Highlights
-
•
Aneurysmal bone cyst occurring in the setting of previously diagnosed fibrous dysplasia is a rare occurrence.
-
•
Repeat investigation with imaging and biopsy was required to obtain accurate diagnosis and exclude malignancy.
-
•
Due to the aggressive nature of the disease and the patient’s medical comorbidities, an above knee amputation was required for disease control.
Keywords: Aneurysmal bone cyst, Fibrous dysplasia
Abstract
Introduction
Aneurysmal bone cyst occurring in the setting of previously diagnosed fibrous dysplasia is rare. While both are benign processes, pain, compression of nearby structures and risk of fracture can require treatment.
Presentation of case
In this report, we describe a 56 year old male who developed an aggressive aneurysmal bone cyst secondary to fibrous dysplasia in the proximal tibia over a period of 8 months. He required an above knee amputation for disease and symptom control due to the aggressive nature of disease and medical comorbidities.
Discussion
The diagnosis of a secondary lesion can prove difficult. It is important to exclude a malignant disease process, particularly when imaging demonstrates an aggressive appearance. In this case, repeat imaging, CT guided biopsies and an open biopsy were performed to exclude malignancy prior to definitive surgical management.
Conclusion
In order to exclude secondary lesions, we suggest further investigation for new onset pain in the setting of a benign lesion.
1. Introduction
Fibrous dysplasia is a benign process where normal bone is replaced with fibrous tissue. It represents 2.5% of primary bone tumours and occurs predominantly in the first 3 decades of life [1]. The gene mutation at the α-subunit of the G-protein receptor results in an increase of cAMP [2,3], causing hyperproliferation of abnormal osteoblasts as well as stimulating cytokine pathways that lead to increased bone resorption by osteoclasts. A combination of these two pathways produce the characteristic lesion. It usually affects the long bones, craniofacial bones and ribs, and can occur as a single lesion (monostotic, 70%), multiple lesions (polyostotic, 30%), or as part of McCune–Albright syndrome involving polyostotic fibrous dysplasia, café-au-lait spots and multiple endocrine dysfunction [3,4,5]. Radiologically, the appearance of fibrous dysplasia includes endosteal scalloping of the inner cortex without periosteal reaction, bony expansion and a ground-glass appearance resulting from the radiolucent bone producing no visible trabecular pattern. There is a classic histological appearance with a low to moderate cellular fibrous stroma surrounding irregular trabeculae of woven bone, arranged in a “Chinese characters” pattern [4,6,7]. In mild cases, treatment consists of surveillance and maintenance of bone density through diet, exercise, and bisphosphonates [2,3]. In severe cases, surgical reinforcement or correction may be required with internal or external fixation, sometimes with the use of cortical allografts [2].
Aneurysmal bone cysts appear as a blood-filled cavity separated by connective tissue septa with fibroblasts and osteoclast-like giant cells. They expand the affected bone, usually occurring at the metaphysis of long bones, flat bones or spinal column [7,8]. Aneurysmal bone cysts are benign, but can be locally aggressive and cause weakening of the bony structure, and expansion can cause pain, swelling, deformity, neurological symptoms and pathological fracture [9,10]. Radiologically, they appear as an eccentric expansile lesion; CT and MRI can show internal separations and fluid levels [8]. While pathogenesis is not completely understood, the development of aneurysmal bone cysts has been linked to tumour-induced vascular processes or as a consequence of trauma [6], and can occur as a secondary vascular phenomenon in areas of a previous lesion [11].
2. Presentation of case
A 56 year old male presented with right proximal tibial pain. He had swelling and localized tenderness in his proximal leg, and required crutches due to pain; he had no skin pigmentations or neurovascular compromise. Two months prior to his presentation, a drug eluting stent had been inserted for treatment of coronary artery disease. He had been commenced on clopidogrel and aspirin.
Imaging was consistent with fibrous dysplasia in the proximal tibia; the lesion measured 15 cm in length and demonstrated endosteal scalloping, ground-glass opacity and medullary expansion without periosteal reaction (Fig. 1). A skeletal survey performed prior to referral to our hospital revealed multiple radiolucent lesions throughout both femoral and right tibial diaphysis, consistent with polyostotic fibrous dysplasia. Thallium scan, which highlights metabolic activity [6,12], showed heterogeneous mild uptake within the tibial lesion and no uptake in other lesions. Core biopsy was performed under CT guidance targeting the area of thallium avidity, and pathology showed spindle cell proliferation and fibrous stroma with immature bone formation (Fig. 1). There were no features of malignancy and the lesion was placed under surveillance after the diagnosis of fibrous dysplasia was made.
