differentiate forms of synaptic plasticity that underpin the symptomatic benefits of l-DOPA and those that contribute to LID. We were also interested in whether there was a difference in synaptic plasticity of dyskinetic animals that were no longer exposed to l-DOPA (i.e. the rodent equivalent to a parkinsonian patient, who experiences dyskinesia but is OFF-l-DOPA). Thus it was necessary to emulate brain activity following first exposure to l-DOPA in a parkinsonian patient, as an indication of the brain activity observed following a symptomatically beneficial dose of l-DOPA devoid of side effects such as dyskinesia. Also, since we were interested in studying synaptic plasticity in the striatum in a rodent equivalent of peak-dose dyskinesia, we needed to expose slices taken from dyskinetic mice to l-DOPA in the recording chamber. To determine a concentration of l-DOPA that was equivalent to those found in the striatum of 6-OHDA-lesioned animals undergoing l-DOPA treatment, previous studies that measured striatal l-DOPA levels in vivo following systemic injection of l-DOPA in parkinsonian rats and peak-dose-dyskinesia were used (Meissner et al., 2006; Lindgren et al., 2010). These studies showed that in parkinsonian and dyskinetic animals, striatal l-DOPA concentrations were similar (180 nM and 250 nM respectively) following systemic administration of l-DOPA. Therefore, to simulate the synaptic environment of the striatum following de novo treatment with a symptomatically effective dose of l-DOPA in Parkinson’s disease (treated parkinsonian) we included l-DOPA (250 nM) in the recording chamber when recording from 6-OHDA-lesioned animals chronically treated with vehicle, while conditions that lead to dyskinesia were mimicked by inclusion of l-DOPA (250 nM) in the recording chamber for slices from 6-OHDA-lesioned animals chronically treated with l-DOPA (4.5 mg/kg) (Table 1).
Assessment of striatal dopamine levels using HPLC
Following slice preparation a striatal slice was snap frozen immediately and stored at −80 °C until HPLC analysis, then dopamine levels were measured (Vanderbilt Neurochemistry Core, Nashville, TN). Striatal slices were thawed on ice, homogenised in 100–750 µl 0.1 Mtric-hloroacetic acid (TCA) containing 10 mM sodium acetate, 100 µM EDTA, and 5 ng/ml isoproterenol and 10.5% methanol (pH 3.8) and centrifuged (10,000 ×g,20 min (4 °C, Eppendorf 58-04R, USA)). The supernatant was removed and stored at −80 °C, and the pellet was saved for protein analysis. The concentration of dopamine was determined utilising an Antex Decade II (oxidation: 0.4 V), with a salt bridge acting as the reference electrode. The supernatant was then thawed and spun for 20 min (10,000 g), before 20 µl was injected into the Water 2707 autosampler onto a Phenomenex Kintex (2.6 U, 100 A) C18 HPLC column (100 × 4.60 mm). Dopamine was eluted with a mobile phase consisting of 89.5% 0.1 M TCA (pH 3.8). Solvent was delivered at 0.6 ml/min using a Waters 515 HPLC pump. HPLC control and data acquisition are managed by Empower software.
Statistical analysis
All data are expressed as mean ± standard error (SEM). To evaluate LTP and LTD expression, paired t tests were used to assess the level of significance between baseline and the last 10 min post-STDP of the recording. To compare between the experimental groups, the last 10 min post-STDP were evaluated using one-way ANOVA followed by Bonferroni’s multiple comparison post-hoc. Data pre and post stimulation paradigms were compared. Significance was assigned where p < 0.05.
Results
Behavioural abnormalities in parkinsonism and l-DOPA induced dyskinesia
To measure the level of parkinsonism, spontaneous 360° rotations were quantified. Sham-operated animals performed approximately equal numbers of ipsiversive (18.3 ± 0.3, n = 26) and contraversive rotations (17.1 ± 0.3, n = 26), whereas 6-OHDA-lesioned animals performed significantly fewer contraversive rotations (1.2 ± 0.1, n = 52) (p < 0.001) (Fig. 1A). Previous studies have shown that the success of 6-OHDA-lesion surgery (>95% dopamine depletion) is reliably predicted by net ipsiversive rotational bias of >70% (see Methods for more details, also, Thiele et al., 2011, 2012). Thus, this was chosen as our behavioural selection criteria for subsequent electrophysiological studies. The extent of striatal dopamine depletion was then quantified in a subset of animals using HPLC post-hoc. In 6-OHDA-lesioned mice, striatal dopamine was reduced by >95% (0.02 ± 0.01 ng/mg, n = 10) compared to sham-operated animals (49.5 ± 5.4 ng/mg, n = 10, p < 0.001) (Fig. 1B).
Fig. 1.
Assessment of parkinsonism and l-DOPA-induced dyskinesia. A) Spontaneous rotational behaviour in parkinsonian mice. Data are shown as the mean number of 360° rotations/40 min performed by shamoperated and 6-OHDA-lesioned animals, immediately post-vehicle injection. B) HPLC analysis revealed nearly a complete depletion of striatal dopamine in 6-OHDA-lesioned animals. Data are shown as mean ± SEM. C)Effect of l-DOPA on abnormal involuntary movements (AIMs) in 6-OHDA-lesioned mice. AIMs were scored following administration of l-DOPA (4.5 mg/kg) over a 21 day treatment period. Forelimb, axial, and orolingual abnormal involuntary movements were scored every 20 min for 2 h, and data are representative of the global AIMs score with a maximal score out of 84. D) Rotational behaviour following administration of l-DOPA (4.5 mg/kg). Mean data are presented as the net number of 360° contraversive rotations made over a 40 min time period ± the SEM (*** indicates p < 0.001).
