Highlights
-
•
Presacral tumors are rare variety of space occupying lesions which due to their location, etiological heterogeneity and difficult surgical approach, present a challenge to treating physician.
-
•
Surgical excision remains the treatment of choice for presacral masses.
-
•
Posterior surgical approach, if applied judicially, in carefully selected cases, remains an attractive option as it provides better surgical exposure, is more direct approach, commodious to adopt, easy to learn with quicker post-operative recovery.
Keywords: Presacral tumors, Posterior approach
Abstract
Introduction
Presacral tumors are a rare variety of space occupying lesions arising in the presacral space. Most of the tumors are congenital in origin. Due to obscure anatomic location, difficult surgical approach and etiological heterogeneity, tumors arising here pose a diagnostic and therapeutic challenge. We report our experience of 10 cases of presacral tumors with posterior approach being used in 6.
Materials and methods
A retrospective analysis was conducted on 10 cases of presacral tumors managed at our hospital during a period of 14 months (May 2013–July 2014). 9 cases were operated while one had advanced disease and was referred for palliative care. Complete en bloc excision of the mass was possible in 8 cases. Finally, presenting complaints, clinical diagnosis, surgical procedure and histopahological findings of the cases were studied.
Results
All of our patients were females in the age group of 18–50 (mean 28.4) years. The pathological findings included schwannoma, leiomyosarcoma, hemangiopericytoma, neurofibroma, paraganglioma and rest were developmental cysts. 6 cases were managed using the posterior approach and rest by anterior approach. There was no major complication or mortality in the follow up.
Conclusion
Complete surgical excision remains the mainstay of therapy. Surgical approach depends upon the location, size, local invasion and surgical expertise of the surgeon. Benign tumors have a good prognosis while the prognosis in malignant tumors remains guarded due to difficulty in obtaining safe resection margins. Posterior approach is an attractive option for low lying, benign tumors that is more direct, with better exposure and quicker recovery.
1. Introduction
Presacral tumors arise in the presacral or retrorectal space. The boundaries are comprised by the fascia propria of the upper 2/3rd of rectum anteriorly, presacral fascia posteriorly and the endopelvic fascia with the ureters and iliac vessels on both lateral aspects. The roof is formed by the peritoneal reflection while the floor by the Waldeyer’s fascia. It constitutes an area for development of multiple embryologic structures between the hindgut and the proctodeum, neural elements and bone and connective tissue. Many tumors, mostly congenital in origin, may arise from these tissues. The incidence has been reported in literature to be between 1 in 40,000 to 1 in 63,000 hospital admissions [1,2]. Due to obscure anatomic location, difficult surgical approach and etiological heterogeneity, tumors arising here pose a diagnostic and therapeutic challenge. We present our experience with 10 cases of presacral tumors with posterior approach being the major surgical approach.
2. Materials and methods
All patients of presacral tumors presenting in the Department of General Surgery, Sawai Man Singh Medical College in a single surgical unit during a time period of 14 months (May 2013–July 2014) were included in the study. Data was collected based on the complete clinical records maintained at our hospital. The clinical presentation, diagnoses, surgical intervention done, post-operative outcome, and histopathological findings were reviewed. Follow up was performed on outpatient visits.
3. Results
Total of 10 patients were included in the study. All patients were routine outpatient admissions. All the patients were females in the age group of 18–50 years (mean 28.4 years). The main symptoms and signs are presented in Table 1.
Table 1.