Fig. 1.
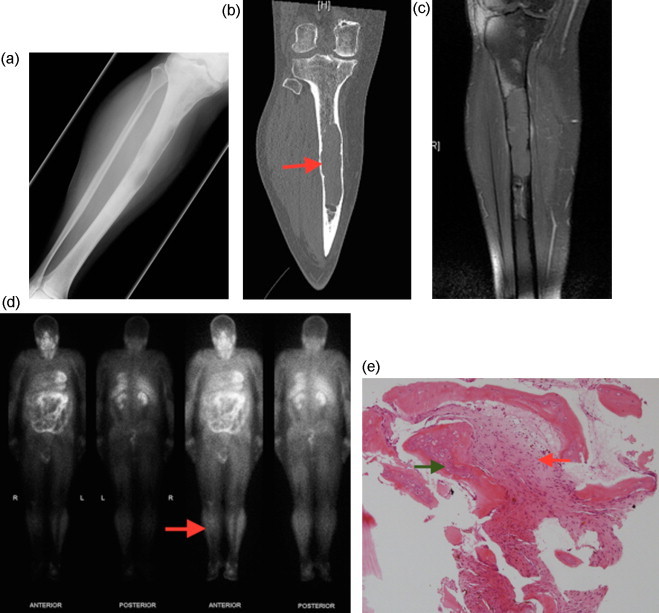
Initial presentation of right tibial diaphyseal lesion:
Radiolucent lesion with medullary expansion and endosteal scalloping shown in: (A) AP radiograph; (B) Coronal CT scan showing a well circumscribed lesion with endosteal scalloping; (C) Coronal T1 with fat saturation MRI; (D) Thallium scan showing moderate heterogeneous uptake in the right proximal tibia on delayed 4 h planar imaging; (E) Core biopsy showing spindle cell proliferation (red arrow) and fibrous stroma with immature bone formation (green arrow), consistent with fibrous dysplasia.
Five months later, he re-presented with increased pain in the right leg. Plain radiographs showed cortical thinning associated with a pathological fracture of the anterior cortex of the tibia and an increase in lesion size (Fig. 2). CT showed cortical breach and periosteal reaction. There was concern of malignant change with MRI features of heterogeneous T2 hyperintensity as well as medullary soft tissue expansion. Fluid-fluid levels were also noted (Fig. 2). A CT guided core biopsy was performed targeting the area of uptake on a repeat thallium scan. This showed a cellular lesion with numerous mulitnucleated giant cells together with smaller mononuclear cells, haemorrhage, fibrin and granulation tissue (Fig. 2). These features were suggestive of solid aneurysmal bone cyst, however, an open biopsy was performed due to clinical concern of malignancy. Intra-operatively it was noted that there was extensive thinning of the bony cortex and replacement of the bony architecture with haematoma. Curettage was performed and analysed, and showed a similar lesion to the previous biopsy. This was consistent with solid aneurysmal bone cyst.
Fig. 2.
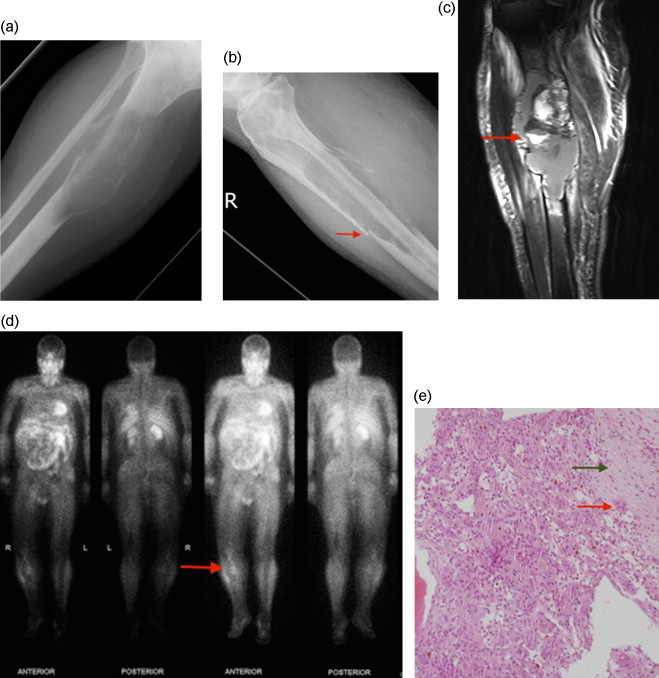
Presentation at 5 month mark:
(A, B) AP and lateral plain radiographs showing cortical erosion, soft tissue extension and minimally displaced pathological fracture (arrow); (C) Coronal STIR weighted MR image showing cortical destruction and medullary soft tissue expansion with associated internal fluid-fluid level (arrow); (D) Thallium scan demonstrates peripheral thallium avidity within the proximal right tibia; (E) Open biopsy showing mulitnucleated giant cells (red arrow) together with smaller mononuclear cells, haemorrhage, fibrin and granulation tissue. Also an area of fibrous stroma is seen (green arrow). These features are consistent with aneurysmal bone cyst in the setting of fibrous dysplasia.
