Abstract
Significance: An ancient anionic phospholipid, cardiolipin (CL), ubiquitously present in prokaryotic and eukaryotic membranes, is essential for several structural and functional purposes. Recent Advances: The emerging role of CLs in signaling has become the focus of many studies. Critical Issues: In this work, we describe two major pathways through which mitochondrial CLs may fulfill the signaling functions via utilization of their (i) asymmetric distribution across membranes and translocations, leading to the surface externalization and (ii) ability to undergo oxidation reactions to yield the signature products recognizable by the executionary machinery of cells. Future Directions: We present a concept that CLs and their oxidation/hydrolysis products constitute a rich communication language utilized by mitochondria of eukaryotic cells for diversified regulation of cell physiology and metabolism as well as for inter-cellular interactions. Antioxid. Redox Signal. 22, 1667–1680.
“Out of intense complexities intense simplicities emerge. Broadly speaking, the short words are the best, and the old words when short are best of all.”
Winston Churchill
Cardiolipins are Ubiquitous Membrane Phospholipids in Prokaryotes and Eukaryotes
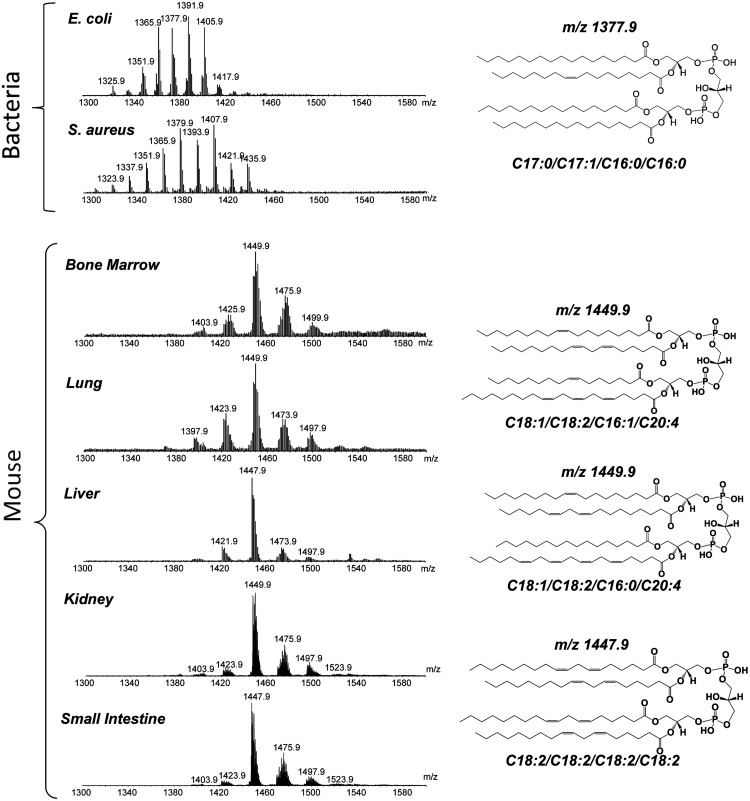
According to a popular concept on the origin of life, spontaneous increase of molecular complexity, specificity, and a combination of self-assembly with self-replication of inanimate matter were the major prerequisites for the transition from prebiotic states to life (56). Among these “precursor” molecules of life, an important role could be played by amphiphillic phospholipids with their ability to self-assemble into organized systems with a beneficial function of compartmentalization (22). Given that cellular life has emerged on our planet more than 3.5 billion years ago, it is interesting to note that one of the ancient groups of phospholipids, cardiolipins (CL), are universally found in both prokaryotes and eukaryotes where they represent important phospholipid components of bacterial membranes and mitochondrial inner membranes, respectively (76). During this very long period of time, the general chemical structure of CLs, [1,3-bis(sn-3′-phosphatidyl)-sn-glycerols] (Fig. 1), remained unchanged with a dimeric moiety of two phosphatidylglycerols connected via a glycerol backbone, thus including two negative charges from the phosphate groups and four acyl chains (76). However, the features of CLs fatty acyls are markedly different: Shorter-chain saturated or monounsaturated residues exist in prokaryotes, and longer-chain polyunsaturated residues are present in eukaryotes (Figs. 1 and 2).
FIG. 1.
Mass spectrometry illustrates diversification of CLs in prokaryotic and eukaryotic cells. Note the presence of shorter chain and uneven carbon number fatty acid residues in bacterial CLs versus the presence of long-chain PUFAs in mouse CLs. MS2 analysis of singly charged molecular ions was used for structural identification of CL as described by Hsu and et al. (33). The MS2 spectra were acquired using isolation width 1.0 m/z. Pulsed-Q dissociation technique (with Q=0.7, and no low mass cutoff for analysis of low-molecular-weight fragment ions) was used for MS2 analysis. Based on the MS fragmentation data, the chemical structures of lipid molecular species were drawn using ChemDraw and confirmed by comparing them with the fragmentation patterns presented in the Lipid Map Data Base (www.lipidmaps.org). CL, cardiolipins; PUFAs, polyunsaturated fatty acids.
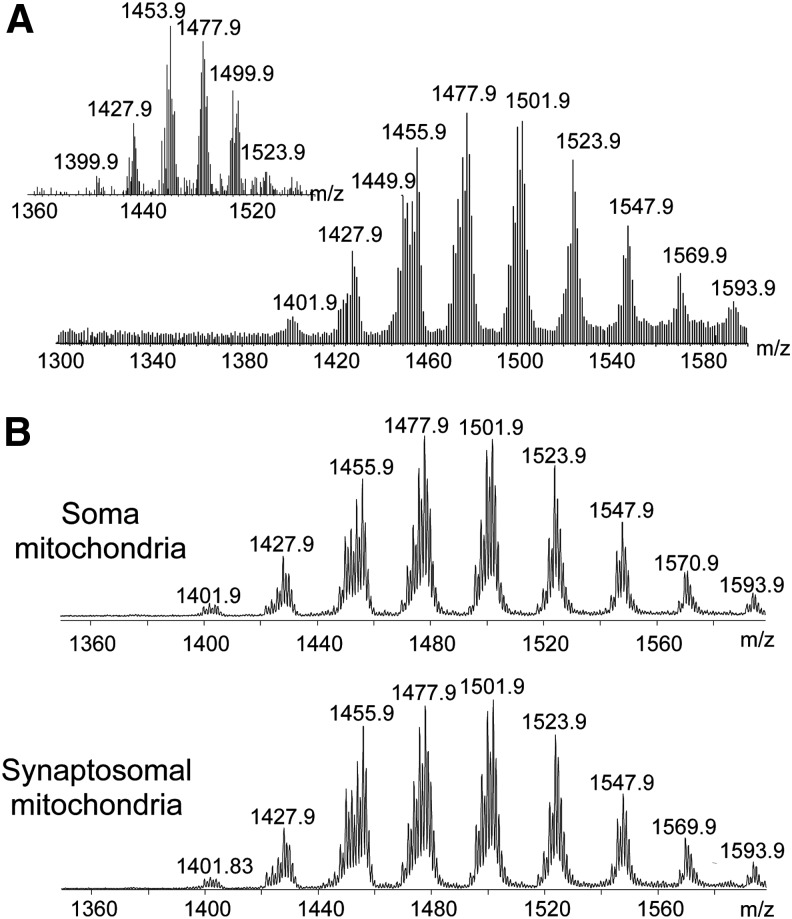
FIG. 2.
Mass spectra of brain CLs demonstrate their high diversification and enrichment with long-chain polyunsaturated species. Mass spectra obtained from mouse (A) and human (inset) brains. (B) Mass spectra of CL from somal and synaptosomal mouse brain mitochondria.
The universal presence of CL in both bacteria and eukaryotic mitochondria is compatible with the endosymbiotic hypothesis of the origin of mitochondria from bacteria (25, 84), and it is also indicative of their important structural and functional roles in highly diversified organisms. The essentiality of CLs for structural and functional organization of energy-producing mechanisms in bacteria and mitochondria has been well documented in several excellent reviews (53, 61, 69, 72, 74). Here, we would like to focus on the recently appreciated signaling functions of CLs with conserved and evolutionarily diverse features in prokaryotes and eukaryotes, respectively.
Asymmetry and Signaling by CL Externalization
There are two types of asymmetry characteristic of CLs—molecular asymmetry and trans-membrane asymmetry (Fig. 3). The former is due to the chirality of the CL molecule. Even apparently structurally symmetrical CL species with identical fatty acid residues such as tetra-linoleoyl-CL or tetra-oleoyl-CL have two chemically distinct phosphatidyl moieties, that is, two chiral centers in the pro-R and pro-S positions relative to the central carbon of the glycerol bridge. As a result, two of the CLs phosphate groups are located in the nonidentical chemical environments. The potential role of this difference in ionization states of CLs in the microenvironments facing the mitochondrial matrix with the basic pH may be quite significant (30). It is tempting to speculate that this propensity of CLs may be responsive to changes in intra-mitochondrial pH/membrane potential and affect CL distribution and trans-membrane asymmetry. For CL species with nonidentical acyls, the molecular asymmetry may lead to even more “dramatic” consequences, resulting not only in structural “fitness” to accommodate the optimal conformations of the CLs bound proteins but also in altered metabolic fate realized via (per)oxidation, hydrolysis reactions, and accumulation of oxidatively modified CL species and lyso-CLs (94).
