Abstract
To demonstrate that pregnancy-related complications are associated with alterations in placental microRNA expression. Gene expression of 15 C19MC microRNAs (miR-512-5p, miR-515-5p, miR-516-5p, miR-517-5p, miR-518b, miR-518f-5p, miR-519a, miR-519d, miR-519e-5p, miR-520a-5p, miR-520h, miR-524-5p, miR-525, miR-526a, and miR-526b) was assessed in placental tissues, compared between groups (21 gestational hypertension [GH], 63 preeclampsia, 36 fetal growth restriction [FGR], and 42 normal pregnancies), and correlated with the severity of the disease with respect to clinical signs, delivery date, and Doppler ultrasound parameters. The expression profile of microRNAs was different between pregnancy-related complications and controls. The downregulation of 4 of 15 (miR-517-5p, miR-519d, miR-520a-5p, and miR-525), 6 of 15 (miR-517-5p, miR-518f-5p, miR-519a, miR-519d, miR-520a-5p, and miR-525), and 11 of 15 (miR-515-5p, miR-517-5p, miR-518b, miR-518f-5p, miR-519a, miR-519d, miR-520a-5p, miR-520h, miR-524-5p, miR-525, and miR-526a) microRNAs was associated with GH, FGR, and preeclampsia, respectively. Sudden onset of severe preeclampsia requiring immediate termination of gestation and mild forms of preeclampsia (persisting for several weeks) were associated with similar microRNA expression profile (downregulation of miR-517-5p, miR-520a-5p, miR-524-5p, and miR-525). In addition, miR-519a was found to be associated with severe preeclampsia. The longer the pregnancy-related disorder lasted, the more extensive was the downregulation of microRNAs (miR-515-5p, miR-518b, miR-518f-5p, miR-519d, and miR-520h). The downregulation of some C19MC microRNAs is a common phenomenon shared between GH, preeclampsia, and FGR. On the other hand, some of the C19MC microRNAs are only downregulated just in preeclampsia.
Introduction
Preeclampsia (PE) and fetal growth restriction (FGR) are major complications affecting 2–10% of pregnancies responsible for maternal and perinatal morbidity and mortality (WHO, 1988; Bamfo and Odibo, 2011). Preeclampsia usually develops after 20 weeks of gestation and is characterized by chronic or gestational hypertension (GH) combined with proteinuria (ACOG practice bulletin, 2002), which results from defective placentation, eliciting inadequate uteroplacental blood perfusion and ischemia (Khong et al., 1986; Khan et al., 2006). The causes of preeclampsia and FGR remain unknown; however, preeclampsia is thought to be an implantation disorder (Miko et al., 2013). The actual hypothesis regarding the etiology of preeclampsia is based on the presumption that inadequate trophoblast invasion and placentation is linked to maladaptation of the local maternal immune response to paternal antigens expressed by extravillous cytotrophoblast at the fetal–maternal interface (Huppertz, 2008; Roberts and Hubel, 2009).
Recent evidence suggests that preeclampsia can be further subdivided into early PE (before 34 weeks of gestation), intermediate PE (between 34 and 37 weeks of gestation), and late PE (after 37 weeks of gestation) (Poon et al., 2010; Akolekar et al., 2011). The concepts of early and late PE are recent; it is widely accepted that these two entities have different etiologies and should be regarded as different forms of the disease, whereas early-onset PE and intrauterine growth restriction (IUGR) are considered placenta-mediated diseases (von Dadelszen et al., 2003; Huppertz, 2008; Valensise et al., 2008).