Previous studies have shown that in 6-OHDA-lesioned rodents, repeated l-DOPA administration results in extensive contraversive rotational behaviour (Papa et al., 1995; Cenci et al., 1998; Duty et al., 1998; Henry et al., 1998a). Here we show that initially (day 1), administration of l-DOPA (4.5 mg/kg) induced mild net contraversive rotational behaviour (23.6 ± 0.3 in 40 min, n = 78). The number of contraversive rotations increased gradually over the 21 day l-DOPA administration period, until it appeared to plateau at day 17 (559.2 ± 1.4 in 40 min, n = 78) (Fig. 1C). In rodents, l-DOPA-induced AIMs are considered the equivalent of l-DOPA-induced dyskinesia in patients; whilst no resemblance exists between the motor abnormalities observed in patients compared to rodents, the abnormalities in basal circuitry activity and neurotransmission are identical (Lundblad et al., 2002; Thiele et al., 2011, 2012). We found that twice daily administration did not lead to the expression of AIMs in the majority of the animals on the first day of treatment, with a small subset of animals exhibiting minimal levels of axial dyskinesia (0.1 ± 0.1, n = 78). However, severe AIMs were observed across axial, forelimb, and orolingual subscales on the fifth day of l-DOPA treatment (17.0 ± 0.6, n = 78), and were stably expressed until day 21, when the behavioural studies were completed (20.6 ± 0.4, n = 78) (Fig. 1D).
Bidirectional synaptic plasticity is lost in parkinsonism
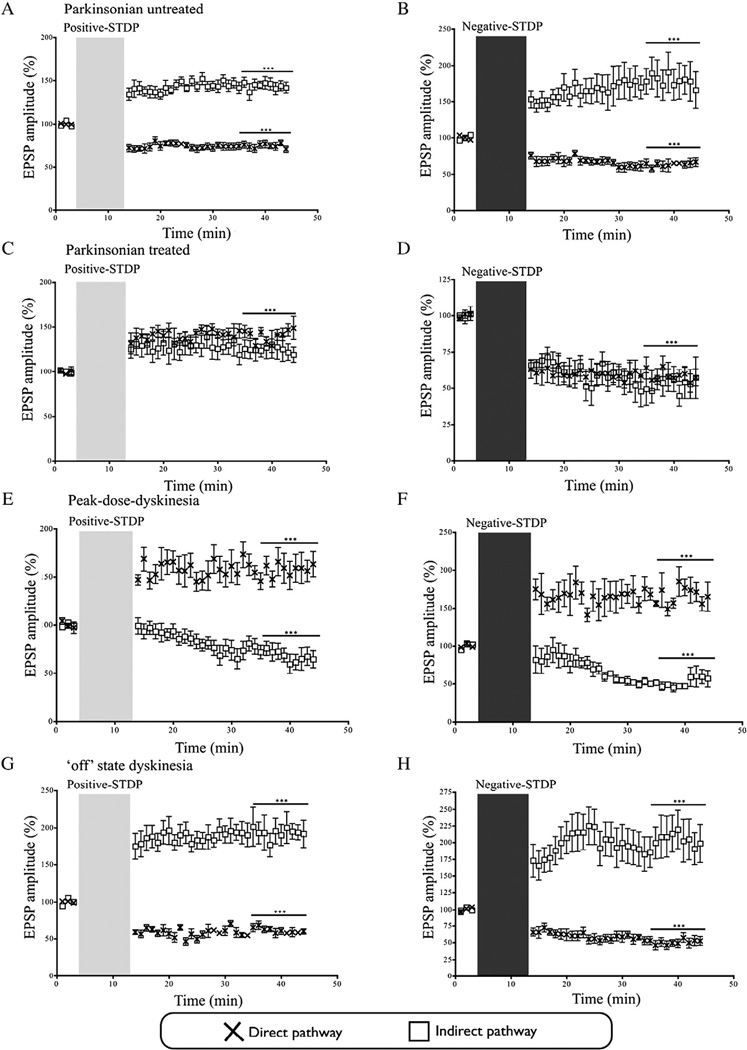
As shown previously (Pawlak and Kerr, 2008),in sham-operated animals, the positive- and negative-timing STDP protocols induced LTP and LTD expression respectively in cortico-striatal synapses in the direct and indirect pathways (Indirect-LTP: 143.1% ± 10.7, n = 8; Indirect-LTD: 61.4% ± 5.3, n = 9; Direct-LTP: 125.2% ± 6.2, n = 5; Direct-LTD: 57.7% ± 5.3, n = 4) (Fig. 2). These levels of synaptic potentiation and depression are comparable to those observed in previous studies (Pawlak and Kerr, 2008; Shen et al., 2008; Fino and Venance, 2010). In untreated parkinsonian mice, following application of the positive-timing protocol, LTP expression was observed at cortico-striatal synapses of the indirect pathway (143.5% ± 14.6, n = 8, p < 0.01) (Fig. 3A). Conversely, in cortico-striatal synapses of the direct pathway of parkinsonian animals, the same positive-timing protocol, resulted in significant depression (74.3% ± 4.0, n = 10, p < 0.001) (Fig. 3A). Application of the negative-timing protocol to the indirect pathway of untreated parkinsonian animals, resulted in expression of LTP rather than LTD (177.7%± 22.3,n= 7,p< 0.001) (Fig.3B). However, LTD was observed when the negative-protocol was applied at cortico-striatal synapses from the direct pathway of untreated parkinsonian animals (63.3% ± 8.7, n = 5, p < 0.001) (Fig. 3B).
Fig. 2.
Synaptic plasticity in naïve animals. A & B) Schematic diagram to show positive and negative timing protocols. C) The positive-timing protocol resulted in an increase of the EPSP amplitude compared to baseline on the indirect pathway. On the direct pathway the positive-timing protocol resulted in an increase in the EPSP amplitude compared to baseline. D) Spike-timing-dependent LTD was induced on the indirect pathway by the negative-timing protocol with a significant decrease in the EPSP amplitude compared to baseline. The use of a negative-timing protocol at direct pathway cortico-striatal synapses induced a decrease in the EPSP amplitude compared to baseline (*** indicates p < 0.001). The light grey vertical bar indicates the start of positive-timing STDP, while the dark grey bar indicates the start of the negative-timing STDP protocol.
Fig. 3.