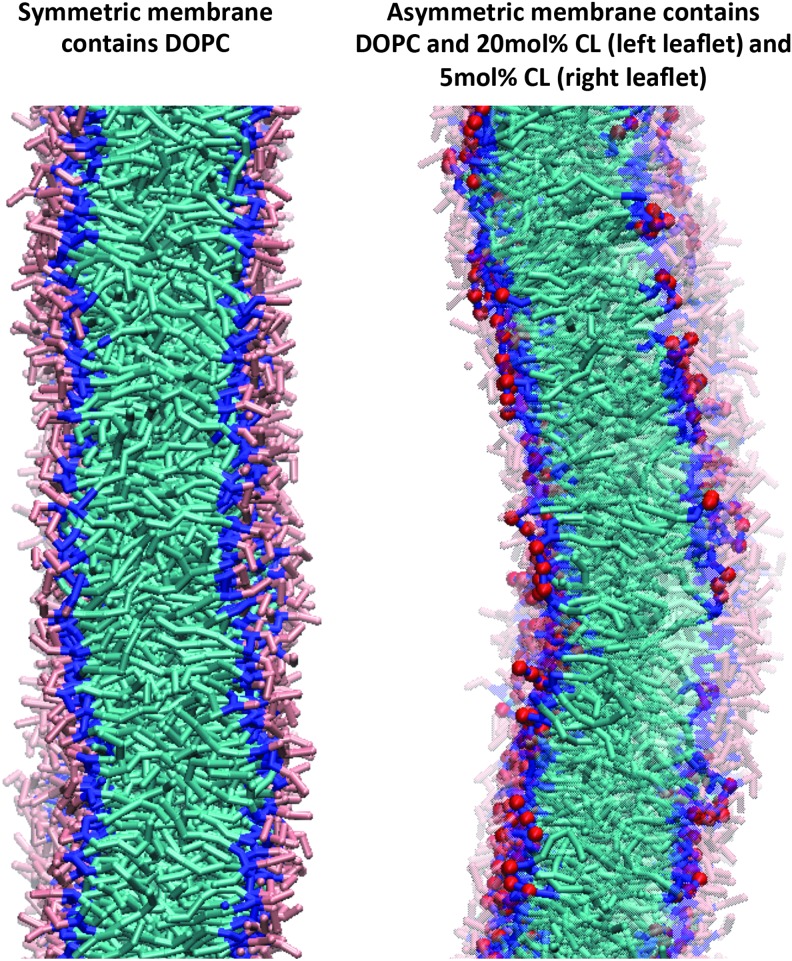
FIG. 3.
Asymmetric distribution of CL between two leaflets induces membrane curvature, characteristic of the IMM and bacterial poles. The final configuration (at t=500 ns) of CG-MD simulations from symmetric distribution of DOPC in the membrane (left) does not display significant curvature, while asymmetric insertion of CL (right) leads to substantial negative curvature toward the CL enriched side. Representation guide: pink, choline and phosphate groups of DOPC; cyan, acyl chains of CL and DOPC; blue, glycerol moiety of DOPC and CL; red, phosphate groups of CL; DOPCs have been represented transparent in the asymmetric membrane; water and ions removed for clarity. CG-MD, coarse-grained molecular dynamics simulations; DOPC, 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine; IMM, inner mitochondrial membrane. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Both in bacteria and in eukaryotes, cellular distribution of CLs is asymmetric. Usually, CLs are found predominantly in the inner membranes of bacterial cells (58, 66). In eukaryotes, this asymmetry is even more robust: CLs are mitochondria-specific and, in functional mitochondria maintaining “healthy” membrane potential, CLs are confined almost exclusively to the inner mitochondrial membrane (IMM) (Fig. 5) with a higher abundance in the matrix leaflet (32). The maintenance of this asymmetry and its disturbances are used for signaling purposes—similar to externalization of another anionic phospholipid, phosphatidylserine (PS), across the plasma membrane during apoptosis (43, 88, 101). Indeed, recent studies have revealed that both in bacteria and in mitochondria, collapse of CL asymmetry represents a signaling event. The exact meaning of this signaling and its significance for bacteria/host interactions have not yet been fully deciphered (20, 27, 52), although biochemical mechanisms involved in CL translocation have been partially identified (1, 21). It has been demonstrated that gram-negative bacteria externalize their CLs on the membrane surface as they encounter host cells (21). Specifically, a transcriptional virulence regulator, PhoPQ of the two-component system of Salmonella typhimurium, induces an increase in CL levels within the outer membrane by facilitating their translocation through the periplasmic space.
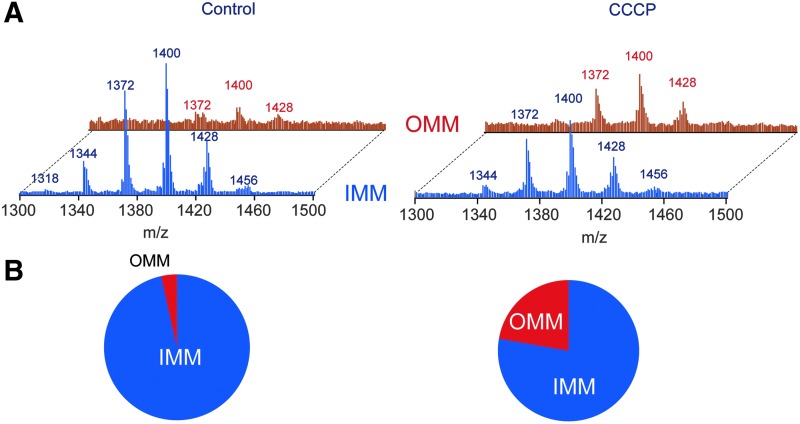
FIG. 5.
LC-MS based assessments of CL asymmetric distribution between the OMM and IMM. Representative mass spectra of CLs (A) and the contents of CLs (B) in mouse lung epithelial (MLE12) cells challenged with a protonophoric uncoupler, CCCP. CCCP, carbonyl cyanide m-chlorophenylhydrazone; MLE12, mouse lung epithelial; OMM, outer mitochondrial membrane. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
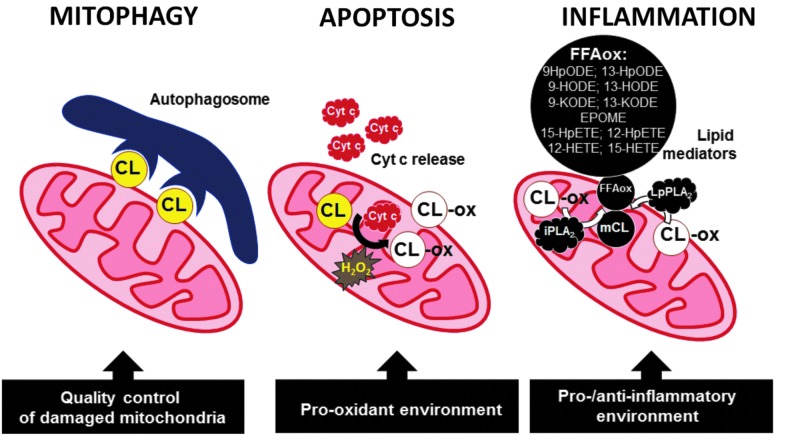
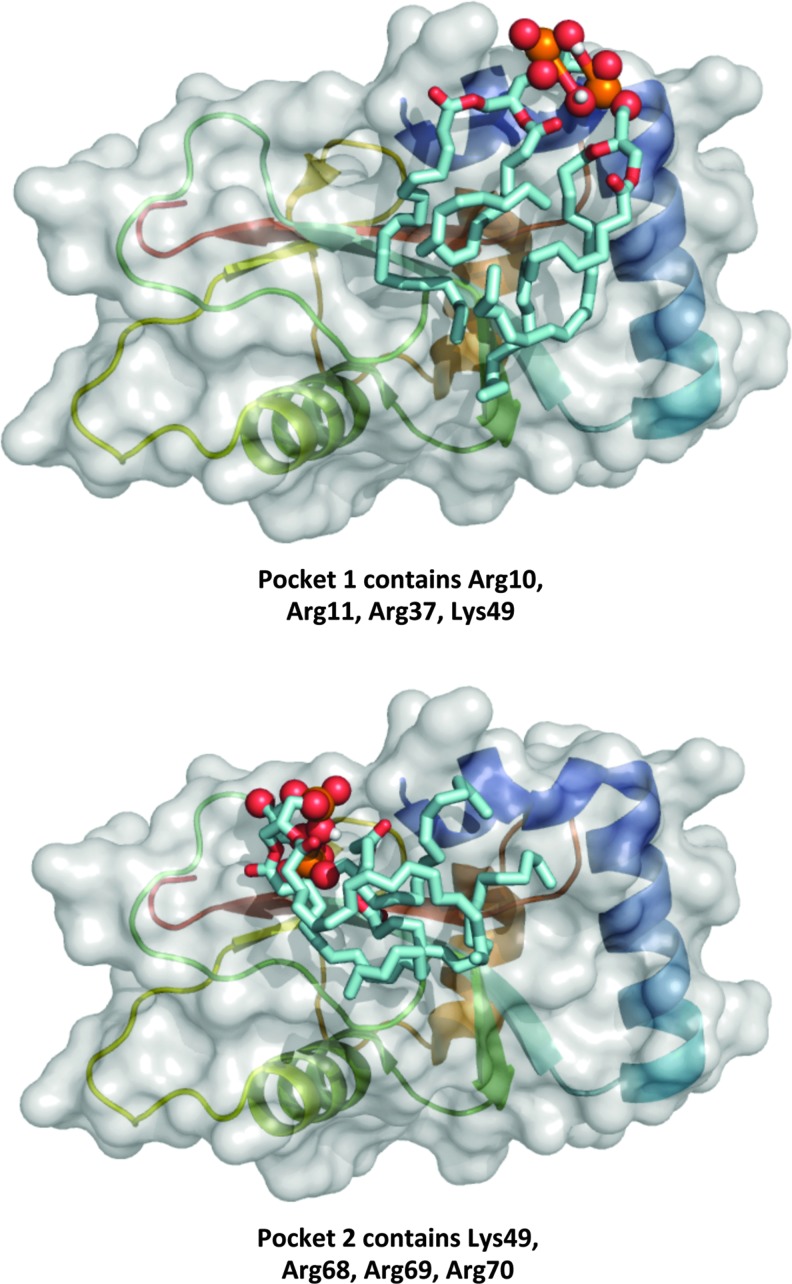
In mitochondria, the role of CL translocation in the mitochondrial surface has been definitively decoded as an “elimination” signal during mitophagy (Fig. 4) (14). It has been established that a partial decrease of membrane potential leads to redistribution of CL from the IMM to the outer mitochondrial membrane (OMM) (Fig. 5) and CL externalization on the mitochondrial surface (Fig. 6) (14). Through the binding to microtubule-associated protein 1 light chain 3 (LC3), an essential protein of the autophagic machinery, externalized CLs act as an “eat-me” signal for mitophagy. With regard to specificity of CL involvement in the signaling process, two points should be noted: (i) Enrichment of the OMM with CLs is not associated with the exposure of its selective molecular species on the surface but rather reflects random trans-membrane translocation of different CL species, and (ii) externalized CLs do not contain increased amounts of oxidized CL species (14). In other words, electrostatic interactions of CLs phosphate groups with a cluster of critical arginine residues of LC3 define the recognition process (46). In line with this, molecular modeling reveals two CL binding pockets on the LC3 protein with similar acyl chains binding poses but different positions of the phosphate groups representing two candidate modes for stabilization of the electrostatic charges (22) (Fig. 7). The strength of the interactions between LC3 and CL in a bilayer membrane is emphasized by the observed CL clustering and membrane deformation due to the binding (Fig. 8).