MicroRNAs belong to the family of small noncoding RNAs (18–25 nucleotides) that regulate gene expression at the post-transcriptional level by degrading or blocking translation of target messenger RNA (Lai, 2002; Bartel, 2004). MicroRNA analyses indicate that a variety of tissues display microRNA expression profiles that are significantly different from normal tissues (Calin and Croce, 2006), which may be useful for a wide range of applications in clinical diagnostics (Rosenfeld et al., 2008). Recent studies have shown that preeclampsia and FGR are associated with alterations in microRNA expression in the placenta (Pineles et al., 2007; Hu et al., 2009; Zhu et al., 2009; Enquobahrie et al., 2011; Maccani et al., 2011; Mayor-Lynn et al., 2011; Noack et al., 2011; Ishibashi et al., 2012; Higashijima et al., 2013). For example, miR-210, upregulated in placental tissues derived from patients with preeclampsia (Pineles et al., 2007; Zhu et al., 2009; Enquobahrie et al., 2011; Ishibashi et al., 2012), was shown to play a role in endothelial cell response to hypoxia, formation of capillary-like structures, vascular endothelial growth factor-driven cell migration, cell differentiation, and survival (Chan et al., 2012). A link between miR-155, also overexpressed in preeclamptic placentas (Pineles et al., 2007; Zhang et al., 2010), and the innate immune response has been suggested. Tili et al. (2007) reported that lipopolysaccharide (LPS) stimulation of macrophages resulted in the upregulation of miR-155 and TNF-α, which is one of the main cytokines involved in the response to LPS. Simultaneously, miR-155 was shown to function as an oncogene and as a common target of a broad range of inflammatory mediators, suggesting that miR-155-inducing signals use the c-Jun N-terminal kinase (JNK) pathway (O'Connell et al., 2007).
Patients with established preeclampsia also had increased levels of miR-181a in placental tissues (Hu et al., 2009; Zhu et al., 2009). T-cell sensitivity to antigen is intrinsically regulated during maturation to ensure proper development of immunity and tolerance. Li et al. (2007) showed that increasing miR-181a expression in mature T cells augmented the sensitivity to peptide antigens, while inhibiting miR-181a expression in immature T cells reduced sensitivity and impaired both positive and negative selection.
Most pregnancy-associated microRNAs have been shown to be encoded by genes located inside chromosome 19 miRNA clusters (C19MC and miR-371-3 cluster) or the chromosome 14 miRNA cluster (C14MC) (Seitz et al., 2004; Bentwich et al., 2005; Liang et al., 2007; Lin et al., 2010; Morales-Prieto et al., 2013).
In this study, we compared gene expression of C19MC microRNAs in normal and complicated pregnancies. Chromosome 19 microRNA cluster involves 46 microRNA genes altogether (Bentwich et al., 2005; Bortolin-Cavaillé et al., 2009; Lin et al., 2010; Morales-Prieto et al., 2013). The study preferentially used those microRNAs that were previously demonstrated to be exclusively expressed in placental tissues (miR-516-5p, miR-517-5p, miR-518b, miR-519a, miR-519d, miR-525, and miR-526b) and those microRNAs that were reported to be highly expressed in placental tissues (miR-512-5p, miR-515-5p, miR-518f-5p, miR-519e-5p, miR-520a-5p, miR-520h, miR-524-5p, and miR-526a) (Hsu et al., 2008; Kotlabova et al., 2011; Hromadnikova et al., 2012).
To our knowledge, no study on C19MC microRNA expression in GH has been carried out. Our study also describes, for the first time, the expression of most members of C19MC microRNAs in preeclampsia (miR-512-5p, miR-515-5p, miR-516-5p, miR-517-5p, miR-519a, miR-519d, miR-520a-5p, miR-520h, miR-524-5p, and miR-526a) or FGR (miR-512-5p, miR-516-5p, miR-517-5p, miR-518f-5p, miR-519a, miR-519e-5p, miR-524-5p, and miR-526a).
The relationship between C19MC microRNA gene expression and identified risk factors for poorer perinatal outcome was analyzed. C19MC microRNA gene expression was analyzed in relation to the severity of the disease with respect to the degree of clinical signs (mild and severe preeclampsia), delivery date (before or after 34 weeks of gestation), and Doppler ultrasonography parameters (the pulsatility index [PI] in the umbilical artery [UA], the PI in the middle cerebral artery [MCA], and the cerebroplacental ratio [CPR]).
Doppler velocimetry of the placental and fetal circulations is currently the main tool used in clinical practice to identify patients at risk for or those with established ischemic placental disease. Doppler velocimetry is considered abnormal when the PIs of the UA and the MCA are >95th and <5th percentiles for a given gestational age. The CPR is calculated by simple division of the MCA by the UA PIs (Cruz-Martinez, 2009). Indeed, animal models have demonstrated that this ratio is better correlated with hypoxia than its individual components (Arbeille et al., 1995).
Consecutively, the comprehensive online databases for miRNA target prediction and functional annotations were used to collect predicted targets of aberrantly expressed C19MC microRNAs in patients with established pregnancy-related complications; the main subjects of interest were predicted targets that were closely related to regulation of the immune system and the inflammatory response.