Synaptic plasticity in parkinsonian and dyskinetic mice.A,C,E,G) The positive-timing protocol was applied in the experimental conditions in the following panels: A) This led to an increase in the EPSP amplitude at indirect cortico-striatal synapses in untreated parkinsonism and a decrease in the EPSP amplitude at direct cortico-striatal synapse. C) An increase in the EPSP amplitude occurred at indirect cortico-striatal synapses in treated parkinsonian animals. An increase in the EPSP amplitude was seen at direct corticostriatal synapses for treated parkinsonian animals. E) In slices representing dyskinesia the EPSP amplitude decreased at indirect cortico-striatal synapses, while the direct-pathway EPSP amplitude increased. G) In slices representing ‘off’ state dyskinesia, a significant increase in the EPSP amplitude was seen at cortico-striatal synapses of the indirect pathway, and at direct cortico-striatal synapses a decrease in the EPSP amplitude occurred. B,D,F,H) The negative timing protocol was applied in the experimental conditions in the following panels: B) This failed to decrease the EPSP amplitude at indirect synapses derived from animals in the untreated parkinsonian state, while a reduction in EPSP amplitude occurred at direct-pathway synapses. D) A decrease in the EPSP amplitude at indirect cortico-striatal synapses occurred in treated parkinsonian animals, as well as at direct pathway synapses. F) A decrease in the EPSP amplitudes at indirect cortico-striatal synapses occurred in dyskinetic animals, and the direct cortico-striatal synapses displayed increased in the EPSP amplitude. H) Slices generated from animals in the ‘off’ state dyskinesia group displayed LTP rather than LTD at indirect synapses, and conversely exhibited depression on the direct pathway. All data is represented as the mean ± the SEM (*** indicates p < 0.001). The light grey vertical bar indicates the start of positive-timing STDP, while the dark grey bar indicates the start of the negative-timing STDP protocol.
l-DOPA de novo restores bidirectional synaptic plasticity in parkinsonian mice
To understand how l-DOPA therapy initially alleviates motor symptoms without producing dyskinesia, we sought to model the striatal conditions that occur when PD patients first receive l-DOPA. To do so, slices prepared from vehicle treated 6-OHDA-lesioned mice were exposed to l-DOPA (250 nM) for the first time in the aCSF of the recording chamber for 15 min prior to stimulation to allow for dopamine synthesis and vesicular packaging (Meissner et al., 2006; Lindgren et al., 2010). The positive-timing protocol induced LTP expression in the indirect pathway (124.4% ± 22.0, n = 5, p < 0.001) and in the direct pathway (140.7% ± 7.3, n = 6, p < 0.001) (Fig. 3C). In cortico-striatal synapses of the indirect pathway, the negative-timing protocol restored LTD expression (52.1% ± 18.5, n = 5, p < 0.001) (Fig. 3D). As expected, application of the negative-timing protocol to the direct pathway in the same group of animals resulted in LTD expression (58.4% ± 6.0, n = 5, p < 0.001) (Fig. 3D).
In dyskinesia, the direction of synaptic response is reversed relative to the parkinsonian state
To simulate dyskinesia, slices prepared from 6-OHDA-lesioned mice, long-term treated with l-DOPA (dyskinetic) were exposed to l-DOPA (250 nM) in the recording chamber. In the initial stages of PD,dopamine derived from l-DOPA therapy, is stored in the remaining presynaptic terminals serving to buffer against major fluctuations in striatal dopamine (Jenner, 2008). However, as an increasing number of dopaminergic terminals degenerate, the conversion of l-DOPA to dopamine occurs in other types of neuronal terminal (Carlsson et al., 2007; Eskow Jaunarajs et al., 2012; Li et al., 2013; Gil et al., 2010; Navailles et al., 2013; Rylander et al., 2010) that do not possess the mechanisms to regulate the release or uptake of dopamine, leading to non-physiological surges in striatal dopamine (Eskow Jaunarajs et al., 2012; Li et al., 2013), even with a near 100% depletion of striatal terminals (Meissner et al., 2006). Application of the positive-timing protocol to cortico-striatal synapses of the indirect pathway resulted in LTD expression (68.9% ± 21.3, n = 7, p < 0.001) (Fig. 3E). LTD expression was also observed on the indirect pathway when the negative-timing protocol was applied (52.0% ± 14.2, n = 5, p < 0.001) (Fig. 3F).
The opposite findings were observed at cortico-striatal synapses of the direct pathway in a situation, which simulates peak-dose dyskinesia in patients. As expected, application of the positive timing protocol led to LTP expression (156.6% ± 13.2, n = 8, p < 0.001) (Fig. 3E). In cortico-striatal synapses of the direct pathway, the negative-timing protocol also induced LTP expression (166.7% ± 15.8, n = 5, p < 0.001) (Fig. 3F).
Synapses of dyskinetic animals in the ‘off-state’ behave like synapses in untreated parkinsonism
We next examined how cortico-striatal synapses of 6-OHDA-lesioned mice behave following chronic l-DOPA-therapy, when l-DOPA has been absent for a prolonged period of time, equivalent to ‘OFF-l-DOPA‘ state dyskinesia in patients. Following stimulation of synapses on the indirect pathway with the positive-timing protocol, LTP was strongly expressed, similar to that observed in the parkinsonian state (194.2% ± 37.6, n = 8, p < 0.001) (Fig. 3G). Synapses of the direct pathway in dyskinesia ‘OFF‘ group also behaved similarly to the parkinsonian state following induction of the positive-timing protocol, and LTD was observed (60.6% ± 4.8, n = 9, p < 0.001) (Fig. 3G).
In the dyskinesia ‘OFF‘ group, the negative-timing protocol enhanced synaptic strength at indirect cortico-striatal synapses (209.0% ± 26.0, n = 13, p < 0.001) (Fig. 3H). Application of the negative-timing protocol to the direct pathway caused significant and robust LTD in this group of animals (50.0% ± 6.8, n = 7, p < 0.001) (Fig. 3H).
Abnormal A2a and D2 receptor transmission underlies dyskinesia
To further understand the mechanisms underlying unidirectional LTD at indirect cortico-striatal synapses in dyskinesia, we also employed pharmacological tools to manipulate to adenosine A2a and dopamine D2 receptors. The positive-timing protocol was performed in the presence of the D2 receptor antagonist (Sulpiride, 10 µM). Sulpiride reversed LTD observed in the dyskinesia group, and LTP was observed (187.5% ± 17.4, n = 5, p > 0.05) (Fig. 4A). Dopamine D2 and adenosine A2a receptors exhibit reciprocal negative regulation on the indirect pathway (Svenningsson et al., 1999; Torvinen et al., 2005a; Morelli et al., 2007). Thus, we also examined whether an adenosine A2a receptor agonist (CGS 21680, 100 nM) could restore LTP on the indirect pathway in the dyskinesia group. Following application of the positive-timing protocol, CGS21680 restored LTP expression (154.2% ± 19.1, n = 4, p > 0.001) (Fig. 4A).