FIG. 4.
Illustrating the major CL-driven intra- and extra-cellular signaling pathways in mitophagy, apoptosis, and inflammation. Externalization of CL on the surface of injured mitochondria serves as an “eat-me” signal recognized by LC3 and leads to the engagement of mitophageal machinery. No CL oxidation takes place during the mitophageal “elimination” of damaged mitochondria. In apoptosis, an interaction of CL with cyt c leads to the formation of peroxidase complexes, leading to the accumulation of CLox products. This leads to the release of pro-apoptotic factors, including cyt c, from mitochondria into the cytosol. CLox can be hydrolyzed by Ca2+-independent PLA2, yielding FFAoxacting as lipid mediators and regulators of the inflammatory response. CLox, oxidized cardiolipin; mCL, monolyso-cardiolipin; FFAox, oxygenated free fatty acids; 9-HpODE, 9-hydroperoxy-octadecadienoic acid; 13-HpODE, 13-hydroperoxy-octadecadienoic acid; 9-KODE, 9-oxo-octadecadienoic acid; 13-KODE,13-oxo-octadecadienoic acid; 9-HODE, 9-hydroxy-octadecadienoic acid; 13-HODE, 13-hydroxy-octadecadienoic acid; EpOME: 9,10-epoxy-octadecanoic acid or 12,13-epoxy-octadecanoic acid; 12-HpETE,12-hydroperoxy-eicosatetraenoic acid, 12-HETE, 12-hydroxy-eicosatetraenoic acid; 15-HpETE, 15-hydroperoxy-eicosatetraenoic acid; 15-HETE, 15-hydroxy-eicosatetraenoic acid; cyt c, cytochrome c; LpPLA2, lipoprotein-associated phospholipase A2; iPLA2γ, Ca2+-independent mitochondrial phospholipase A2; LC3, light chain 3; PLA2, phospholipases A2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 6.
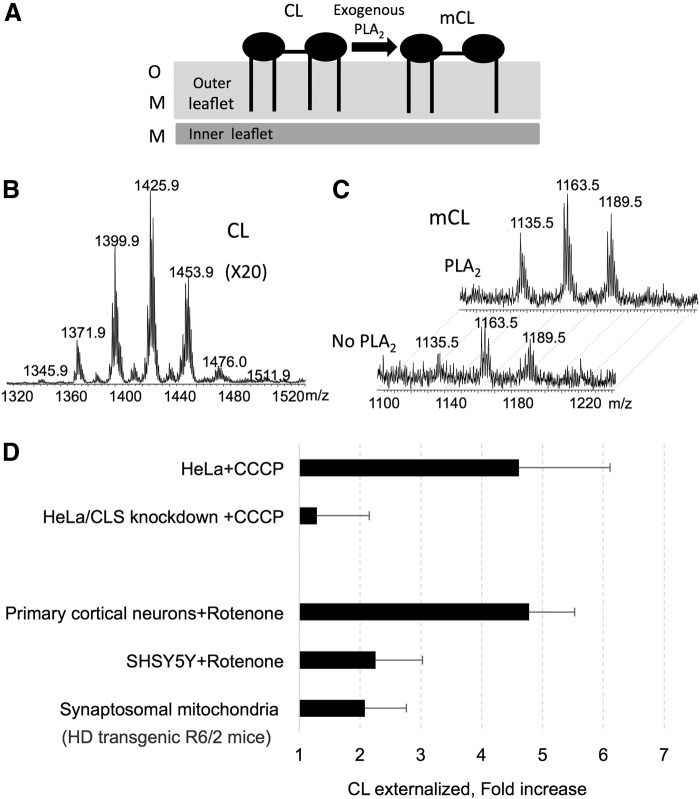
Determination of CL externalization on the mitochondrial surface using treatment with exogenous PLA2 and LC/MS-based analysis of mCL as hydrolysis products. (A) A schema explaining the PLA2-catalyzed hydrolysis of accessible CLs externalized on the outer leaflet of OMM. (B) Mass spectrum of CL obtained from mitochondria of HeLa cells. Mass spectrum of CL from control (untreated with PLA2) mitochondria is presented. (C) Mass spectra of mCL obtained from mitochondria of HeLa cells before and after treatment with PLA2. (D) Typical examples of assessments of CL externalization to the mitochondrial surface after mitochondrial injury induced by a protonophoric uncoupler, CCCP, in HeLa cells (14); a mitochondrial complex I inhibitor, rotenone, in primary rat cortical neurons and SHSY5Y cells (14), and in synaptosomal mitochondria from brain of transgenic Huntington disease R6/2 mice. Note that decreased levels of CL in HeLa cells on CLS knockdown resulted in a diminished externalization response to CCCP and decreased levels of mitophagy (14). Data are presented as fold increase versus untreated cells (mean±SD, n=3). For synaptosomal mitochondria isolated from transgenic R6/2 mice, the data are compared with synaptosomal mitochondria from brain of wild-type mice (mean±SD, n=5). CLS, cardiolipin synthase; PLA2, phospholipiase A2; LC/MS, liquid chromatography mass spectrometry; mCL, monolysocardiolipin.
FIG. 7.
Two proposed CL binding sites on LC3. Note that the externalized CLs act as an “eat-me” signal for mitophagy (14). Upper panel shows the top-ranked model, and lower panel represents the alternative-binding site. Representation guide: cyan, acyl chains of CL; red and orange, oxygen and phosphorus in CL phosphate groups; gray, surface representation of LC3; cartoon representation of LC3 also has been added to surface representation, colored blue>red from N>C terminus. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 8.