Materials and Methods
Patients
The study was retrospective. The studied cohort consisted of 162 consecutive Caucasian pregnant women involving 63 pregnancies with clinically established preeclampsia (PE), 36 pregnancies complicated by FGR, 21 patients with GH, and 42 normal pregnancies. Of the 63 patients with preeclampsia, 30 had symptoms of mild preeclampsia and 33 were diagnosed with severe preeclampsia. Twenty-two preeclamptic patients required delivery before 34 weeks of gestation, and 41 patients delivered after 34 weeks of gestation. Preeclampsia both occurred in previously normotensive patients (44 cases) and was superimposed on preexisting hypertension (19 cases). Eight growth-retarded fetuses were delivered before 34 weeks of gestation, and 28 were delivered after 34 weeks of gestation. Oligohydramnios or anhydramnios were present in 12 growth-restricted fetuses.
An examination of blood flow (Doppler ultrasonography) showed an abnormal PI in the UA (6 preeclampsia and 17 FGR) and/or in the MCA (1 preeclampsia and 8 FGR). The CPR, expressed as a ratio between the MCA and the UA PIs, was below the fifth percentile in nine cases (nine FGR). Absent or reversed end-diastolic velocity waveforms in the UA occurred in four cases (one preeclampsia and three FGR). The clinical characteristics of the normal and complicated pregnancies are presented in Table 1.
Table 1.
Clinical Characteristics of the Normal and Complicated Pregnancies
| Healthy pregnant women (n=42) | Preeclamptic patients (n=63) | FGR patients (n=36) | GH patients (n=21) | |
|---|---|---|---|---|
| Age (years) | 30.6 (4.4) | 31.7 (5.0) | 30.4 (5.5) | 29.4 (2.9) |
| Blood pressure (mmHg) | ||||
| Systolic | 121.6 (14.1) | 152.6 (14.2) | 123.1 (16.4) | 149.8 (9.2) |
| Diastolic | 75.1 (8.2) | 96.8 (8.9) | 79.0 (11.6) | 95.2 (9.8) |
| Proteinuria (g/24 h) | None | 2.7 (6.1) | None | None |
| Gestational age at delivery (weeks) | 39.0 (1.9) | 34.3 (4.3) | 35.9 (3.7) | 38.5 (1.6) |
| Pregnancy body mass index | 26.9 (3.2) | 29.6 (4.8) | 26.3 (3.8) | 31.5 (6.0) |
| Fetal birth weight (g) | 3440.7 (427.1) | 2030.6 (889.3) | 2111.3 (690.0) | 3173.7 (477.9) |
| Mode of delivery | ||||
| Vaginal | 36 (85.7%) | 9 (14.3%) | 8 (22.2%) | 15 (71.4%) |
| Cesarean section | 6 (14.3%) | 54 (85.7%) | 28 (77.8%) | 6 (28.6%) |
| Fetal sex | ||||
| Boy | 26 (61.9%) | 23 (36.5%) | 20 (55.6%) | 7 (33.3%) |
| Girl | 16 (38.1%) | 40 (63.5%) | 16 (44.4%) | 14 (66.7%) |
| Glucose status | ||||
| Normal | 38 (90.5%) | 60 (95.2%) | 36 (100%) | 21 (100%) |
| GDM/DM | 4 (9.5%) | 3 (4.8%) | 0 (0%) | 0 (0%) |
Data are presented as mean and (standard deviation) for continuous variables and as number (percent) for categorical variables.
FGR, fetal growth restriction; GDM/DM, gestational diabetes mellitus/diabetes mellitus; GH, gestational hypertension.
Women with normal pregnancies were defined as those without medical, obstetrical, or surgical complications at the time of the study and who subsequently delivered full-term, singleton healthy infants weighing >2500 g after 37 completed weeks of gestation. Preeclampsia was defined as blood pressure >140/90 mmHg in two determinations 4 h apart that was associated with proteinuria >300 mg/24 h after 20 weeks of gestation (ACOG practice bulletin, 2002). Severe preeclampsia was diagnosed by the presence of one or more of the following findings: (1) a systolic blood pressure >160 mmHg or a diastolic blood pressure >110 mmHg, (2) proteinuria greater than 5 g of protein in a 24-h sample, (3) very low urine output (less than 500 mL in 24 h), (4) signs of respiratory problems (pulmonary edema or cyanosis), (5) impairment of liver function, (6) signs of central nervous system problems (severe headache, visual disturbances), (7) pain in the epigastric area or right upper quadrant, (8) thrombocytopenia, and (9) the presence of severe FGR (ACOG practice bulletin, 2002).