Due to the anticoagulants preventing definitive surgical intervention, angiographic embolisation was attempted but was unsuccessful [13]. The patient was therefore temporarily placed into a bi-valved thermoplastic splint and commenced on zoledronic acid in an attempt to control the growth of the lesion [3,14,15]. His symptoms were controlled with this therapy and he was able to mobilise with crutches.
Over a time-period of 3 months he developed increasing pain requiring admission and this was unable to be controlled despite maximal analgesia. Repeat imaging showed extensive disease progression with increased lesion size, cortical destruction, soft tissue extension into all compartments of the leg and encapsulation of the neurovascular bundle (Fig. 3). It was elected to perform an above knee amputation for local control as it was felt a limb salvage procedure was not possible. Histology on the whole specimen confirmed extensive aneurysmal bone cyst in association with fibrous dysplasia extending into soft tissue, again with no features of malignancy (Fig. 3).
Fig. 3.
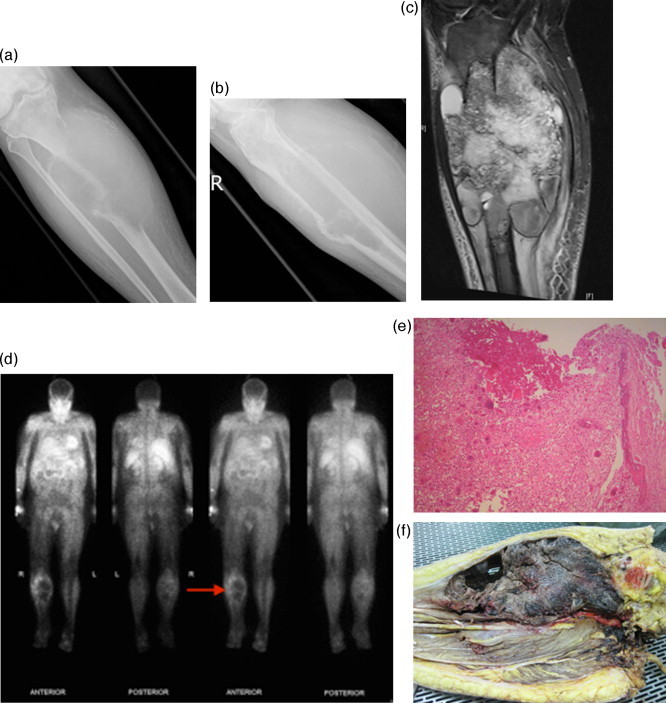
Presentation at 8 month mark:
(A, B) AP and lateral plain radiographs showing large radiolucent lesion with cortical erosion and soft tissue extension; (C) Coronal STIR weighted MR image demonstrates marrow replacement by soft tissue lesion with soft tissue extension; (D) Thallium scan demonstrates peripheral thallium avidity of the proximal right tibia on 4 h planar images; (E) Histology confirms aneurysmal bone cyst; (F) Clinical photo of resection specimen showing bony replacement and soft tissue expansion.
3. Discussion
Aneurysmal bone cyst occurring in the setting of previously diagnosed fibrous dysplasia is a rare presentation. We describe a patient with known polyostotic fibrous dysplasia who developed an aggressive secondary aneurysmal bone cyst that presented a diagnostic and management dilemma. Sarcoma was included in the list of differential diagnoses due to the development of new symptoms, aggressive radiological appearance and rapid rate of change. It has been well documented that fibrous dysplasia can undergo malignant transformation; osteosarcoma, fibrosarcoma and malignant fibrous histiocytoma are the most commonly diagnosed [2,16,17]. Ruggieri et al. [17] reported 28 sarcomas in a review of 1122 histologically diagnosed cases of fibrous dysplasia, and the rate of malignant transformation was higher for polyostotic disease than for monostotic disease (6.7% versus 1.9%). Suspicion for malignant change in a patient with known fibrous dysplasia is raised when there is increased pain or radiologic changes seen on routine follow-up [17], and this should prompt re-staging; however, these changes can also be caused by pathological fracture or other secondary benign lesions such as aneurysmal bone cyst.