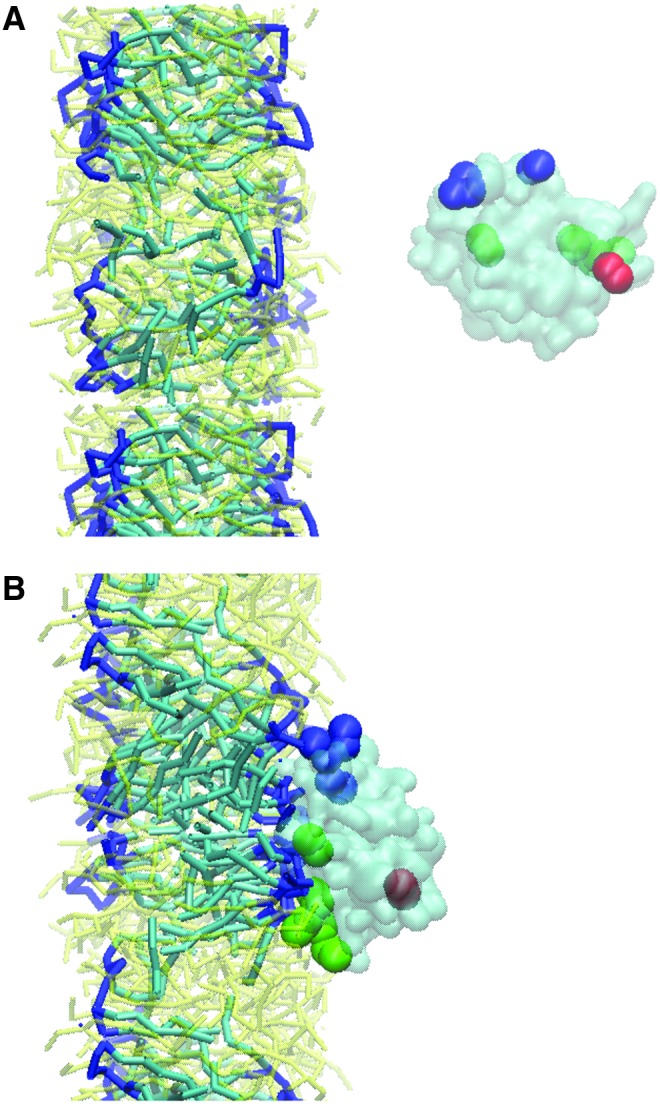
CG-MD simulations suggest that LC3 triggers lateral diffusion of CL and its clustering in the membrane. The curvature formation caused by protein binding emphasizes the strong interactions (14). (A) Initial configuration - LC3 was placed ∼3 nm away from the membrane; (B) Final configuration - LC3 is interacting with CL-containing membrane. Color guide: head groups of CL, dark blue sticks; acyl chain of CL, light blue sticks; LC3, cyan (transparent); N-terminus of LC3 (K5, R10-R11), blue spheres; K49, R68-R69-R70, green spheres; Glu117, red sphere. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
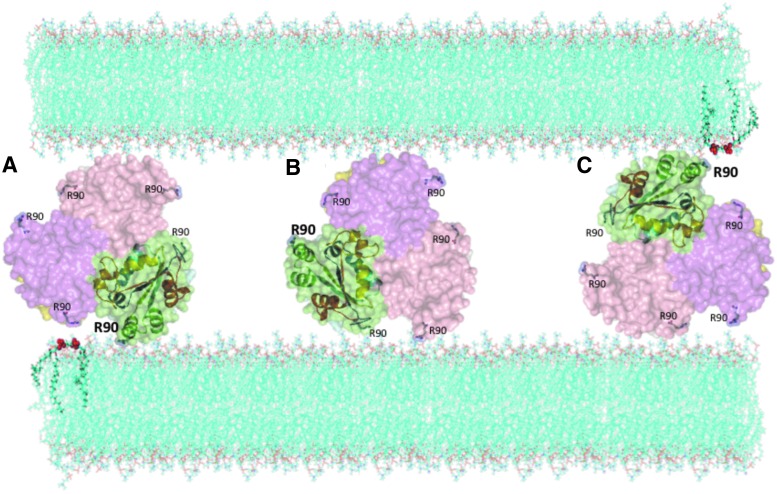
The mechanisms of the overall process leading to CL externalization to the surface of mitochondria remain enigmatic whereby three consecutive CL translocations have to be identified: (i) from the inner to the outer leaflet of the inner membrane, (ii) from the outer leaflet of the inner membrane to the inner leaflet of the outer membrane, and (iii) from the inner to the outer leaflet of the outer membrane. While several candidate proteins have been proposed for these translocation stages—adenine nucleotide translocator (ANT), uncoupling proteins, mitochondrial nucleoside diphosphate kinase NM23-H4/NDPK-D, creatine phosphokinase (CPK), truncated-BH3 interacting domain death agonist (t-Bid), and scramblase-3—the firm experimental evidence unequivocally supporting their participation is still lacking. The role of the NDPK-D hexamer in facilitating the most energetically “difficult” transgression stage—re-distribution of CL between the inner and the outer membranes likely occurring at the contact sites—has recently been confirmed in cells (77). The proposed mechanism emphasizes the critical involvement of R90 in CL binding—via the interaction with the phosphate groups of the phospholipid (Fig. 9)—and transfer by a “rotary activity” of the protein hexameric complex.
FIG. 9.
A computational model explaining the CL transfer mechanisms by Nm23-H4/NDPK-D. The predicted orientation of Nm23-H4 with respect to the membrane confirms the experimentally observed interaction with Arg-90 in the basic RRK triad (26). (A–C) Different protein orientations illustrating “rotary activity” of NDPK-D. Representation guide: Different chains are indicated by different colors, and one chain contains a cartoon representation as well as emphasizes the rotating model. Surface representation is used. Arg90 is highlighted in all chains. CL is indicated in green inside the membrane, with phosphate groups highlighted as red spheres. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Because mitochondrial damage is frequently associated with the excessive production of reactive oxygen species (ROS), there is a tendency to believe that oxidative stress is almost an obligatory stage of mitochondrial responses to perilous environments [reviewed in Refs. (12, 15, 17, 19, 35, 67, 70, 78)]. While commonly accepted, this association has only been partially validated, as there are many examples when mitochondrial dysfunction emerges and this process occurs independently of ROS production (11, 40, 98). In fact, our previous work has demonstrated that in cytochrome c (cyt c)-deficient HeLa cells, staurosporine-triggered autophagy/mitophagy was not accompanied by either generation of superoxide radicals or accumulation of hydrogen peroxide (H2O2). Accordingly, polyethylene glycol, conjugated superoxide dismutase, and catalase did not affect mitochondrial degradation. Moreover, staurosporine induced mitophagy in DNA-deficient ρ° HeLa1.2 cells in which both electron transport and ROS generation were completely disrupted (40). Similarly, in primary cortical neurons and SH-SY5Y cells, stimulation of mitophagy by several distinct stimuli—staurosporine, rotenone, 6-hydroxydopamine—caused robust externalization of CL without causing its oxidation (14).
There is also an accumulating number of examples for CLs participation in pro-mitophageal signaling independent of oxidant mechanisms. Interestingly, mitophagy of dysfunctional mitochondria in Huntington's disease may also be associated with CL externalization. A recent study documented causative relationships between the abnormal polyglutamine expansion in huntingtin (Htt) and the TIM23 mitochondrial protein import complex, resulting in a protein import defect (103). Notably, synaptosomal mitochondria from the brains of transgenic R6/2 mice with a pronounced protein import defect revealed significantly higher levels of externalized CLs compared with controls (Fig. 6). Notably, in all these cases of mitochondrial injury leading to externalization of CLs and mitophagy, no detectable accumulation of CL oxidation products was observed.
CL Oxidation in Apoptotic Signaling
Cell and tissue damage has long been associated with the development of oxidative stress characterized by the accumulation of a variety of (phospho)lipid oxidation products (42). Notably, despite a relatively low abundance of CLs, they displayed the highest sensitivity to oxidation (47, 86). Moreover, the very high selectivity toward CLs was in stark contrast to other more abundant phospholipids such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), which either remained nonoxidized or were only marginally oxidized (e.g., PI, phosphatidylinositol) (91, 92).
In vivo, this type of selective CL oxidation has been documented in several tissues—in the lungs of mice exposed to hyperoxia (91), to nanoparticles (92), or to lethal total body irradiation (93); in the small intestine of irradiated mice (89); and in the acutely injured brain after traumatic or ischemic insults [e.g., after controlled cortical impact (CCI)] (4, 38). Using the rotenone model of Parkinson's disease (68), we were also able to document oxidation of CLs in the substantia nigra (87). While in all these cases CLs constituted the major target for oxidative attack, the scale of oxidative response as well as the composition of oxidation products varied quite significantly. For example, CCI resulted in robust oxidation of CLs with the formation of ∼100 new oxidatively modified species of CL (CLox) (38) and relatively low levels of CLox hydrolysis products—oxygenated free fatty acids (FFAs) and mono-lyso-CL (mCLs) (94). In contrast, cardiac arrest-induced modification of CLs in the brain resulted in accumulation of FFAox and mCLs with a relatively low level of detectable CLox. Accumulation of FFAox and mCLs was also detected in the small intestine of irradiated mice (94). Overall, it is likely that not only direct oxidation but also secondary metabolic conversions of CLox, particularly hydrolysis of CLox, defines the specific features of products detectable in different types of cells and tissues. Because the first and essential catalytic step is CL oxidation, this raises the issue of the nature of the catalysis and mechanisms of CL oxidation.
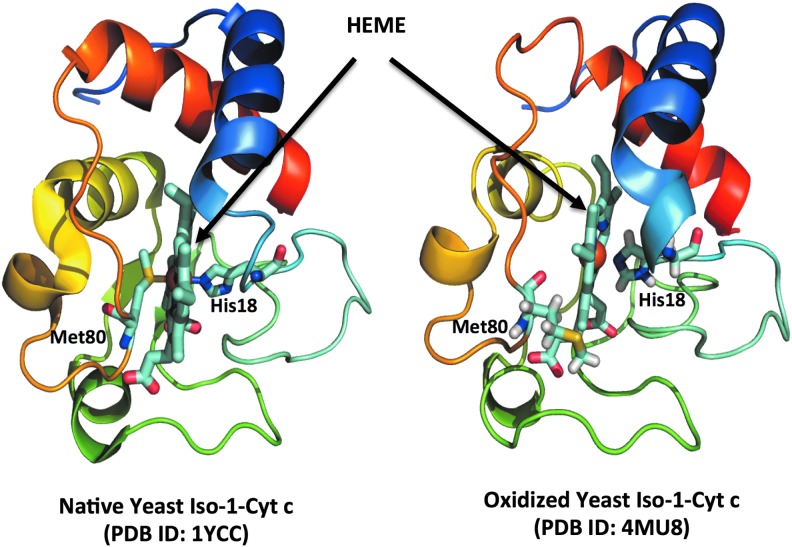
In eukaryotic cells, CLs are mitochondria specific; thus, one may assume that there should be a mitochondrial catalyst of these selective reactions of CL oxidation. Indeed, several studies have identified the intermembrane space heme-protein, cyt c, as an effective catalyst of CL oxidation due to the ability of CL to form peroxidase complexes with the protein (5). Usually, cyt c has a very low peroxidase activity owing to the stable hexa-coordinate structure of the heme-iron (59, 99). The distal ligand, Met80, is located only 2.5 Å away from Fe, thus precluding access to the heme in the native protein by H2O2 or other peroxides (83). On binding and partial unfolding of cyt c by CL, Met80 moves away from the heme Fe atom and releases the sixth Fe coordination bond. This displacement of Met80 out of the heme crevice in the protein leads to enhanced access of the heme catalytic site to small molecules acting as sources of oxidizing equivalents, such as H2O2 and lipid hydroperoxides (60).