Fig. 4.
On the indirect pathway A2a and D2 receptors cause dyskinesia. A) Inslices representing dyskinesia, inclusion of a D2 antagonist in the aCSF (Sulpiride, 10µM) and application of the positive-timing protocol resulted in an increase in the EPSP amplitude, that was significantly higher than those observed in sham-operated animals. The application of an A2a agonist (CGS 21680, 100 nM) to slices from dyskinetic animals resulted in an increase in the EPSP amplitude following a positive-timing protocol. B) In the dyskinetic OFF state, the application of a positive-timing protocol resulted in no change in the EPSP amplitude in the presence of the A2a antagonist (SCH58261) (10 µM). In the presence of the D2 agonist (Quinpirole, 10 µM) spike-timing-dependent LTP induced by the positive timing protocol resulted in a significant decrease in the EPSP amplitude in slices from dyskinetic animals in the OFF state. All data is represented as the mean ± the SEM (*** indicates p < 0.001). The light grey vertical bar indicates the start of positive-timing STDP, while the dark grey bar indicates the start of the negative-timing STDP protocol.
We also attempted to pinpoint the receptors responsible for elevated levels of LTP at cortico-striatal synapses of the indirect pathway in the ‘OFF-DOPA‘ dyskinetic group. The positive-timing protocol was applied in the presence of the A2a receptor antagonist SCH58261 (100 nM) in slices generated from animals in this group. SCH58261 resulted in a loss of the potentiation previously observed, bringing postsynaptic activity back to baseline levels (97.4% ± 12.9, n = 4, p < 0.001) (Fig. 4B). We also examined whether application of a dopamine D2 receptor agonist (Quinpirole, 10 µM,) during the positive-timing protocol, could provide the necessary balance to normalize LTP levels in the ‘ OFF l-DOPA‘ dyskinetic state. However, application of Quinpirole resulted in a significant depression (68.2% ± 10.9, n = 4, p > 0.05) (p < 0.001) (Fig. 4B).
Discussion
The idea that over-activity of the indirect pathway and under-activity of the direct pathway might underlie the generation of parkinsonism was proposed more than 30 years ago and has become a keystone of thinking on the mechanisms of control of movement by the basal ganglia (Pan et al., 1985; Albin et al., 1989, 1995; Crossman, 1989, 1990; DeLong, 1990). The idea that opposite changes occur in dyskinesia was not far behind (Mitchell et al., 1985a,b; Henry et al., 1998a, 1999). The concept of differential activity of direct and indirect pathways in parkinsonism and dyskinesia, has been backed up by a wealth of studies, that have demonstrated changes in metabolism and gene expression on each pathway (Brotchie et al., 1991, 2005; Maneuf et al., 1994, 1997; Wichmann et al., 1994; Henry et al., 1998b, 1999; Nash et al., 2000; Aubert et al., 2005; Guigoni et al., 2005; Berthet et al., 2009) and has reached the status of dogma. However, despite the almost universal acceptance of the phenomena, none of these studies identified the synaptic mechanisms by which these circuitry abnormalities were generated. Physiological changes in dopamine govern important aspects of action selection (Reynolds et al., 2001; Dang et al., 2006; Yin et al., 2009; Cui et al., 2013), and the present study highlights that chronic disruption of dopamine transmission leads to widespread adaptations in parameters that modify neuronal excitability in the striatum. Our results cast light on the synaptic basis of abnormalities in the direct and indirect striatal output pathways in parkinsonism and dyskinesia. These studies suggest that in PD and dyskinesia, non-physiological dopamine-mediated transmission causes loss of bidirectional plasticity and only unidirectional changes in synaptic strength are generated. Thus in parkinsonism the indirect pathway exhibits only LTP, providing a basis for its over-activity, while the direct pathway, exhibiting only LTD will become underactive. By contrast, in the dyskinetic state, the direct pathway exhibits only LTP, becoming generally overactive, and the indirect pathway exhibits only LTD.
Methodological considerations
The primary objectives of this study were to define changes in synaptic plasticity that correlate with the parkinsonian state, the symptomatic benefits of l-DOPA, and the production of LID. Thus it was necessary to emulate, in vitro, the situations observed following both a symptomatically beneficial dose of l-DOPA devoid of dyskinesia and in peak-dose dyskinesia. To this end striatal slices were taken from previously-untreated parkinsonian mice and parkinsonian mice that had received repeated l-DOPA therapy and developed AIMS, a rodent correlate of dyskinesia, then these slices were exposed to l-DOPA in the recording chamber. To determine a clinically relevant concentration of striatal l-DOPA, we looked to previous studies that measured striatal l-DOPA levels in vivo following systemic injection of l-DOPA in parkinsonian rats and during dyskinesia were used (Meissner et al., 2006; Lindgren et al., 2010). These studies showed that striatal l-DOPA concentrations were similar in parkinsonian and dyskinetic animals (180 nM and 250 nM respectively). Therefore, we included 250 nM of l-DOPA in the recording chamber.
To ensure our studies were physiologically relevant, spike-timing dependant plasticity paradigms were used (Pawlak and Kerr, 2008) that most likely mimic in vivo stimulation patterns observed during striatal based learning (DeCoteau et al., 2007a,b; Tort et al., 2008; Hawes et al., 2013). Methods employed to generate slices preserved thalamic and cortical inputs onto striatal output neurons, and also retained output basal ganglia nuclei, thus retaining striatal inputs and outputs as well as feedback loops (Beurrier et al., 2006).
Effects of 6-OHDA lesion compared to control animals
As shown previously, application of positive-timing and negative-timing protocols induced LTP and LTD expression respectively in both output pathways in the dopamine-intact striatum (Pawlak and Kerr, 2008; Shen et al., 2008). Also, as previously demonstrated, striatal dopamine depletion caused a loss of bidirectional plasticity, such that each pathway persistently exhibited only one form of synaptic plasticity (Shen et al., 2008). Thus, in parkinsonism, irrespective of the type of stimulation protocol, cortico-striatal synapses of the direct and indirect pathways exhibit only LTD or LTP respectively. This loss of synaptic flexibility likely contributes to under-activity of the direct pathway and over-activity of the indirect pathway respectively that is thought to underlie symptoms of PD.