Demographic and clinical profile of the patients.
| S. no. | Age | Sex | Presenting complaints | Duration of symptoms (Mo) |
|---|---|---|---|---|
| 1 | 18 | F | Lump abdomen, pain abdomen | 2 |
| 2 | 45 | F | Lump abdomen, lower limb edema | 1 |
| 3 | 21 | F | Lump abdomen, lower limb pain | 24 |
| 4 | 42 | F | Lump abdomen | 15 |
| 5 | 18 | F | Body ache, headache, palpitations | 36 |
| 6 | 25 | F | Perineal Swelling | 3 |
| 7 | 38 | F | Swelling lower back, pain in swelling | 5 |
| 8 | 27 | F | Swelling coccygeal region | 2 |
| 9 | 30 | F | Swelling lower back | 3 |
| 10 | 20 | F | Lump abdomen, pain abdomen | 2 |
The most common presenting symptom was lump lower abdomen (5/10) followed by swelling in lower back and perineal region (Fig. 1). One patient presented with features of adrenergic overactivity manifested as body ache, headache and palpitations. Pressure symptoms due to mass effect were present in two patients. One had edema of the left lower limb and second had neuropathic pain in left lower limb. One patient also complained of burning micturition. Duration of symptoms ranged from 1 to 36 months (median of 3 months).
Fig. 1.

Preoperative photograph of the patient 7 showing swelling over lower back.
Three out of the 10 patients had been operated previously for similar findings and a recurrence was suspected. Patient no.1 in Table 1 had been operated previously for lump abdomen and Histopathological Examination (HPE hereafter) report was in favor of Schwannoma; patient no. 4 had been previously operated twice and HPE examination was in favor of leiomyosarcoma; patient no. 5 had undergone surgery for lump abdomen with HPE suggestive of paraganglioma.
The swelling was palpable per rectally in 6/10 patients, and it was possible to reach the upper limit of swelling in all of these.
Diagnostic evaluation of the patients was done by clinical examination, ultrasonography of the abdomen and pelvis followed by CT/MRI abdomen and pelvis whereever required (Fig. 2).
Fig. 2.

MRI abdomen and pelvis of patient 10 showing well defined presacral mass.
Ultrsonography of the abdomen revealed the presence of hepatic, pulmonary and omental metastatic deposits along with ascites in patient no. 4 along with complex pelvic mass displacing the rectum anterolaterally. She was diagnosed to have diffuse peritoneal leiomyomatosis with metastases and was referred for palliative treatment. In patient no. 2, the CECT abdomen and pelvis was inconclusive about the involvement of major pelvic blood vessels which was revealed on subsequent laparotomy.
Surgical intervention was done in 9 patients. The surgical approach, histopathological diagnosis, ICU requirement, postoperative complication, length of hospital stay is summarized in Table 2. Out of the 9 patients operated, 6 were by posterior approach and rest by anterior approach. In one case, i.e. patient no. 1, the previous surgery was done by anterior approach while the redo surgery was done by posterior approach. Complete en bloc excision of the mass was achieved in all cases except one; patient no. 2, the mass was found to be encasing the ureters and major pelvic vasculature and hence only a biopsy was taken.
Table 2.
Findings on DRE, final histopathological diagnosis, post – operative recovery of the patients.
| S. no. | Mass felt on DRE | Surgical approach | Histopahology | ICU requirement | Duration of hospital stay (Days) | Follow up (Mo) | Bladder/bowel function | Residual nerve weakness |
|---|---|---|---|---|---|---|---|---|
| 1 | Yes | Post. | Schwannomaa | Yes | 10 | 2 | Normal | None |
| 2 | No | Ant. | Lymphoma | No | 8 | 12 | Normal | None |
| 3 | No | Ant. | Neurofibroma | No | 7 | 12 | Normal | None |
| 4 | No | N/A | Leiomyosarcomaa | N/A | 3 | N/A | N/A | N/A |
| 5 | No | Ant. | Paragangliomaa | Yes | 9 | 3 | Normal | None |
| 6 | Yes | Post. | Epidermoid | No | 2 | 3 | Normal | None |
| 7 | Yes | Post. | Epidermoid | No | 2 | 2 | Normal | None |
| 8 | Yes | Post. | Dermoid | No | 2 | 2 | Normal | None |
| 9 | Yes | Post. | Epidermoid | No | 2 | 2 | Normal | None |
| 10 | Yes | Post. | Hemangiopericytoma | No | 7 | 1 | Normal | None |
Recurrent cases.