FGR was diagnosed when the estimated fetal weight, calculated using the Hadlock formula (Astraia Software GmbH), was below the tenth percentile for the evaluated gestational age; adjustments were made for the appropriate population standards of the Czech Republic.
Patients with a complicated gestation demonstrating premature rupture of membranes, in utero infections, fetal anomalies or chromosomal abnormalities, and fetal demise in utero or stillbirth were excluded from the study.
All patients who participated in this study provided written informed consent. The study was approved by the Ethics Committee of the Third Faculty of Medicine, Charles University in Prague.
Processing of samples, preparation of microRNAs
Samples of placenta were collected at the Institute for the Care of Mother and Child and stored at −80°C until further processing.
Total RNA was extracted from 25 mg of placental tissue preserved in RNAlater (Ambion) followed by an enrichment procedure for small RNAs (siRNAs, microRNAs), according to the manufacturer's instructions using a mirVana microRNA Isolation kit (Ambion). To minimize DNA contamination, we treated the eluted RNA with 5 μL of DNase I (Fermentas International) for 30 min at 37°C. Using this novel approach, an RNA fraction highly enriched in RNA species <200 nt was obtained, whose concentration and quality was assessed using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). The A(260/280) absorbance ratio of isolated RNA was 1.8–2.0, demonstrating that the RNA fraction was pure and could be used for analysis. In addition, the A(260/230) ratio was greater than 1.6, demonstrating negligible contamination by polysaccharides.
Reverse transcriptase reaction using a stem-loop primer
Each of the 15 microRNAs (miR-512-5p, miR-515-5p, miR-516-5p, miR-517-5p, miR-518b, miR-518f-5p, miR-519a, miR-519d, miR-519e-5p, miR-520a-5p, miR-520h, miR-524-5p, miR-525, miR-526a, and miR-526b) was reverse transcribed into complementary DNA (cDNA) using a TaqMan MicroRNA Assay, containing microRNA-specific stem-loop reverse transcription (RT) primers (Table 2), and a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) in a total reaction volume of 32 μL, according to the manufacturer's instructions. Reverse transcriptase reactions were performed using a 7500 Real-Time PCR system (Applied Biosystems) with the following thermal cycling parameters: 30 min at 16°C, 30 min at 42°C, and 5 min at 85°C, and then held at 4°C. Finally, 20 ng of the RNA template was used for each RT reaction.
Table 2.
Characteristics of Selected C19MC microRNAs
| Assay name | miRBase ID | NCBI location chromosome | microRNA sequence | Expression in placenta |
|---|---|---|---|---|
| hsa-miR-512-5p | hsa-miR-512-5p | Chr.19: 54169933-54170016 [+] | 5′-CACUCAGCCUUGAGGGCACUUUC-3′ | High expression |
| hsa-miR-515-5p | hsa-miR-515-5p | Chr.19: 54182257-54182339 [+] | 5′-UUCUCCAAAAGAAAGCACUUUCUG-3′ | High expression |
| hsa-miR-516-5p | hsa-miR-516b-5p | Chr.19: 58920508-58920592 [+] | 5′-CAUCUGGAGGUAAGAAGCACUUU-3′ | Exclusively expressed |
| hsa-miR-517* | hsa-miR-517-5p | Chr.19: 54215522-54215608 [+] | 5′-CCUCUAGAUGGAAGCACUGUCU-3′ | Exclusively expressed |
| hsa-miR-518b | hsa-miR-518b | Chr.19: 54205991-54206073 [+] | 5′-CAAAGCGCUCCCCUUUAGAGGU-3′ | Exclusively expressed |
| hsa-miR-518f* | hsa-miR-518f-5p | Chr.19: 54203269-54203355 [+] | 5′-CUCUAGAGGGAAGCACUUUCUC-3′ | High expression |
| hsa-miR-519a | hsa-miR-519a-3p | Chr.19: 54255651-54255735 [+] | 5′-AAAGUGCAUCCUUUUAGAGUGU-3′ | Exclusively expressed |
| hsa-miR-519d | hsa-miR-519d | Chr.19: 54216601-54216688 [+] | 5′-CAAAGUGCCUCCCUUUAGAGUG-3′ | Exclusively expressed |