Several CL binding sites have been proposed for cyt c/CL interactions, yet the exact molecular features of the complex formation are not fully understood. It is likely that more than one CL molecule is required for the functionally significant structural re-arrangements of cyt c, resulting in its conversion from the hexa- to penta-coordinated state (Fig. 10), gain of the peroxidase function, dramatic changes in the redox potential (3), and associated loss of the electron-transporting capacity of the protein (8). Notably, direct oxidation of Met80—likely realized during oxidative stress events—can also confer peroxidase catalytic activity on the protein in the absence of CL or other anionic phospholipids (37). Thus, both CL binding and protein partial unfolding, along with the possible oxidative modification of the protein's Met80, can contribute to the catalytic peroxidase mechanism that is effective in CL oxidation. However, there is one apparent obstacle for its realization with regard to CL involvement—the lack of physical proximity of the protein-localized exclusively in the intermembrane space—and CLs confined predominantly to the inner leaflet of the IMM (32, 44). It should be noted that during the initial stages of apoptosis, translocation of CL from the inner to the OMM takes place (26, 44, 77), thus allowing its interaction with cyt c. As a result of this translocation, cyt c in the intermembrane space gains access to CL pools localized in the outer leaflet of the inner membrane as well as in the inner leaflet of the outer membrane. It is also possible that the inner and outer membranes contact sites with the concentrated nonbilayer hexagonal organization of CL are the preferred sites where the formation of peroxidase cyt c/CL complexes takes place (69).
FIG. 10.
CL-cyt c complex formation initiates the Met80 move out of the heme crevice, resulting in the enhanced access of the heme catalytic site to small molecules such as H2O2. The native (55) and oxidized (60) structures of yeast iso-1-cyt c with hexa- and penta-coordinated iron atom have been shown in cartoon representation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In the resulting complex, cyt c loses its electron-transporting function but gains peroxidase activity toward polyunsaturated species of CL (44, 46). Oxidation of CL is essential for further transduction of apoptotic signals by facilitating detachment of cyt c from the mitochondrial membrane and formation of the mitochondrial permeability transition pore that ultimately leads to the release of pro-apoptotic factors from mitochondria into the cytosol and completion of the intrinsic apoptotic program (Fig. 4) (44). It should be also mentioned that CL has been identified as a required platform for caspase-8 involvement in extrinsic apoptotic mechanisms (28, 48). In this case, however, CL oxidation does not play any significant role.
The reaction of CL oxidation by cyt c is an enzymatic reaction with a marked selectivity toward its substrates. This selectivity includes not only preferential oxidation of CLs versus other classes of more abundant phospholipids (PC and PE) but also specificity within diversified CL species. For example, C18:2 molecular species of CLs are predominantly oxidized even in the presence of species that are more readily “oxidizable” in random chemical reactions, including C20:4-, C22:5-, and C22:6-containing CL species (89, 91, 93). Moreover, in mixed polyunsaturated CLs containing C18:2 along with more polyunsaturated fatty acid (PUFA) residues within the same molecule, the oxidation process leads to more robust oxidation of C18:2 versus other fatty acid chains (91, 93). The mechanisms of this selectivity needs further clarification based on structural modeling of cyt c interactions with diversified CLs. Interestingly, computational docking indicates potential differences between binding of tetra-linoleoyl-cardiolipin (TLCL) versus CL species with four different fatty acid chains.
Assuming that the peroxidase activity and CL oxidation are essential for the execution of apoptosis, cyt c/CL complexes might represent promising targets for anti-apoptotic drug discovery. Indeed, several classes of new inhibitors of CL oxidation via the peroxidase pathway have been developed with significant propensities in vitro—in cell cultures—and in vivo (2). Given the mitochondrial localization of the cyt c/CL complexes, there is a common requirement for their mitochondrial targeting. This has been achieved through conjugation of the drugs with either an organic cation–triphenylphosphonium—that facilitates “electrophoresis” of the conjugates into mitochondria or with a moiety with high affinity to one of the intramitochondrial components. In the latter paradigm, a peptide mimicking fragment of an antibacterial antibiotic, gramicidine, has been employed (102). One series of mitochondria-targeted compounds was a nitroxide-based electron scavenger capable of preventing the formation of superoxide radicals and H2O2—the source of oxidizing equivalents for the peroxidase reaction (5, 7, 39). The other group of compounds include different homologues of imidazole-substituted fatty acids whereby the imidazole moiety on the acyl chain protruding into the hydrophobic pocket of cyt c interacts with the heme-Fe to lock the catalytic site and to form a high-affinity complex (2, 41). The third series of inhibitors includes analogs of (2-hydroxyamino-vinyl)-triphenyl-phosphonium activatable by the peroxidase function of cyt c/CL complexes to yield nitric oxide NO• (6). The released NO• represents additional opportunities for the suppression of the peroxidase activity of cyt c/CL complexes.
While CL redistribution seems to be essential for its enhanced oxidation during apoptosis, it is also possible that a fraction of CL usually present on the outer leaflet of the IMM will bind cyt c and, hence, function as a peroxidase mechanism in mitochondria of nonapoptotic cells. Interestingly, the early work of Cortese et al. indicated the presence of relatively small amounts of hydrophobically bound cyt c (about 10%) that could not be removed from mitochondria by high ionic strength buffers (18). Assuming the presence of CL on the outer leaflet of the inner membrane exposed to the intermembrane space, the high affinity of cyt c for CL (5) would favor their interaction. The functions of these usually present peroxidase cyt c/CL complexes have to be further established but may be relevant to the production of signaling lipid mediators (94) as discussed next.
CL Oxidation Products as Precursors of Lipid Mediators
The major universal bioenergetic instrument of aerobic life in eukaryotic cells—from protozoan to mammals—is mitochondria. This organelle is filled with machinery that is capable of oxygen-driven “burning” of different oxidizable substrates in a coupled enzymatic and electrochemical process associated with a highly effective generation and preservation of energy in the form of adenosine triphosphate (ATP). In addition to their function as the powerhouse of cells, mitochondria are currently viewed as the major regulatory platform involved in numerous intra- and extracellular functions, from coordination of energy metabolism, catabolism, and cell death to immune responses (9, 36, 45, 65, 96). Hence, there is an intuitive necessity for the existence of a rich and specific language for mitochondrial communications. Because of the presence of four distinct acyl chains in CLs, the potential for their diversification and complexity is enormous. This diversification of CL molecular species can be further enhanced by a large number of oxygenated CL species with several types of oxygen-containing groups—hydroperoxy-, hydroxy-, epoxy-, and oxo-—attached in different positions of the long hydrocarbon chain, thus forming multiple stereo-isomers (4, 85, 94).
In eukaryotic cells, CLs are confined to the IMM where it is known to play prominent structural roles in the organization of respiratory complexes (62, 69) and other lipid–protein interactions. These structural commitments do not require CL diversification and may be fulfilled by a limited number of molecular species of CLs. Indeed, in several tissues and organs (e.g., heart, muscles, liver), a well-known process of CL “maturation” eliminates the majority of its nascent diversified species, limiting them to predominantly (∼80%–85%) CL species containing 18:2 acyls, tetra-linoleoyl CL (75). The majority of tissues and cells, however, express a rich assortment of CLs commonly represented by at least several dozens of molecular species (81, 90). The brain demonstrates an unprecedented diversification of mitochondria-specific CLs—incomparable to other tissues—whereby hundreds of polyunsaturated individual molecular species of CLs have been identified (4, 13, 38, 49). It is tempting to speculate that CLs and their numerous metabolites constitute the basis for mitochondrial communications, that is, represent an unknown mitochondrial language—which is particularly important for coordination of complex brain function. The remarkably diversified four-acyl chains in CLs may represent a quaternary numerical system, in a way analogous to the four DNA nucleotides genetic code of DNA. With >20 fatty acid residues available for integration into CLs, the total theoretical number of possible isomers will be 204. In addition to this, oxidation of fatty acid chains in CLs and enzymatic cleavage of oxidized fatty acid chains with resultant lyso-CLs will present unlimited opportunities for refined and meaningful signaling and, simultaneously, represents a real challenge for experimental studies. As mentioned earlier, acute traumatic brain injury leads to the accumulation of >102 new oxygenated molecular species of CLox as well as their secondary metabolites. Many of them are essential signals triggering different responses—from apoptotic and nonapoptotic cell death to phagocytosis, innate immune reactions, and inflammation. But which of these multiple signals are meaningful words in the drama of brain injury and which are accompanying nonessential exclamations remains obscure. Unfortunately, the current state of knowledge does not have reasonable answers to these questions as yet. But we do know that complete suppression of CLox formation drastically changes the biochemical characteristics of brain responses to trauma and is associated with functional protection against organ injury (38)?