We used the transverse incision in all cases of posterior approach, the location and length of the incision depending upon the size and extent of the tumor. Finger dissection was done after the skin incision with minimal use of electrosurgical devices and space was created between the tumor, presacral fascia and surrounding structures. There was no need of sacrectomy in any of the cases while coccygectomy was done in 1 case.
Post-operative recovery of the patients was uneventful (Fig 3). Intensive care monitoring was required for two patients. There were no major complications like pelvic hematoma, nerve injury, bowel or visceral injury. Minor local wound complications were noted in two patients. Mean length of hospital stay was 5.2 days. In follow up, there was no evidence of bowel or bladder dysfunction in any of the patients operated by posterior approach; neither was there any residual limb weakness or sensation loss.
Fig. 3.
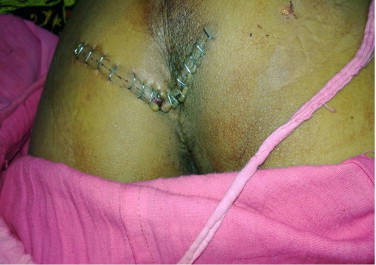
Post operative photograph showing the transverse incision used for posterior approach.
The HPE findings have been illustrated in Table 2. Margins were negative.
4. Discussion
Preascral tumors are a rare variety of space occupying lesions whose true incidence is not known because most of the series have been published from tertiary care referral centers. Despite this, various authors report an incidence between 1.4 and 6.3 cases per year [2–6]. The incidence of benign tumors has been reported to be more in females while malignant tumors have a slight male predominance [2,3,4,7–9]. Several classification systems have been proposed but most commonly the retrorectal masses are classified based on their origin into congenital (55–65%), neurogenic (10–12%), osseous (5–11%), inflammatory (5%) and miscellaneous (12–16%).
The congenital tumors are most common and arise from all three germ layers. These are more common in females. These may arise from ectoderm (dermoids and epidermoids), sequestration of hindgut remnants (tail gut cysts, rectal duplication cysts), neural tube defects (anterior sacral meningocele), and notochord (chordomas). Clinical presentation is nonspecific depending on the size and location of the mass and tissue of origin. A careful digital rectal examination (DRE hereafter) is diagnostic in 90%. Chordoma is the most common malignant presacral mass.
Neurogenic lesions typically arise from the peripheral nerves. Ependymomas are the most common histological type. Neuropathic pain and nerve weakness are the presenting symptoms. They may give rise to pelvic mass effect when large in size.
Osseous tumors may be both benign and malignant. Symptoms most often arise due to compression of nearby neurological structures causing radicular pain. Biological behavior is similar to osseous tumors elsewhere in the body. Complete resection is recommended due to high rates of recurrence.
Symptoms arising due to presacral masses depend on the size, location and origin of the tumor and also on whether the tumor is benign or malignant. In a review of five largest case series, 43% of benign tumors were free from symptoms while only 7% of malignant tumors were symptomless [8,10]. A careful DRE is the initial examination of choice. In a series of 120 cases from Mayo clinic, 97% of the tumors were palpable per rectally [2,10]. If the superior limit of the tumor is palpable on DRE, a posterior surgical approach may be feasible. Plain radiographs may sometimes be useful and demonstrate sacral destruction (chordomas), calcifications within the mass (teratomas) or pathognomic signs like the “Scimitar sign” (anterior sacral meningocele). Computed tomography of the pelvis is widely used and is capable of differentiating solid from cystic lesions, vascular, adjacent organ involvement and bony destruction. Contrast enhanced MRI pelvis is the gold standard investigation of choice and the most sensitive and specific investigation also. Limitations of MRI are inability to distinguish between fibrosis and recurrent pelvic carcinoma in patients managed with surgery or radiation therapy. Other investigations like CT/MR myelography may be done when there is involvement of CNS. In cases of major vessel involvement, preoperative angiography may be necessary to expose the major feeders of the tumor. Endorectal ultrasound and lower GI endoscopy can help in evaluating the involvement of layers of rectum and superior extent of the lesion as well as the consistency of the tumor [11].