| hsa-miR-519e* | hsa-miR-519e-5p | Chr.19: 54183194-54183277 [+] | 5′-UUCUCCAAAAGGGAGCACUUUC-3′ | High expression |
| hsa-miR-520a* | hsa-miR-520a-5p | Chr.19: 54194135-54194219 [+] | 5′-CUCCAGAGGGAAGUACUUUCU-3′ | High expression |
| hsa-miR-520h | hsa-miR-520h | Chr.19: 54245766-54245853 [+] | 5′-ACAAAGUGCUUCCCUUUAGAGU-3′ | High expression |
| hsa-miR-524-5p | hsa-miR-524-5p | Chr.19: 54214256-54214342 [+] | 5′-CUACAAAGGGAAGCACUUUCUC-3′ | High expression |
| hsa-miR-525 | hsa-miR-525-5p | Chr.19: 54200787-54200871 [+] | 5′-CUCCAGAGGGAUGCACUUUCU-3′ | Exclusively expressed |
| hsa-miR-526a | hsa-miR-526a | Chr.19: 54209506-54209590 [+] | 5′-CUCUAGAGGGAAGCACUUUCU-3′ | High expression |
| hsa-miR-526b | hsa-miR-526b-5p | Chr.19: 54197647-54197729 [+] | 5′-CUCUUGAGGGAAGCACUUUCUGU-3′ | Exclusively expressed |
miRNAMap 2.0 designers themselves performed the quantitative polymerase chain reaction (Q-PCR) experiments for monitoring the expression profiles of 224 human miRNAs in 18 major normal tissues in humans, including placental tissues. The miRNAMap release 2.0 is now available at http://miRNAMap.mbc.nctu.edu.tw/ (Hsu et al., 2008).
Relative quantification of microRNAs by real-time polymerase chain reaction
Fifteen microliters of cDNA, corresponding to each selected microRNA, were mixed with specific primers and the TaqMan MGB probe (TaqMan MicroRNA Assay; Applied Biosystems), and the ingredients of the TaqMan Universal PCR Master Mix (Applied Biosystems) in a total reaction volume of 35 μL. TaqMan polymerase chain reaction (PCR) conditions were set as described in the TaqMan guidelines. The analysis was performed using a 7500 Real-Time PCR System. All polymerase chain reactions (PCRs) were performed in duplicate. Multiple negative controls such as no template control (NTC) (water instead of cDNA sample), no amplification control (NAC) (nontranscribed RNA samples), and genomic DNA (isolated from equal biological samples) did not generate any signals during PCR reactions. Each sample was considered positive if the amplification signal occurred before the 40th threshold cycle (Ct<40).
The expression of particular microRNA was determined using the comparative Ct method (Livak and Schmittgen, 2001) relative to synthetic Caenorhabditis elegans microRNA (cel-miR-39; Qiagen), which was used as an internal control for variations during the preparation of RNA, cDNA synthesis, and real-time PCR. Due to a lack of generally accepted standards, all experimental real-time quantitative real-time polymerase chain reaction (qRT-PCR) data were normalized to cel-miR-39, as it shows no sequence homology to any human microRNA. One microliter of 100 nM cel-miR-39 was spiked in after incubation with miRNA Homogenate Additive (a component of mirVana microRNA Isolation kit; Ambion) to the human placental samples.
RNA isolated from a randomly selected placenta derived from a normal gestation was chosen as a reference for each comparison. RNA that was highly enriched with small RNA, isolated from the fetal part of the placenta (the part of the placenta derived from the chorionic sac that encloses the embryo, consisting of the chorionic plate and villi), was used as a reference sample for relative quantification throughout the study. The difference (ΔCt) between the Ct values of particular microRNA and the internal control (cel-miR-39) was calculated for each sample. The comparative ΔΔCt calculation involved finding the difference between each sample's ΔCt and the reference's ΔCt. Finally, ΔΔCt values were transformed to absolute values using the formula 2−ΔΔCt. This distinctive approach allows long-term, large-scale analysis composed of multiple analyses performed at different periods.