The beneficial effects of l-DOPA in a non-dyskinetic situation
Exposure of a symptomatically beneficial concentration of l-DOPA to the striatum from previously-untreated parkinsonian mice normalised striatal output plasticity. Thus, both striatal output pathways expressed LTD and LTP when stimulated with negative- and positive-timing STDP protocols respectively. These results suggest that bidirectional synaptic plasticity is required for normal motor control.
Synaptic plasticity in dyskinesia
Current studies suggest that a major component of the mechanism driving LID is over-activity of the direct pathway (Brotchie, 2000; Aubert et al., 2005; Westin et al., 2007; Berthet et al., 2009; Guigoni and Bezard, 2009). From a synaptic plasticity perspective this is supported to some extent by the fact that a D1 receptor antagonist reverses the inability of neurons to depotentiate in slices prepared from 6-OHDA-lesioned rats with AIMs, D1 receptors being selectively localised on the direct pathway (Picconi et al., 2003, 2008). However, as the identity of neurons, with respect to the direct and indirect pathway segregation was not defined, and the studies were conducted with the absence of l-DOPA in the recording chamber, this conclusion cannot be substantiated and indeed the relevance to the expression of dyskinetic symptom is unclear. In a recent study by Cerovic et al., it was shown that Ras-extracellular signal-regulated kinase (Ras-ERK) is crucial for the induction of LTP and depotentiation in the direct striatal pathway, suggesting that other factors may control ERK signalling in the indirect pathway (Cerovic et al., in press). It was also shown in synapses from dyskinetic mice that Ras-ERK signalling impedes rather than facilitates depotentiation of LTP, confirming a derangement of ERK-mediated mechanisms in this neurophysiological correlate of LID. Furthermore, it seems quite feasible that both striatal output pathways could contribute to LID (Brotchie et al., 2005).We show, that in a mouse model equivalent of dyskinesia, both pathways show abnormalities in synaptic plasticity, and no matter what the stimulation paradigm, only LTP and LTD can be expressed on the direct and indirect pathways respectively. Thus peak-dose dyskinesia represents the opposite of the circuitry abnormalities observed in the parkinsonian state. In comparing our findings in the dyskinesia state to the ‘OFF‘ l-DOPA state, in dyskinesia-primed animals, slices from mouse models of LID were generated, but assessed with no l-DOPA in the recording chamber. These mice exhibited unilateral synaptic plasticity that was comparable to that observed in the parkinsonian, untreated state, though with the positive-timing protocol, the level of LTP was greater compared to both naive and untreated, parkinsonian animals (approximately 35% higher than both). This enhancement is comparable to a study, in the absence of l-DOPA, carried out by Picconi and colleagues between levels of potentiation observed at the coinciding time-point on day 21 (Picconi et al., 2008).
Findings in PD patients and animal models have linked A2A receptors with LID (Zeng et al., 2000; Calon et al., 2004; Castrioto et al., 2011). On the indirect pathway, A2a and D2 receptors interact physically (Kull et al., 1999; Svenningsson et al., 1999; Canals et al., 2004; Morelli et al., 2007), and activation of one of these receptors causes inhibition of the other. Thus, we hypothesised that A2A and D2 receptors may be responsible for the abnormal expression of synaptic plasticity in our models of LID (Schiffmann et al., 2003; Torvinen et al., 2005b; Flajolet et al., 2008; Shen et al., 2008; Higley and Sabatini, 2010). Since in peak-dose dyskinesia, cortico-striatal synapses of the indirect pathway only express LTD, and previous studies have shown that induction of LTD on this pathway requires activation of dopamine D2 receptors, and limited or absent A2A receptor stimulation (Shen et al., 2008; Lerner et al., 2010) we sought to reinstate LTP expression through blockade of D2 receptors, which indeed was the case. LTP could also be rescued by application of an A2A receptor agonist. Given the importance of A2A receptors in LTP expression on the indirect pathway (Schiffmann et al., 1991; Flajolet et al., 2008; Shen et al., 2008) we next examined the impact of an A2a receptor antagonist in animals in the ‘OFF‘ dyskinesia state. Blockade of A2A receptors inhibited LTP, and the postsynaptic response returned to baseline levels, suggesting that the enhanced levels of potentiation observed in this group are linked to altered A2A receptor transmission. Since loss of D2 receptor activation leads to enhanced A2A receptor signalling (Shen et al., 2008),we tested whether addition of a D2 receptor agonist could attenuate LTP expression. Under these conditions, presence of the D2 agonist led to the positive-timing protocol being over-ridden, as LTD expression was observed, and was comparable to that observed in the dyskinesia group. Our current studies provide more functional evidence that disruption of adenosine A2a and dopamine D2 receptor interactions may lead to imbalanced activity on the indirect pathway.
These studies suggest that in parkinsonism and dyskinesia, non-physiological dopamine receptor-mediated transmission causes loss of bidirectional plasticity and only unidirectional changes in synaptic strength are generated. Inconclusion, by unravelling the synaptic mechanisms underpinning parkinsonism, and also the beneficial and pathological effects of l-DOPA we have revealed how striatal synapses must function to enable normal motor function. In the future this assay may help to expose novel pharmaceutical agents that are able to block LID without hindering the symptomatic benefits of l-DOPA.
Acknowledgments
We would like to thank the Natural Sciences and Engineering Research Council (NSERC) and the Parkinson’s Society Canada for funding this research. Also, Vanderbilt Neurochemistry Core Facility (Nashville, TN) is acknowledged for completing the HPLC.