The role of preoperative biopsy is controversial. A preoperative biopsy should only be performed if it is likely to change the management and surgical approach. Currently, there is no indication for biopsy of a cystic lesion [11,12]. Adequate resection is diagnostic as well as therapeutic. Preoperative percutaneous biopsy is, however, indicated for solid or heterogeneous tumors to facilitate decision making for neoadjuvant therapy and surgical planning [12]. Biopsy, if indicated, should never be done transperitoneally, transretroperitoneally, transrectally or transvaginally [11].
Three main surgical approaches are used: anterior, posterior and combined. The anterior or trans abdominal approach is recommended for very large tumors, when there is suspicion of malignancy and when the lower limit of the tumor is above the S3 vertebra. Advantages of an anterior approach include better visualization of pelvic structures, bleeding control and easy mobilization of rectum.
The posterior approach, first described by Paul Kraske (1885), may be inter-sphincteric, trans-sphincteric parasacrococcygeal, trans-sacral, trans-sacrococcygeal, trans-anorectal, trans-vaginal. It is indicated in low lying tumors with upper limit below S3, when there is evidence of sacral involvement, in small tumors and when malignancy is not suspected. Coccygectomy – indicated for better exposure as well as for complete removal of a potential communication route and consequent recurrence of cystic lesions or teratomas. Advantages include a more direct approach, more commodious to adopt, operating field of the tumor is clearer, adverse influences of bowel coming in between are avoided, quicker post-operative recovery. Potential drawbacks include absence of control over pelvic vessels, chances of injury to pelvic nerves and reduced working space.
A combined abdomino-sacral approach is recommended for larger lesions that extend both above and below S3, large masses, and suspected malignancy.
A perineal endoscopic approach has also been described to access the presacral space and perform excision biopsies [13].
Complications of surgery include bleeding, post-operative urinary and fecal incontinence, nerve root injury leading to weakness and chances of abscess or fistula formation. Rates of complications are higher in patients undergoing some form of sacral resection. Even, if sacrectomy is necessary, care must be taken to preserve at least one side of S2 to reduce postoperative complications arising out of nerve damage.
5. Conclusion
Adequate surgical resection is the only treatment for presacral masses as most of the tumors eventually enlarge in size; there is always a risk of malignancy and infection; and they have the potential of causing dystocia in women of child bearing age. Posterior surgical approach is an attractive option as most common variety of presacral masses is low lying developmental cysts. Rate of complications can be avoided by careful selection of cases. The prognosis of benign tumors is excellent after complete excision while that of malignant tumors is poor due to high rates of recurrence.
Conflicts of interest
No conflicts of interest.
Sources of funding
No sources of funding.
Ethical approval
Ethical approval not required.
Consent
Written informed consent was obtained from the patients for publication of this case series and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Dr. Dhananjay Saxena – Study design, data acquisition, data analysis, writing, revision.
Dr. Abhinav Pandey – Writing, revision.
Dr. Rajendra Prasad Bugalia – data collection, design, revision.
Dr. Mahendra Kumar – data analysis, writing.
Dr. Raju Kadam – data collection, analysis.
Dr. Vipul Agarwal – Article revision.
Dr. Amit Goyal – Article revision.
Dr. Jeevan Kankaria – Design, revision.
Dr. R. K. Jenaw – Data analysis, article revision, approval.
Guarantor
Dr. Dhananjay Saxena, the contributing author, is the Guarantor.
Contributor Information
Dhananjay Saxena, Email: dr.dhananjaysaxena@gmail.com.
Abhinav Pandey, Email: drabhinavpandey1987@gmail.com.
Rajendra Prasad Bugalia, Email: drrpbugalia@gmail.com.
Mahendra Kumar, Email: drmahendrakhichar983@gmail.com.
Raju Kadam, Email: drr4jk4d4m@gmail.com.