Statistical analysis
Data normality was assessed using the Shapiro–Wilk test, which showed that our data followed a normal distribution. Therefore, microRNA levels were compared between groups using parametric tests. To test for a significant difference among multiple groups, the one-way analysis of variance and the post hoc Bonferronis test was applied. The Benjamini–Hochberg (BH) correction was applied to the normalized expression data to adjust for multiple comparisons in order to limit a false discovery rate (FDR). The significance level was established at an adjusted p-value (q*=0.022 for group comparison; q*=0.022 for comparison of preeclampsia severity with respect to clinical signs; q*=0.025 for comparison of preeclampsia severity with respect to delivery date; q* is the corrected significance level after Benjamini and Hochberg for multiple testing). Similarly, the two-tailed Students t-test was used for the comparisons between two groups. Again, if necessary, the FDR approach was used to correct for multiple comparisons (q*=0.0045 for comparison of disease severity with respect to Doppler ultrasonography of UA flow).
Data analysis was performed, and box plots were generated using Statistica software (version 9.0; StatSoft, Inc.). Each box encompasses the mean (dark horizontal line) of log-normalized gene expression values for microRNAs of interest in cohorts, one standard error above and below the mean in the box, and the 95% confidence interval in the bars (standard deviation). Outliers are indicated by circles, and extremes are indicated by asterisks.
C19MC microRNA target prediction
The predicted targets of studied C19MC microRNAs were collected from the miRDB database (http://mirdb.org/miRDB/) and the miRWalk database (www.umm.uni-heidelberg.de/apps/zmf/mirwalk). miRDB is an online database for miRNA target prediction and functional annotations. All the targets were predicted using MirTarget2 bioinformatics tool, which was developed by analyzing thousands of genes impacted by miRNAs with an SVM learning machine (Wang and El Naqa, 2008). miRDB hosts predicted miRNA targets in five species: human, mouse, rat, dog, and chicken.
The miRDB database is connected to the NCBI database (www.ncbi.nlm.nih.gov/gene), where the description of proteins encoded by predicted genes is provided. Comprehensive and systematic search for each predicted target of a particular C19MC microRNA, which have been shown to be downregulated in patients with the onset of GH, preeclampsia, and FGR in relation to the regulation of the immune system and inflammatory response, was made using the PubMed database (www.ncbi.nlm.nih.gov/pubmed).
Further, miRWalk database (www.umm.uni-heidelberg.de/apps/zmf/mirwalk) and the Validated Targets module were used to provide information on an experimentally verified interaction between appropriate microRNA and specific genes (Dweep et al., 2011). miRWalk is a comprehensive database that provides information on miRNA from human, mouse, and rat on their predicted as well as validated binding sites on their target genes.
Results
After correction for FDR (q*=0.043), overall, significantly decreased expression of miR-515-5p (F=3.893, df 3,160, p=0.01), miR-516-5p (F=3.135, df 3,160, p=0.027), miR-517-5p (F=10.532, df 3,160, p<0.001), miR-518b (F=3.950, df 3,160, p=0.009), miR-518f-5p (F=4.989, df 3,160, p=0.002), miR-519a (F=5.449, df 3,160, p=0.001), miR-519d (F=5.179, df 3,160, p=0.002), miR-519e-5p (F=3.316, df 3,160, p=0.021), miR-520a-5p (F=8.162, df 3,160, p<0.001), miR-520h (F=5.122, df 3,160, p=0.002), miR-524-5p (F=4.645, df 3,160, p=0.004), miR-525 (F=6.810, df 3,160, p<0.001), and miR-526a (F=4.377, df 3,160, p=0.006) was observed in women with pregnancy-related complications (GH, preeclampsia, and FGR) compared with all together with normal pregnancies. Bonferonni correction for multiple comparisons (q*=0.0033) confirmed downregulation of the following microRNA biomarkers (miR-517-5p, miR-518f-5p, miR-519a, miR-519d, miR-520a-5p, miR-520h, and miR-525).