There is also a good possibility that the CLs/CLox-based communication language may be realized, at least in part, through its utilization as a precursor/source of signals in previously identified signaling systems, such as a well-known language of oxygenated FFAs-based lipid mediators. A plethora of lipid mediators, with diversified effects on normal cellular homeostasis and responses to stress and disease, is generated through oxygenation of free PUFAs. Biosynthetic machinery involved in the production of these bioactive compounds has been assigned mostly to the cytosol (10). In spite of the well appreciated role of mitochondria as an intracellular regulatory platform, its role and participation in the generation of lipid mediators has not been identified (29).
Oxygenation of free PUFAs requires the hydrolysis and release of free substrates—a process controlled and catalyzed by Ca2+-dependent phospholipases A2 (PLA2) (23). These reactions define the overall rate and effectiveness of the biosynthesis of lipid mediators whereby oxygenation reactions are catalyzed by several enzymes, including cyclooxygenases (COXs), lipoxygenases (LOXs), cytochrome P450 isoforms, and peroxidases (73, 82). The products of these oxygenation reactions are numerous and include, among others, well-known regulators of the major intra- and extra-cellular functions and responses such as prostaglandins, prostacyclins, thromboxanes, resolvins, protectins, maresins, leukotrienes, lipoxines, lipoxenes, and levuloglandins (80, 82). Interestingly, one of the major phospholipid precursors of PUFA substrates—an anionic phospholipid, PS—is not present in mitochondria due to its remarkably effective decarboxylation to PE (97). In spite of this, mitochondria are important sites for the production of lipid mediators although via entirely different pathways. Our recent studies identified one of them whereby PUFA-containing CLs and their oxidation hydrolysis products undergo a series of enzymatic transformation—cyt c-catalyzed oxidation followed by subsequent hydrolysis of CLox by Ca2+-independent PLA2 (94). These tandem reactions constitute a novel biosynthetic pathway with minimal or possibly negligible effects of Ca2+ (Fig. 4). In fact, the recognition of hydrolyzable CLox is based on the stereo-complementarity of catalytic sites of the respective PLA2—lipoprotein-associated PLA2 VIIA (LpPLA2) (its cytosolic isoform) (23) and mitochondrial iPLA2γ (63). While the involvement of the mitochondrial enzyme seems appropriate, a question can be raised about mutual accessibility of LpPLA2 and CLox. One can assume that not only oxidation but also externalization of CL are important regulators of this process. Most importantly, the formation of lipid mediators and their respective reaction counterparts, mCLs, has been documented in the acutely injured brain and in the small intestine of irradiated animals (94). Importantly, the accumulation of these products was affected by an inhibitor of iPLA2-γ R-BEL [6E-(bromoethylene)tetrahydro-3R-(1-naphthalenyl)-2H-pyran-2-one] (94).
The existence of a Ca2+-independent pathway of CL-derived lipid mediators is intriguing, as it demonstrates the previously unpredicted complexity of mitochondrial regulatory mechanisms. It is possible that the well-known ROS signaling by mitochondria involves reactions of CL oxidation, leading to the formation of multiple products with known signaling functions (such as already identified lipid mediators) as well as yet to be identified derivatives, for example, oxygenated forms of lyso-CLs, whose heralding significance has not yet been established.
It is known that accumulation of mono-lyso-CL (mCL) is a hallmark of Barth syndrome—a complex metabolic disorder caused by mutations in the mitochondrial transacylase, tafazzin (16, 54, 57, 100). In fact, three major biochemical consequences of tafazzin deficiency are characteristic of Barth syndrome: (i) a decrease in CL, (ii) an increase in mCL, and (iii) an altered composition of CL molecular species. Recent lipidomics studies have revealed additional features of dysregulation in an inducible tafazzin shRNA knockdown mouse model: significant changes in the levels of eicosanoids and prostanoids, as well as oxidized linoleic and docosahexaenoic metabolites (50). Thus, whether specific accumulation of mCL or general alterations in metabolism of CL and FA are responsible for the mitochondrial impairments in Barth syndrome is still far from being clear.
Another important pathway leading to the formation of lipid mediators from esterified PUFA may involve special isoforms of LOX-12/15-ALOX, which has been shown to catalyze oxidation of arachidonic residues in PC and PE (31, 34, 51). While the role of 12/15-ALOX in oxidation of CLs is less studied, it is worth mentioning an earlier work by Rapoport et al. (71), where the role of the enzyme in elimination of mitochondria in maturing reticulocytes has been established. Of note, the 12/15-ALOX pathway has been associated with the execution of a caspase-independent nonapoptotic form of cell death (79), now referred to as ferroptosis (24). An interesting regulatory feature of this oxidative pathways is its sensitivity to glutathione peroxidase 4 (Gpx4)—an enzyme catalyzing the conversion of phospholipid hydroperoxides (including CL hydroperoxides) (64) into their respective hydroxyl-derivatives (95). This connects the CL-based signaling pathways with another important redox regulatory system of thiols (104). While more work is required for understanding the role of 12/15-ALOX in generating lipid mediators from CLs, it is interesting that this biosynthetic pathway may represent an unusual combination of Ca2+-sensitive (12/15-ALOX) and Ca2+-insensitive (iPLA2γ, LpPLA2) pathways.
Innovation and Concluding Remarks
Emergence of the group of small-molecule anionic phospholipids with four fatty acyls was a remarkable achievement of bio-organic evolution on our planet. Their role in self-assembly of membrane structures was essential for the creation and optimization of energy-producing machinery. They were also important for compartmentalization of metabolic reactions and fulfillment of simple signaling roles based on changes in asymmetric->symmetric distribution of CLs—along with some other lipids—in single-cell prokaryotes. In eukaryotes, the preserved structural role of CLs was enriched by complex signaling functions necessary for precise spatiotemporal coordination of multiple biochemical reactions and intra- and inter-cellular communications. The diversity of CLs and oxidation/hydrolysis products formed from their polyunsaturated molecular species along with the simplicity of molecular structure of this phospholipid were essential for the creation of meaningful words in the rich mitochondrial dictionary of eukaryotic life. Nowadays, deciphering of these signaling roles of CLs and decoding of the language and the meaning of its words are still in its infancy. However, the availability of powerful LC-MS-based analytical tools of oxidative lipidomics provides an excellent opportunity to interpret the molecular behavior of CLs and the sophisticated mechanisms of CLs/CLoxs participation in coordinating nonlinear processes of fundamental importance in cellular metabolism.
Abbreviations Used
- ANT
adenine nucleotide translocator
- ATP
adenosine triphosphate
- CCI
controlled cortical impact
- CL
cardiolipin
- COXs
cyclooxygenases
- CPK
creatine phosphokinase
- cyt c
cytochrome c
- FFAs
free fatty acids
- Gpx4
glutathione peroxidase 4
- H2O2
hydrogen peroxide
- Htt
huntingtin
- IMM
inner mitochondrial membrane
- LC3
light chain 3
- LOXs
lipoxygenases
- LpPLA2
lipoprotein-associated PLA2
- mCL
mono-lyso-CL
- OMM
outer mitochondrial membrane
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
- PLA2
phospholipases A2
- PS
phosphatidylserine
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- TLCL
tetra-linoleoyl-cardiolipin
Acknowledgments
This study was supported by NIH: PO1HL114453, CA165065, ES020693, U19AIO68021, NS076511, NS061817; NIOSH: OH008282, HFSP-RGP0013/2014, a Merit Review award from the US Department of Veterans Affairs and Alexander von Humboldt Stiftung (to AJPF) and Marie Curie Actions grant 626470 MPFP FP7-People-IIF.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Arechaga I. Membrane invaginations in bacteria and mitochondria: common features and evolutionary scenarios. J Mol Microbiol Biotechnol 23: 13–23, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Atkinson J, Kapralov AA, Yanamala N, Tyurina YY, Amoscato AA, Pearce L, Peterson J, Huang Z, Jiang J, Samhan-Arias AK, Maeda A, Feng W, Wasserloos K, Belikova NA, Tyurin VA, Wang H, Fletcher J, Wang Y, Vlasova II, Klein-Seetharaman J, Stoyanovsky DA, Bayir H, Pitt BR, Epperly MW, Greenberger JS, and Kagan VE. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation-induced death. Nat Commun 2: 497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battistuzzi G, Borsari M, Cowan JA, Ranieri A, and Sola M. Control of cytochrome c redox potential: axial ligation and protein environment effects. J Am Chem Soc 124: 5315–5324, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bayir H, Tyurin VA, Tyurina YY, Viner R, Ritov V, Amoscato AA, Zhao Q, Zhang XJ, Janesko-Feldman KL, Alexander H, Basova LV, Clark RS, Kochanek PM, and Kagan VE. Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis. Ann Neurol 62: 154–169, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, and Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry 45: 4998–5009, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belikova NA, Jiang JF, Stoyanovsky DA, Glumac A, Bayir H, Greenberger JS, and Kagan VE. Mitochondria-targeted (2-hydroxyamino-vinyl)-triphenyl-phosphonium releases no center dot and protects mouse embryonic cells against irradiation-induced apoptosis. FEBS Lett 583: 1945–1950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belikova NA, Tyurina YY, Borisenko G, Tyurin V, Samhan Arias AK, Yanamala N, Furtmuller PG, Klein-Seetharaman J, Obinger C, and Kagan VE. Heterolytic reduction of fatty acid hydroperoxides by cytochrome c/cardiolipin complexes: antioxidant function in mitochondria. J Am Chem Soc 131: 11288–11289, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Birk AV, Chao WM, Bracken C, Warren JD, and Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 171: 2017–2028, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blake R. and Trounce IA. Mitochondrial dysfunction and complications associated with diabetes. Biochim Biophys Acta 1840: 1404–1412, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Bozza PT, Bakker-Abreu I, Navarro-Xavier RA, and Bandeira-Melo C. Lipid body function in eicosanoid synthesis: an update. Prostaglandins Leukot Essent Fatty Acids 85: 205–213, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Campello S, Strappazzon F, and Cecconi F. Mitochondrial dismissal in mammals, from protein degradation to mitophagy. Biochim Biophys Acta 1837: 451–460, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Chen YR. and Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res 114: 524–537, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng H, Mancuso DJ, Jiang X, Guan S, Yang J, Yang K, Sun G, Gross RW, and Han X. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochemistry 47: 5869–5880, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Qiang Wang KZ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, and Kagan VE. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 15: 1197–1205, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claypool SM. Cardiolipin, a critical determinant of mitochondrial carrier protein assembly and function. Biochim Biophys Acta 1788: 2059–2068, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claypool SM, Whited K, Srijumnong S, Han XL, and Koehler CM. Barth syndrome mutations that cause tafazzin complex lability. J Cell Biol 192: 447–462, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claypool SM. and Koehler CM. The complexity of cardiolipin in health and disease. Trends Biochem Sci 37: 32–41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortese JD, Voglino AL, and Hackenbrock CR. Ionic strength of the intermembrane space of intact mitochondria as estimated with fluorescein-BSA delivered by low pH fusion. J Cell Biol 113: 1331–1340, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crimi M. and Esposti MD. Apoptosis-induced changes in mitochondrial lipids. Biochim Biophys Acta 1813: 551–557, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Curtis MM. and Sperandio V. A complex relationship: the interaction among symbiotic microbes, invading pathogens, and their mammalian host. Mucosal Immunol 4: 133–138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalebroux ZD, Matamouros S, Whittington D, Bishop RE, and Miller SI. Phopq regulates acidic glycerophospholipid content of the Salmonella typhimurium outer membrane. Proc Natl Acad Sci U S A 111: 1963–1968, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deamer DW. Role of amphiphilic compounds in the evolution of membrane structure on the early earth. Orig Life Evol Biosph 17: 3–25, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Dennis EA, Cao J, Hsu YH, Magrioti V, and Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 111: 6130–6185, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, III, and Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emelyanov VV. Rickettsiaceae, rickettsia-like endosymbionts, and the origin of mitochondria. Biosci Rep 21: 1–17, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Garcia Fernandez M, Troiano L, Moretti L, Nasi M, Pinti M, Salvioli S, Dobrucki J, and Cossarizza A. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ 13: 449–455, 2002 [PubMed] [Google Scholar]
- 27.Gold VA, Robson A, Bao H, Romantsov T, Duong F, and Collinson I. The action of cardiolipin on the bacterial translocon. Proc Natl Acad Sci U S A 107: 10044–10049, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ, Petit PX, Vaz FM, and Gottlieb E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol 183: 681–696, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutierrez J, Ballinger SW, Darley-Usmar VM, and Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res 99: 924–932, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Haines TH. A new look at cardiolipin. Biochim Biophys Acta 1788: 1997–2002, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Hammond VJ, Morgan AH, Lauder S, Thomas CP, Brown S, Freeman BA, Lloyd CM, Davies J, Bush A, Levonen AL, Kansanen E, Villacorta L, Chen YE, Porter N, Garcia-Diaz YM, Schopfer FJ, and O'Donnell VB. Novel keto-phospholipids are generated by monocytes and macrophages, detected in cystic fibrosis, and activate peroxisome proliferator-activated receptor-gamma. J Biol Chem 287: 41651–41666, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horvath SE. and Daum G. Lipids of mitochondria. Prog Lipid Res 52: 590–614, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Hsu FF, Turk J, Rhoades ER, Russell DG, Shi Y, and Groisman EA. Structural characterization of cardiolipin by tandem quadrupole and multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom 16: 491–504, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Hutchins PM. and Murphy RC. Cholesteryl ester acyl oxidation and remodeling in murine macrophages: formation of oxidized phosphatidylcholine. J Lipid Res 53: 1588–1597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huttemann M, Pecina P, Rainbolt M, Sanderson TH, Kagan VE, Samavati L, Doan JW, and Lee I. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion 11: 369–381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichinohe T, Yamazaki T, Koshiba T, and Yanagi Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc Natl Acad Sci U S A 110: 17963–17968, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivanetich KM, Bradshaw JJ, and Kaminsky LS. Methionine sulfoxide cytochrome c. Biochemistry 15: 1144–1153, 1976 [DOI] [PubMed] [Google Scholar]
- 38.Ji J, Kline AE, Amoscato A, Samhan-Arias AK, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio AM, Okonkwo DO, Cheng JP, Alexander H, Clark RS, Kochanek PM, Wipf P, Kagan VE, and Bayir H. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat Neurosci 15: 1407–1413, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang J, Stoyanovsky DA, Belikova NA, Tyurina YY, Zhao Q, Tungekar MA, Kapralova V, Huang Z, Mintz AH, Greenberger JS, and Kagan VE. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat Res 172: 706–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang J, Maeda A, Ji J, Baty CJ, Watkins SC, Greenberger JS, and Kagan VE. Are mitochondrial reactive oxygen species required for autophagy? Biochem Biophys Res Commun 412: 55–60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang JF, Bakan A, Kapralov AA, Silva KI, Huang ZT, Amoscato AA, Peterson J, Garapati VK, Saxena S, Bayir H, Atkinson J, Bahar I, and Kagan VE. Designing inhibitors of cytochrome c/cardiolipin peroxidase complexes: mitochondria-targeted imidazole-substituted fatty acids. Free Radic Biol Med 71: 221–230, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kagan VE. Lipid Peroxidation in Biomembranes. Boca Raton, FL: CRC Press, 1988 [Google Scholar]
- 43.Kagan VE, Borisenko GG, Serinkan BF, Tyurina YY, Tyurin VA, Jiang J, Liu SX, Shvedova AA, Fabisiak JP, Uthaisang W, and Fadeel B. Appetizing rancidity of apoptotic cells for macrophages: oxidation, externalization, and recognition of phosphatidylserine. Am J Physiol Lung Cell Mol Physiol 285: L1–L17, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, and Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1: 223–232, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, and Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med 46: 1439–1453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kagan VE, Chu CT, Tyurina YY, Cheikhi A, and Bayir H. Cardiolipin asymmetry, oxidation and signaling. Chem Phys Lipids 179: 64–69, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapralov AA, Kurnikov IV, Vlasova II, Belikova NA, Tyurin VA, Basova LV, Zhao Q, Tyurina YY, Jiang J, Bayir H, Vladimirov YA, and Kagan VE. The hierarchy of structural transitions induced in cytochrome c by anionic phospholipids determines its peroxidase activation and selective peroxidation during apoptosis in cells. Biochemistry 46: 14232–14244, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Kasahara A. and Scorrano L. Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol 24: 761–770, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Kiebish MA, Han X, and Seyfried TN. Examination of the brain mitochondrial lipidome using shotgun lipidomics. Methods Mol Biol 579: 3–18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiebish MA, Yang K, Liu X, Mancuso DJ, Guan S, Zhao Z, Sims HF, Cerqua R, Cade WT, Han X, and Gross RW. Dysfunctional cardiac mitochondrial bioenergetic, lipidomic, and signaling in a murine model of Barth syndrome. J Lipid Res 54: 1312–1325, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobe MJ, Neau DB, Mitchell CE, Bartlett SG, and Newcomer ME. The structure of human 15-lipoxygenase-2 with a substrate mimic. J Biol Chem 289: 8562–8569, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kozjak-Pavlovic V, Ross K, and Rudel T. Import of bacterial pathogenicity factors into mitochondria. Curr Opin Microbiol 11: 9–14, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Lanciano P, Khalfaoui-Hassani B, Selamoglu N, Ghelli A, Rugolo M, and Daldal F. Molecular mechanisms of superoxide production by complex III: a bacterial versus human mitochondrial comparative case study. Biochim Biophys Acta 1827: 1332–1339, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li GL, Chen SL, Thompson MN, and Greenberg ML. New insights into the regulation of cardiolipin biosynthesis in yeast: implications for Barth syndrome. Biochim Biophys Acta 1771: 432–441, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Louie GV. and Brayer GD. High-resolution refinement of yeast iso-1-cytochrome c and comparisons with other eukaryotic cytochromes c. J Mol Biol 214: 527–555, 1990 [DOI] [PubMed] [Google Scholar]
- 56.Luisi PL, Walde P, and Oberholzer T. Lipid vesicles as possible intermediates in the origin of life. Curr Opinion Coll Interface Sci 4: 33–39, 1999 [Google Scholar]