References
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Ahmed MR, Berthet A, Bychkov E, Porras G, Li Q, Bioulac BH, Carl YT, Bloch B, Kook S, Aubert I, Dovero S, Doudnikoff E, Gurevich VV, Gurevich EV, Bezard E. Lentiviral overexpression of GRK6 alleviates l-dopa-induced dyskinesia in experimental Parkinson’s disease. Sci. Transl. Med. 2010;2:28ra28. doi: 10.1126/scitranslmed.3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–64. [PubMed] [Google Scholar]
- Anden NE, Carlsson A, Kerstell J, Magnusson T, Olsson R, Roos BE, Steen B, Steg G, Svanborg A, Thieme G, Werdinius B. Oral l-dopa treatment of parkinsonism. Acta Med. Scand. 1970;187:247–255. doi: 10.1111/j.0954-6820.1970.tb02939.x. [DOI] [PubMed] [Google Scholar]
- Aubert I, Guigoni C, Hakansson K, Li Q, Dovero S, Barthe N, Bioulac BH, Gross CE, Fisone G, Bloch B, Bezard E. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann. Neurol. 2005;57:17–26. doi: 10.1002/ana.20296. [DOI] [PubMed] [Google Scholar]
- Cerovic M, Bagetta V, Pendolino V, Ghiglieri V, Fasano S, Morella I, Hardingham N, Heuer A, Papale A, Marchisella F, Giampà C, Calabresi P5, Picconi B, Brambilla R. Derangement of Ras-Guanine Nucleotide-Releasing Factor 1 (Ras-GRF1) and Extracellular Signal-Regulated Kinase (ERK) dependent striatal plasticity in l-DOPA-induced dyskinesia. Biol. Psychiatry (in press) 2014 doi: 10.1016/j.biopsych.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Berthet A, Porras G, Doudnikoff E, Stark H, Cador M, Bezard E, Bloch B. Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of l-DOPA-induced dyskinesia. J. Neurosci. 2009;29:4829–4835. doi: 10.1523/JNEUROSCI.5884-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Ben-Ari Y, Hammond C. Preservation of the direct and indirect pathways in an in vitro preparation of the mouse basal ganglia. Neuroscience. 2006;140:77–86. doi: 10.1016/j.neuroscience.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat. Rev. Neurosci. 2001;2:577–588. doi: 10.1038/35086062. [DOI] [PubMed] [Google Scholar]
- Brotchie JM. The neural mechanisms underlying levodopa-induced dyskinesia in Parkinson’s disease. Ann. Neurol. 2000;47:S105–S112. (discussion S112-104) [PubMed] [Google Scholar]
- Brotchie JM. Nondopaminergic mechanisms in levodopa-induced dyskinesia. Mov. Disord. 2005;20:919–931. doi: 10.1002/mds.20612. [DOI] [PubMed] [Google Scholar]
- Brotchie JM, Lee J, Venderova K. Levodopa-induced dyskinesia in Parkinson’s disease. J. Neural Transm. 2005;112:359–391. doi: 10.1007/s00702-004-0251-7. [DOI] [PubMed] [Google Scholar]
- Brotchie JM, Mitchell IJ, Sambrook MA, Crossman AR. Alleviation of parkinsonism by antagonism of excitatory amino acid transmission in the medial segment of the globus pallidus in rat and primate. Mov. Disord. 1991;6:133–138. doi: 10.1002/mds.870060208. [DOI] [PubMed] [Google Scholar]
- Calon F, Dridi M, Hornykiewicz O, Bedard PJ, Rajput AH, Di Paolo T. Increased adenosine A2A receptors in the brain of Parkinson’s disease patients with dyskinesias. Brain. 2004;127:1075–1084. doi: 10.1093/brain/awh128. [DOI] [PubMed] [Google Scholar]
- Canals M, Burgueno J, Marcellino D, Cabello N, Canela EI, Mallol J, Agnati L, Ferre S, Bouvier M, Fuxe K, Ciruela F, Lluis C, Franco R. Homodimerization of adenosine A2A receptors: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Neurochem. 2004;88:726–734. doi: 10.1046/j.1471-4159.2003.02200.x. [DOI] [PubMed] [Google Scholar]
- Carlsson T, Carta M, Winkler C, Bjorklund A, Kirik D. Serotonin neuron transplants exacerbate l-DOPA-induced dyskinesias in a rat model of Parkinson’s disease. J. Neurosci. 2007;27:8011–8022. doi: 10.1523/JNEUROSCI.2079-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch. Neurol. 2011;68:1550–1556. doi: 10.1001/archneurol.2011.182. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Ratings of l-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson’s disease in rats and mice. Curr. Protoc. Neurosci. 2007 doi: 10.1002/0471142301.ns0925s41. (Supplement 41, Chapter 9: Unit 9 25) [DOI] [PubMed] [Google Scholar]
- Cenci MA, Konradi C. Maladaptive striatal plasticity in l-DOPA-induced dyskinesia. Prog. Brain Res. 2010;183:209–233. doi: 10.1016/S0079-6123(10)83011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur. J. Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- Crossman AR. Neural mechanisms in disorders of movement. Comp. Biochem. Physiol. A Comp. Physiol. 1989;93:141–149. doi: 10.1016/0300-9629(89)90201-6. [DOI] [PubMed] [Google Scholar]
- Crossman AR. A hypothesis on the pathophysiological mechanisms that underlie levodopa- or dopamine agonist-induced dyskinesia in Parkinson’s disease: implications for future strategies in treatment. Mov. Disord. 1990;5:100–108. doi: 10.1002/mds.870050203. [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang MT, Yokoi F, Yin HH, Lovinger DM, Wang Y, Li Y. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoteau WE, Thorn C, Gibson DJ, Courtemanche R, Mitra P, Kubota Y, Graybiel AM. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc. Natl. Acad. Sci. U. S. A. 2007a;104:5644–5649. doi: 10.1073/pnas.0700818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoteau WE, Thorn C, Gibson DJ, Courtemanche R, Mitra P, Kubota Y, Graybiel AM. Oscillations of local field potentials in the rat dorsal striatum during spontaneous and instructed behaviors. J. Neurophysiol. 2007b;97:3800–3805. doi: 10.1152/jn.00108.2007. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of l-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav. Brain Res. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Duty S, Henry B, Crossman AR, Brotchie JM. Topographical organization of opioid peptide precursor gene expression following repeated apomorphine treatment in the 6-hydroxydopamine-lesioned rat. Exp. Neurol. 1998;150:223–234. doi: 10.1006/exnr.1997.6771. [DOI] [PubMed] [Google Scholar]