Aneurysmal bone cyst can originate from other precursor lesions, either as the result of trauma or a tumour-induced vascular process. Approximately 30% of cases arise as a secondary lesion in the presence of a primary lesion [18]. We found radiological and pathological evidence of fibrous dysplasia being the primary diagnosis. Our patient presented multiple times with new symptoms requiring repeat investigation, and serial imaging showed the change from a benign lesion to an aggressive lesion, but we were unable to differentiate between malignant and benign disease based on imaging alone. However, by performing repeated biopsies we have shown the clear progression from fibrous dysplasia to aneurysmal bone cyst, supporting the precursor theory of secondary aneurysmal bone cyst development. From our search of the literature, we were unable to find other cases that clearly show the development of the secondary aneurysmal bone cyst.
Nguyen [18] presented a case with secondary aneurysmal bone cyst occurring in known fibrous dysplasia of the proximal radius. There were difficulties with the diagnosis due to aggressive but non-specific imaging findings. This patient was successfully treated with curettage after frozen section was performed on an open biopsy. Montalti [7] also presented a case of a patient who developed aneurysmal bone cyst in known fibrous dysplasia of the proximal femur. Imaging and CT-guided biopsy were unable to exclude malignancy, but trochar biopsy confirmed the diagnosis. These cases, along with our case, show the difficulties of early accurate diagnosis, as well as the benefits when this can be achieved. In our patient, attempts were made to treat the lesion conservatively but we were unable to control disease progression. It can be seen that over a time period of 8 months the lesion spreads to involve all compartments of the leg as well as the neurovascular bundle. This, together with medical comorbidities, lead to above knee amputation. As far as we are aware, this is the only reported case of above knee amputation for control of rapidly progressive and destructive aneurysmal bone cyst. One other case of amputation for benign disease was reported by Diercks et al., in which above knee amputation was required for a functionless limb resulting from multiple fractures due to polyostotic fibrous dysplasia [19]. Literature review revealed 35 cases of aneurysmal bone cyst occurring secondary to fibrous dysplasia. These lesions were in the skull, maxilla, mandible, ribs, ilium, femur and radius. To the best of our knowledge this is the first published case of aneurysmal bone cyst complicating fibrous dysplasia of the tibia.
4. Conclusion
We describe a rare case of aggressive aneurysmal bone cyst occurring in the setting of previously diagnosed fibrous dysplasia. Due to the potential for aggressive secondary lesions, we encourage prompt further investigation in patients with fibrous dysplasia who develop new symptoms, particularly increased pain or fracture, or who develop changes on routine follow-up imaging. Early intervention may avoid the need for amputation.
Conflict of interest
The authors state that they have no conflict of interest.
Funding
None.
Author’s contributions
Nathan Anderson
Group1 - Conception and design, Acquisition of data, Analysis and interpretation of data.
Group 2 - Drafting the article, Critical revision of the article.
Group 3 - Final approval of the version to be published.
Claudia DiBella
Group1 - Conception and design, Analysis and interpretation of data.
Group 2 - Critical revision of the article.
Group 3 - Final approval of the version to be published.
Marcus Pianta
Group1 - Analysis and interpretation of data.
Group 2 - Critical revision of the article.
Group 3 - Final approval of the version to be published.
John Slavin
Group1 - Acquisition of data, Analysis and interpretation of data.
Group 2 - Critical revision of the article.
Group 3 - Final approval of the version to be published.
Peter Choong
Group1 - Conception and design, Analysis and interpretation of data.
Group 2 - Critical revision of the article.
Group 3 - Final approval of the version to be published.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Guarantor
The guarantor for this project is Nathan Anderson.
Contributor Information
Nathan Anderson, Email: nathananderson9@gmail.com.
Claudia DiBella, Email: claudibella@hotmail.it.
Marcus Pianta, Email: Marcus.Pianta@svhm.org.au.
John Slavin, Email: John.Slavin@svhm.org.au.
Peter Choong, Email: sarcoma@bigpond.net.au.