- 57.Ma L, Vaz FM, Gu Z, Wanders RJ, and Greenberg ML. The human TAZ gene complements mitochondrial dysfunction in the yeast taz1delta mutant. Implications for Barth syndrome. J Biol Chem 279: 44394–44399, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Maloney E, Madiraju SC, Rajagopalan M, and Madiraju M. Localization of acidic phospholipid cardiolipin and DnaA in mycobacteria. Tuberculosis (Edinb) 91 Suppl 1: S150–S155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margoliash E. and Schejter A. Cytochrome c. Adv Protein Chem 21: 113–286, 1966 [DOI] [PubMed] [Google Scholar]
- 60.McClelland LJ, Mou TC, Jeakins-Cooley ME, Sprang SR, and Bowler BE. Structure of a mitochondrial cytochrome c conformer competent for peroxidase activity. Proc Natl Acad Sci U S A 111: 6648–6653, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mileykovskaya E. and Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta 1788: 2084–2091, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mileykovskaya E. and Dowhan W. Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem Phys Lipids 179: 42–48, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moon SH, Jenkins CM, Liu X, Guan S, Mancuso DJ, and Gross RW. Activation of mitochondrial calcium-independent phospholipase a2gamma (ipla2gamma) by divalent cations mediating arachidonate release and production of downstream eicosanoids. J Biol Chem 287: 14880–14895, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nomura K, Imai H, Koumura T, Kobayashi T, and Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem J 351: 183–193, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Neill LA. Cardiolipin and the nlrp3 inflammasome. Cell Metab 18: 610–612, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Oliver PM, Crooks JA, Leidl M, Yoon EJ, Saghatelian A, and Weibel DB. Localization of anionic phospholipids in escherichia coli cells. J Bacteriol 196: 3386–3398, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Osman C, Voelker DR, and Langer T. Making heads or tails of phospholipids in mitochondria. J Cell Biol 192: 7–16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, and Greenamyre JT. Rotenone model of parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem 280: 42026–42035, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Paradies G, Paradies V, De Benedictis V, Ruggiero FM, and Petrosillo G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta 1837: 408–417, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Patil VA. and Greenberg ML. Cardiolipin-mediated cellular signaling. Adv Exp Med Biol 991: 195–213, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Rapoport SM, Schewe T, Wiesner R, Halangk W, Ludwig P, Janickehohne M, Tannert C, Hiebsch C, and Klatt D. Lipoxygenase of reticulocytes—purification, characterization and biological dynamics of the lipoxygenase—its identity with the respiratory inhibitors of the reticulocyte. Eur J Biochem 96: 545–561, 1979 [DOI] [PubMed] [Google Scholar]
- 72.Ren M, Phoon CK, and Schlame M. Metabolism and function of mitochondrial cardiolipin. Prog Lipid Res 55C: 1–16, 2014 [DOI] [PubMed] [Google Scholar]
- 73.Rouzer CA. and Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes p450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev 111: 5899–5921, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schagger H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim Biophys Acta 1555: 154–159, 2002 [DOI] [PubMed] [Google Scholar]
- 75.Schlame M, Shanske S, Doty S, Konig T, Sculco T, DiMauro S, and Blanck TJ. Microanalysis of cardiolipin in small biopsies including skeletal muscle from patients with mitochondrial disease. J Lipid Res 40: 1585–1592, 1999 [PubMed] [Google Scholar]
- 76.Schlame M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J Lipid Res 49: 1607–1620, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schlattner U, Tokarska-Schlattner M, Ramirez S, Tyurina YY, Amoscato AA, Mohammadyani D, Huang Z, Jiang J, Yanamala N, Seffouh A, Boissan M, Epand RF, Epand RM, Klein-Seetharaman J, Lacombe ML, and Kagan VE. Dual function of mitochondrial nm23-h4 protein in phosphotransfer and intermembrane lipid transfer: a cardiolipin-dependent switch. J Biol Chem 288: 111–121, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schumacker PT, Gillespie MN, Nakahira K, Choi AM, Crouser ED, Piantadosi CA, and Bhattacharya J. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol 306: L962–L974, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W, Bornkamm GW, Schweizer U, and Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and aif-mediated cell death. Cell Metab 8: 237–248, 2008 [DOI] [PubMed] [Google Scholar]
- 80.Serhan CN, Chiang N, and Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8: 349–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sparagna GC. and Lesnefsky EJ. Cardiolipin remodeling in the heart. J Cardiovasc Pharmacol 53: 290–301, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Stables MJ. and Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res 50: 35–51, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Stevens JM. Cytochrome c as an experimental model protein. Metallomics 3: 319–322, 2011 [DOI] [PubMed] [Google Scholar]
- 84.Tian HF, Feng JM, and Wen JF. The evolution of cardiolipin biosynthesis and maturation pathways and its implications for the evolution of eukaryotes. BMC Evol Biol 12: 32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tyurin VA, Tyurina YY, Feng W, Mnuskin A, Jiang J, Tang M, Zhang X, Zhao Q, Kochanek PM, Clark RS, Bayir H, and Kagan VE. Mass-spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J Neurochem 107: 1614–1633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tyurin VA, Tyurina YY, Jung MY, Tungekar MA, Wasserloos KJ, Bayir H, Greenberger JS, Kochanek PM, Shvedova AA, Pitt B, and Kagan VE. Mass-spectrometric analysis of hydroperoxy- and hydroxy-derivatives of cardiolipin and phosphatidylserine in cells and tissues induced by pro-apoptotic and pro-inflammatory stimuli. J Chromatogr B Analyt Technol Biomed Life Sci 877: 2863–2872, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tyurina Y, Vikulina A, Kapralova V, Winnica D, Sanders L, Greenamyre T, Tyurin V, and Kagan V. Oxidized cardiolipins as a biomarker of mitochondrial dysfunction triggered by pesticide, rotenone. Suppl Toxicol Sci 138: 398, 2014 [Google Scholar]
- 88.Tyurina YY, Shvedova AA, Kawai K, Tyurin VA, Kommineni C, Quinn PJ, Schor NF, Fabisiak JP, and Kagan VE. Phospholipid signaling in apoptosis: peroxidation and externalization of phosphatidylserine. Toxicology 148: 93–101, 2000 [DOI] [PubMed] [Google Scholar]
- 89.Tyurina YY, Tyurin VA, Epperly MW, Greenberger JS, and Kagan VE. Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic Biol Med 44: 299–314, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Tyurina YY, Tyurin VA, Kapralova VI, Amoscato AA, Epperly MW, Greenberger JS, and Kagan VE. Mass-spectrometric characterization of phospholipids and their hydroperoxide derivatives in vivo: effects of total body irradiation. Methods Mol Biol 580: 153–183, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Tyurina YY, Tyurin VA, Kaynar AM, Kapralova VI, Wasserloos K, Li J, Mosher M, Wright L, Wipf P, Watkins S, Pitt BR, and Kagan VE. Oxidative lipidomics of hyperoxic acute lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Am J Physiol Lung Cell Mol Physiol 299: L73–L85, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tyurina YY, Kisin ER, Murray A, Tyurin VA, Kapralova VI, Sparvero LJ, Amoscato AA, Samhan-Arias AK, Swedin L, Lahesmaa R, Fadeel B, Shvedova AA, and Kagan VE. Global phospholipidomics analysis reveals selective pulmonary peroxidation profiles upon inhalation of single-walled carbon nanotubes. ACS Nano 5: 7342–7353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tyurina YY, Tyurin VA, Kapralova VI, Wasserloos K, Mosher M, Epperly MW, Greenberger JS, Pitt BR, and Kagan VE. Oxidative lipidomics of gamma-radiation-induced lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Radiat Res 175: 610–621, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tyurina YY, Poloyac SM, Tyurin VA, Kapralov AA, Jiang JF, Anthonymuthu TS, Kapralova VI, Vikulina AS, Jung MY, Epperly MW, Mohammadyani D, Klein-Seetharaman J, Jackson TC, Kochanek PM, Pitt BR, Greenberger JS, Vladimirov YA, Bayir H, and Kagan VE. A mitochondrial pathway for biosynthesis of lipid mediators. Nat Chem 6: 542–552, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ursini F, Maiorino M, and Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta 839: 62–70, 1985 [DOI] [PubMed] [Google Scholar]
- 96.van Vliet AR, Verfaillie T, and Agostinis P. New functions of mitochondria associated membranes in cellular signaling. Biochim Biophys Acta 1843: 2253–2262, 2014 [DOI] [PubMed] [Google Scholar]
- 97.Vance JE. and Tasseva G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim Biophys Acta 1831: 543–554, 2013 [DOI] [PubMed] [Google Scholar]
- 98.Venditti P, Di Stefano L, and Di Meo S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 13: 71–82, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Weinkam P, Zimmermann J, Romesberg FE, and Wolynes PG. The folding energy landscape and free energy excitations of cytochrome c. Acc Chem Res 43: 652–660, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whited K, Baile MG, Currier P, and Claypool SM. Seven functional classes of Barth syndrome mutation. Hum Mol Genet 22: 483–492, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williamson P. and Schlegel RA. Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim Biophys Acta 1585: 53–63, 2002 [DOI] [PubMed] [Google Scholar]
- 102.Wipf P, Xiao J, Jiang J, Belikova NA, Tyurin VA, Fink MP, and Kagan VE. Mitochondrial targeting of selective electron scavengers: synthesis and biological analysis of hemigramicidin-tempo conjugates. J Am Chem Soc 127: 12460–12461, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Yano H, Baranov SV, Baranova OV, Kim J, Pan Y, Yablonska S, Carlisle DL, Ferrante RJ, Kim AH, and Friedlander RM. Inhibition of mitochondrial protein import by mutant huntingtin. Nat Neurosci 17: 822–831, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yin F, Sancheti H, and Cadenas E. Mitochondrial thiols in the regulation of cell death pathways. Antioxid Redox Signal 17: 1714–1727, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]