- Eskow Jaunarajs KL, George JA, Bishop C. L-DOPA-induced dysregulation of extrastriatal dopamine and serotonin and affective symptoms in a bilateral rat model of Parkinson’s disease. Neuroscience. 2012;218:243–256. doi: 10.1016/j.neuroscience.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Venance L. Spike-timing dependent plasticity in the striatum. Front Synaptic Neurosci. 2010;2:6. doi: 10.3389/fnsyn.2010.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor J, Wallach I, Nairn AC, Surmeier DJ, Greengard P. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat. Neurosci. 2008;11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJPG. The Mouse Brain in Stereotaxic Coordinates. Academic Press; 2007. [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gil S, Park C, Lee J, Koh H. The roles of striatal serotonin and l-amino-acid decar-boxylase on l-DOPA-induced dyskinesia in a hemiparkinsonian rat model. Cell. Mol. Neurobiol. 2010;30:817–825. doi: 10.1007/s10571-010-9509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Bezard E. Involvement of canonical and non-canonical D1 dopamine receptor signalling pathways in l-dopa-induced dyskinesia. Parkinsonism Relat. Disord. 2009;15(Suppl. 3):S64–S67. doi: 10.1016/S1353-8020(09)70783-7. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Aubert I, Li Q, Gurevich VV, Benovic JL, Ferry S, Mach U, Stark H, Leriche L, Hakansson K, Bioulac BH, Gross CE, Sokoloff P, Fisone G, Gurevich EV, Bloch B, Bezard E. Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism Relat. Disord. 2005;11(Suppl. 1):S25–S29. doi: 10.1016/j.parkreldis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Hawes SL, Gillani F, Evans RC, Benkert EA, Blackwell KT. Sensitivity to theta-burst timing permits LTP in dorsal striatal adult brain slice. J. Neurophysiol. 2013;110:2027–2036. doi: 10.1152/jn.00115.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B, Crossman AR, Brotchie JM. Characterization of enhanced behavioral responses to l-DOPA following repeated administration in the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Exp. Neurol. 1998a;151:334–342. doi: 10.1006/exnr.1998.6819. [DOI] [PubMed] [Google Scholar]
- Henry B, Crossman AR, Brotchie JM. Characterization of a rodent model in which to investigate the molecular and cellular mechanisms underlying the pathophysiology of l-dopa-induced dyskinesia. Adv. Neurol. 1998b;78:53–61. [PubMed] [Google Scholar]
- Henry B, Crossman AR, Brotchie JM. Effect of repeated l-DOPA, bromocriptine, or lisuride administration on preproenkephalin-A and preproenkephalin-B mRNA levels in the striatum of the 6-hydroxydopamine-lesioned rat. Exp. Neurol. 1999;155:204–220. doi: 10.1006/exnr.1998.6996. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat. Neurosci. 2010;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) in the central nervous system and its relation to the Parkinson syndrome in man. Dtsch. Med. Wochenschr. 1962;7:1807–1810. doi: 10.1055/s-0028-1114024. [DOI] [PubMed] [Google Scholar]
- Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. The pharmacology of l-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol. Rev. 2013;65:171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- Jenner P. Preventing and controlling dyskinesia in Parkinson’s disease–a view of current knowledge and future opportunities. Mov. Disord. 2008;23(Suppl. 3):S585–S598. doi: 10.1002/mds.22022. [DOI] [PubMed] [Google Scholar]
- Konradi C, Westin JE, Carta M, Eaton ME, Kuter K, Dekundy A, Lundblad M, Cenci MA. Transcriptome analysis in a rat model of l-DOPA-induced dyskinesia. Neurobiol. Dis. 2004;17:219–236. doi: 10.1016/j.nbd.2004.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J. Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull B, Ferre S, Arslan G, Svenningsson P, Fuxe K, Owman C, Fredholm BB. Reciprocal interactions between adenosine A2A and dopamine D2 receptors in Chinese hamster ovary cells co-transfected with the two receptors. Biochem. Pharmacol. 1999;58:1035–1045. doi: 10.1016/s0006-2952(99)00184-7. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Horne EA, Stella N, Kreitzer AC. Endocannabinoid signaling mediates psychomotor activation by adenosine A2A antagonists. J. Neurosci. 2010;30:2160–2164. doi: 10.1523/JNEUROSCI.5844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Qiu G, Ding S, Zhou FM. Serotonin hyperinnervation and upregulated 5-HT2A receptor expression and motor-stimulating function in nigrostriatal dopamine-deficient Pitx3 mutant mice. Brain Res. 2013;1491:236–250. doi: 10.1016/j.brainres.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA. L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: temporal and quantitative relationship to the expression of dyskinesia. J. Neurochem. 2010;112:1465–1476. doi: 10.1111/j.1471-4159.2009.06556.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of l-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol. Dis. 2004;16:110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur. J. Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Usiello A, Carta M, Hakansson K, Fisone G, Cenci MA. Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp. Neurol. 2005;194:66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Maneuf YP, Crossman AR, Brotchie JM. The cannabinoid receptor agonist WIN 55,212-2 reduces D2, but not D1, dopamine receptor-mediated alleviation of akinesia in the reserpine-treated rat model of Parkinson’s disease. Exp. Neurol. 1997;148:265–270. doi: 10.1006/exnr.1997.6645. [DOI] [PubMed] [Google Scholar]
- Maneuf YP, Mitchell IJ, Crossman AR, Brotchie JM. On the role of enkephalin cotransmission in the GABAergic striatal efferents to the globus pallidus. Exp. Neurol. 1994;125:65–71. doi: 10.1006/exnr.1994.1007. [DOI] [PubMed] [Google Scholar]
- Meissner W, Ravenscroft P, Reese R, Harnack D, Morgenstern R, Kupsch A, Klitgaard H, Bioulac B, Gross CE, Bezard E, Boraud T. Increased slow oscillatory activity in substantia nigra pars reticulata triggers abnormal involuntary movements in the 6-OHDA-lesioned rat in the presence of excessive extracellular striatal dopamine. Neurobiol. Dis. 2006;22:586–598. doi: 10.1016/j.nbd.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Sambrook MA, Crossman AR. Subcortical changes in the regional uptake of [3H]-2-deoxyglucose in the brain of the monkey during experimental choreiform dyskinesia elicited by injection of a gamma-aminobutyric acid antagonist into the subthalamic nucleus. Brain. 1985a;108(Pt 2):405–422. doi: 10.1093/brain/108.2.405. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Jackson A, Sambrook MA, Crossman AR. Common neural mechanisms in experimental chorea and hemiballismus in the monkey. Evidence from 2-deoxyglucose autoradiography. Brain Res. 1985b;339:346–350. doi: 10.1016/0006-8993(85)90102-7. [DOI] [PubMed] [Google Scholar]