References
- 1.Edgerton M., Persing J., Jane J. The surgical treatment of fibrous dysplasia with emphasis on recent contributions from cranio-maxillo-facial surgery. Ann Surg. 1985;202:459–479. doi: 10.1097/00000658-198510000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riddle N., Bui M. Fibrous Dysplasia. Arch. Pathol. Lab. Med. 2013;137:134–138. doi: 10.5858/arpa.2012.0013-RS. [DOI] [PubMed] [Google Scholar]
- 3.Mohan H., Mittal P., Mundi I., Kumar S. Fibrous dysplasia of bone: a clinicopathologic review. Pathol. Lab. Med. Int. 2011;3(3):31–42. [Google Scholar]
- 4.DiCaprio M., Enneking W. Fibrous dysplasia: pathophysiology, evaluation and treatment. J. Bone Joint Surg. Am. 2005;87(8):1848–1864. doi: 10.2106/JBJS.D.02942. [DOI] [PubMed] [Google Scholar]
- 5.Albright F., Butler A., Hampton A., Smith P. Syndrome characterized by osteitis fibrosa disseminate, areas of pigmentation and endocrine dysfunction, with precocious puberty in females: report of five cases. N. Engl. J. Med. 1937;216:727–747. [Google Scholar]
- 6.Goto Y., Ihara K., Kawauchi S., Ohi R., Sasaki K., Kawai S. Clinical significance of thallium-201 scintigraphy in bone and soft tissue tumours. J. Arthroplasty. 2002;3:304–312. doi: 10.1007/s007760200052. [DOI] [PubMed] [Google Scholar]
- 7.Montalti M., Alberghini M., Ruggieri P. Secondary aneurysmal bone cyst in fibrous dysplasia of the proximal femur. Orthopaedics. 2009;32(5):363. doi: 10.3928/01477447-20090501-10. [DOI] [PubMed] [Google Scholar]
- 8.Vergel De Dios A., Bond J., Shives T., McLeod R., Unni K. Aneurysmal bone cyst: a clinicopathologic study of 238 cases. Cancer. 1992;69(12):2921–2931. doi: 10.1002/1097-0142(19920615)69:12<2921::aid-cncr2820691210>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Clayer M. Injectable form of calcium sulphate as treatment of aneurysmal bone cyst. ANZ J. Surg. 2008;78(5):336–370. doi: 10.1111/j.1445-2197.2008.04479.x. [DOI] [PubMed] [Google Scholar]
- 10.Burch S., Hu S., Berven S. Aneurysmal bone cysts of the spine. Neurosurg. Clin. N. Am. 2008;19(1):41–47. doi: 10.1016/j.nec.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Enneking W. A system of staging for musculoskeletal neoplasms. Clin. Orthop. Relat. Res. 1986;204:9–24. [PubMed] [Google Scholar]
- 12.Caluser C., Abdel-Dayem M., Macapinlac H., Scott A., Healey J., Huvos A. The value of thallium and three-phase bone scan in the evaluation of bone and soft tissue sarcomas. EJNMMI. 1994;21(11):1198–1205. doi: 10.1007/BF00182353. [DOI] [PubMed] [Google Scholar]
- 13.De Cristofaro R., Biagini S., Boriani S., Ricci P., Ruggieri P., Rossi G. Selective arterial embolization in the treatment of aneurysmal bone cyst and angioma of bone. Skeletal Radiol. 1992;21(8):523–527. doi: 10.1007/BF00195235. [DOI] [PubMed] [Google Scholar]
- 14.Simm P., O'sullivan M., Zacharin M. Successful treatment of a sacral aneurysmal bone cyst with zoledronic acid. J. Paediatr. Orthop. 2013;33(5):61–64. doi: 10.1097/BPO.0b013e318285c3a7. [DOI] [PubMed] [Google Scholar]
- 15.Balke M., Campanacci L., Gebert C., Picci P., Gibbons M., Taylor R. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC cancer. 2010;10:462–470. doi: 10.1186/1471-2407-10-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen M., Moffat J. Osteosarcoma of the skull base after radiation therapy in a patient with McCune–Albright syndrome: case report. Skull Base. 2003;13(2):79–83. doi: 10.1055/s-2003-820562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruggieri P., Sim F., Bond J., Unni K. Malignancies in fibrous dysplasia. Cancer. 1994;73(5):1411–1424. doi: 10.1002/1097-0142(19940301)73:5<1411::aid-cncr2820730516>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen B., Lugo-Olivieri C., McCarthy E., Frassica F., Ma L., Zerhouni E. Fibrous dysplasia with secondary aneurysmal bone cyst. Skeletal Radiol. 1996;25(1):88–91. doi: 10.1007/s002560050041. [DOI] [PubMed] [Google Scholar]
- 19.Diercks R., Sauter A., Mallens W. Aneurysmal bone cyst in association with fibrous dysplasia: a case report. J. Bone Joint Surg. Br. 1986;68(1):144–146. doi: 10.1302/0301-620X.68B1.3941131. [DOI] [PubMed] [Google Scholar]