Vipul Agarwal, Email: vipul_bmc@yahoo.co.in.
Amit Goyal, Email: amitgoyal0304@gmail.com.
Jeevan Kankaria, Email: jeevan.kankaria@gmail.com.
Raj Kamal Jenaw, Email: jenawrk@yahoo.com.
References
- 1.Whittaker L.D., Pemberton J.D. Tumors ventral to the sacrum. Ann. Surg. 1938;107(1):96–106. doi: 10.1097/00000658-193801000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jao S.W., Beart R.W., Jr., Spencer R.J., Reiman H.M., Ilstrup D.M. Retrorectal tumors mayo clinic experience 1960–1979. Dis. Colon Rectum. 1985;28(9):644–652. doi: 10.1007/BF02553440. [DOI] [PubMed] [Google Scholar]
- 3.Uhlig B.E., Johnson R.L. Presacral tumors and cysts in adults. Dis. Colon Rectum. 1975;18:581–589. doi: 10.1007/BF02587141. [DOI] [PubMed] [Google Scholar]
- 4.Cody H.S., 3rd, Marcove R.C., Quan S.H. Malignant retrorectal tumors: 28 years’ experience at Memorial Sloan-Kettering Cancer Center. Dis. Colon Rectum. 1981;24(7):501–506. doi: 10.1007/BF02604308. [DOI] [PubMed] [Google Scholar]
- 5.Böhm B.J.W.M., Fazio V.W., Lavery C., Church J.M., Oakley J.R. Our approach to the management of congenital presacral tumors in adults. Int. J. Colorectal Dis. 1993;8(3):134–138. doi: 10.1007/BF00341185. [DOI] [PubMed] [Google Scholar]
- 6.Freier D.T., Stanley J.C., Thompson N.W. Retrorectal tumors in adults. Surg. Gynecol. Obstet. 1971;132(4):681–686. [PubMed] [Google Scholar]
- 7.Hobson K.G., Ghaemmaghami V., Roe J.P., Goodnight J.E., Khatri V.P. Tumors of the retrorectal space. Dis. Colon Rectum. 2005;48(10):1964–1974. doi: 10.1007/s10350-005-0122-9. [DOI] [PubMed] [Google Scholar]
- 8.Glasgow S.C., Birnbaum E.H., Lowney J.K., Fleshman J.W., Kodner I.J., Mutch D.G., Lewin S., Mutch M.G., Dietz D.W. Retrorectal tumors: a diagnostic and therapeutic challenge. Dis. Colon Rectum. 2005;48(8):1581–1587. doi: 10.1007/s10350-005-0048-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang J.Y., Hsu C.H., Changchien C.R., Chen J.S., Hsu K.C., You Y.T., Tang R., Chiang J.M. Presacral tumor: a review of forty five cases. Am. Surg. 1995;61(4):310–315. [PubMed] [Google Scholar]
- 10.Devine, RM . Managing presacral tumours. In: Zbar A.P., Wexner S.D., editors. Coloproctology. Springer; London: 2010. pp. 81–93. [Google Scholar]
- 11.Hassan I., Wietfeldt E.D. Presacral tumors diagnosis and management. Clin. Colon Rectal Surg. 2009;22(2):84–93. doi: 10.1055/s-0029-1223839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merchea A., Larson D.W., Hubner M., Wenger D.E., Rose P.S., Dozois E.J. The value of preoperative biopsy in the management of solid presacral tumors. Dis. Colon Rectum. 2013;56(June (6)):756–760. doi: 10.1097/DCR.0b013e3182788c77. [DOI] [PubMed] [Google Scholar]
- 13.Nieuwenhuis, Dorothée H., Gagner, Michel, Consten, Esther C.J. The Endoscopic Perineal Approach to the Presacral Space: an excision biopsy. J. Laparoendosc. Adv. Surg. Tech. 2009;19(December (6)) doi: 10.1089/lap.2008.0224. p799. [DOI] [PubMed] [Google Scholar]