- Morelli M, Di Paolo T, Wardas J, Calon F, Xiao D, Schwarzschild MA. Role of adenosine A2A receptors in parkinsonian motor impairment and l-DOPA-induced motor complications. Prog. Neurobiol. 2007;83:293–309. doi: 10.1016/j.pneurobio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Nash JE, Fox SH, Henry B, Hill MP, Peggs D, McGuire S, Maneuf Y, Hille C, Brotchie JM, Crossman AR. Antiparkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson’s disease. Exp. Neurol. 2000;165:136–142. doi: 10.1006/exnr.2000.7444. [DOI] [PubMed] [Google Scholar]
- Navailles S, Lagiere M, Contini A, De Deurwaerdere P. Multisite intracerebral microdialysis to study the mechanism of l-DOPA induced dopamine and serotonin release in the parkinsonian brain. ACS Chem. Neurosci. 2013;4:680–692. doi: 10.1021/cn400046e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HS, Penney JB, Young AB. Gamma-aminobutyric acid and benzodiazepine receptor changes induced by unilateral 6-hydroxydopamine lesions of the medial forebrain bundle. J. Neurochem. 1985;45:1396–1404. doi: 10.1111/j.1471-4159.1985.tb07205.x. [DOI] [PubMed] [Google Scholar]
- Papa SM, Boldry RC, Engber TM, Kask AM, Chase TN. Reversal of levodopa-induced motor fluctuations in experimental parkinsonism by NMDA receptor blockade. Brain Res. 1995;701:13–18. doi: 10.1016/0006-8993(95)00924-3. [DOI] [PubMed] [Google Scholar]
- Park YS, Kim HY, Chang WS, Lee PH, Sohn YH, Chang JW. A comparison of LEDD and motor scores following STN-DBS treatment in patient with young onset vs late onset Parkinson’s disease. Neuromodulation. 2010;13:255–260. doi: 10.1111/j.1525-1403.2009.00273.x. [DOI] [PubMed] [Google Scholar]
- Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J. Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in l-DOPA-induced dyskinesia. Nat. Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Picconi B, Paille V, Ghiglieri V, Bagetta V, Barone I, Lindgren HS, Bernardi G, Angela Cenci M, Calabresi P. l-DOPA dosage is critically involved in dyskinesia via loss of synaptic depotentiation. Neurobiol. Dis. 2008;29:327–335. doi: 10.1016/j.nbd.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Picconi B, Bagetta V, Ghiglieri V, Paille V, Di Filippo M, Pendolino V, Tozzi A, Giampa C, Fusco FR, Sgobio C, Calabresi P. Inhibition of phosphodiesterases rescues striatal long-term depression and reduces levodopa-induced dyskinesia. Brain. 2011;134:375–387. doi: 10.1093/brain/awq342. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Rylander D, Parent M, O’Sullivan SS, Dovero S, Lees AJ, Bezard E, Descarries L, Cenci MA. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann. Neurol. 2010;68:619–628. doi: 10.1002/ana.22097. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Libert F, Vassart G, Vanderhaeghen JJ. Distribution of adenosine A2 receptor mRNA in the human brain. Neurosci. Lett. 1991;130:177–181. doi: 10.1016/0304-3940(91)90391-6. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Dassesse D, d’Alcantara P, Ledent C, Swillens S, Zoli M. A2A receptor and striatal cellular functions: regulation of gene expression, currents, and synaptic transmission. Neurology. 2003;61:S24–S29. doi: 10.1212/01.wnl.0000095207.66853.0d. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog. Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Thiele SL, Warre R, Nash JE. Development of a unilaterally-lesioned 6-OHDA mouse model of Parkinson’s disease. J. Vis. Exp. 2012;14:3234. doi: 10.3791/3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele SL, Warre R, Khademullah CS, Fahana N, Lo C, Lam D, Talwar S, Johnston TH, Brotchie JM, Nash JE. Generation of a model of l-DOPA-induced dyskinesia in two different mouse strains. J. Neurosci. Methods. 2011;197:193–208. doi: 10.1016/j.jneumeth.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torvinen M, Marcellino D, Canals M, Agnati LF, Lluis C, Franco R, Fuxe K. Adenosine A2A receptor and dopamine D3 receptor interactions: evidence of functional A2A/D3 heteromeric complexes. Mol. Pharmacol. 2005a;67:400–407. doi: 10.1124/mol.104.003376. [DOI] [PubMed] [Google Scholar]
- Torvinen M, Torri C, Tombesi A, Marcellino D, Watson S, Lluis C, Franco R, Fuxe K, Agnati LF. Trafficking of adenosine A2A and dopamine D2 receptors. J. Mol. Neurosci. 2005b;25:191–200. doi: 10.1385/JMN:25:2:191. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- Utley JD, Carlsson A. Relative effects of l-DOPA and its methyl ester given orally or intraperitoneally to reserpine-treated mice. Acta Pharmacol. Toxicol. 1965;23:189–193. doi: 10.1111/j.1600-0773.1965.tb03584.x. [DOI] [PubMed] [Google Scholar]
- Valastro B, Dekundy A, Krogh M, Lundblad M, James P, Danysz W, Quack G, Cenci MA. Proteomic analysis of striatal proteins in the rat model of l-DOPA-induced dyskinesia. J. Neurochem. 2007;102:1395–1409. doi: 10.1111/j.1471-4159.2007.04655.x. [DOI] [PubMed] [Google Scholar]
- Warre R, Thiele S, Talwar S, Kamal M, Johnston TH, Wang S, Lam D, Lo C, Khademullah CS, Perera G, Reyes G, Sun XS, Brotchie JM, Nash JE. Altered function of glutamatergic cortico-striatal synapses causes output pathway abnormalities in a chronic model of parkinsonism. Neurobiol. Dis. 2011;41:591–604. doi: 10.1016/j.nbd.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of l-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol. Psychiatry. 2007;62:800–810. doi: 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J. Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat. Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng BY, Pearce RK, MacKenzie GM, Jenner P. Alterations in preproenkephalin and adenosine-2a receptor mRNA, but not preprotachykinin mRNA correlate with occurrence of dyskinesia in normal monkeys chronically treated with l-DOPA. Eur. J. Neurosci. 2000;12:1096–1104. doi: 10.1046/j.1460-9568.2000.00988.x. [DOI] [PubMed] [Google Scholar]