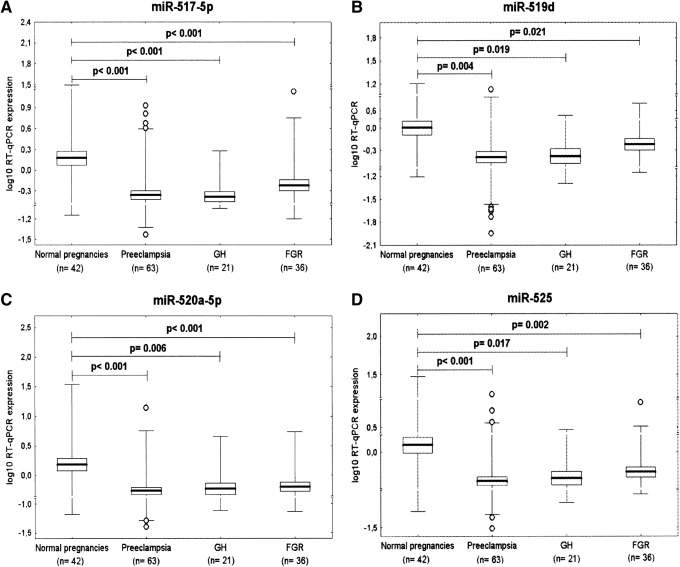
Downregulation of miR-517-5p, miR-519d, miR-520a-5p, and miR-525 is a common feature of GH, preeclampsia, and FGR
After an FDR adjustment for multiple testing (q*=0.022), it was found that the expression of miR-517-5p, miR-519d, miR-520a-5p, and miR-525 differed significantly between the control group and pregnancies affected with pregnancy complications. Lower expression rates were detected in patients with GH (miR-517-5p: 0.589±0.666 vs. 3.821±4.938, p<0.001; miR-519d: 0.671±0.772 vs. 2.423±3.454, p=0.019; miR-520a-5p: 1.066±1.609 vs. 4.320±6.586, p=0.006; miR-525: 0.901±1.872 vs. 4.089±6.931, p=0.017), preeclampsia (miR-517-5p: 0.873±1.452 vs. 3.821±4.938, p<0.001; miR-519d: 0.934±1.823 vs. 2.423±3.454, p=0.004; miR-520a-5p: 1.093±1.867 vs. 4.320±6.586, p<0.001; miR-525: 0.905±2.236 vs. 4.089±6.931, p<0.001), and FGR (miR-517-5p: 1.239±2.242 vs. 3.821±4.938, p<0.001; miR-519d: 0.959±0.974 vs. 2.423±3.454, p=0.021; miR-520a-5p: 1.061±1.075 vs. 4.320±6.586, p<0.001; miR-525: 0.904±1.352 vs. 4.089±6.931, p=0.002) (Fig. 1). Using the more stringent Bonferroni-corrected significance level for multiple comparison (q*=0.001), a significant decrease in miR-517-5p expression was confirmed for all three diagnoses.
FIG. 1.
Downregulation of (A) miR-517-5p, (B) miR-519d, (C) miR-520a-5p, and (D) miR-525 is a common feature of gestational hypertension, preeclampsia, and fetal growth restriction (FGR). GH, gestational hypertension.
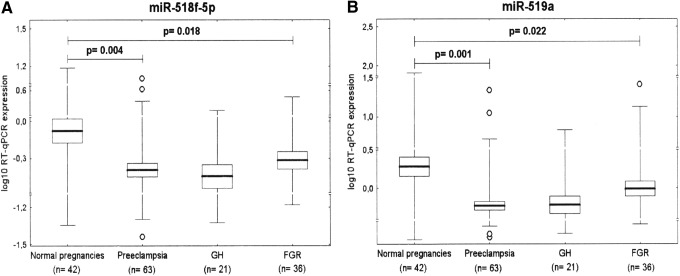
Downregulation of miR-518f-5p and miR-519a represents a common feature of preeclampsia and FGR
After FDR analysis (q*=0.022), a significant difference in miR-518f-5p and miR-519a expression was found between the control group and pregnancies affected with preeclampsia (0.798±1.643 vs. 2.262±3.475, p=0.004; 1.258±2.954 vs. 9.992±21.452, p=0.001) and FGR (0.793±0.872 vs. 2.262±3.475, p=0.018; 2.508±4.829 vs. 9.992±21.452, p=0.022) (Fig. 2).
FIG. 2.
Downregulation of (A) miR-518f-5p and (B) miR-519a represents a common feature of preeclampsia and FGR.
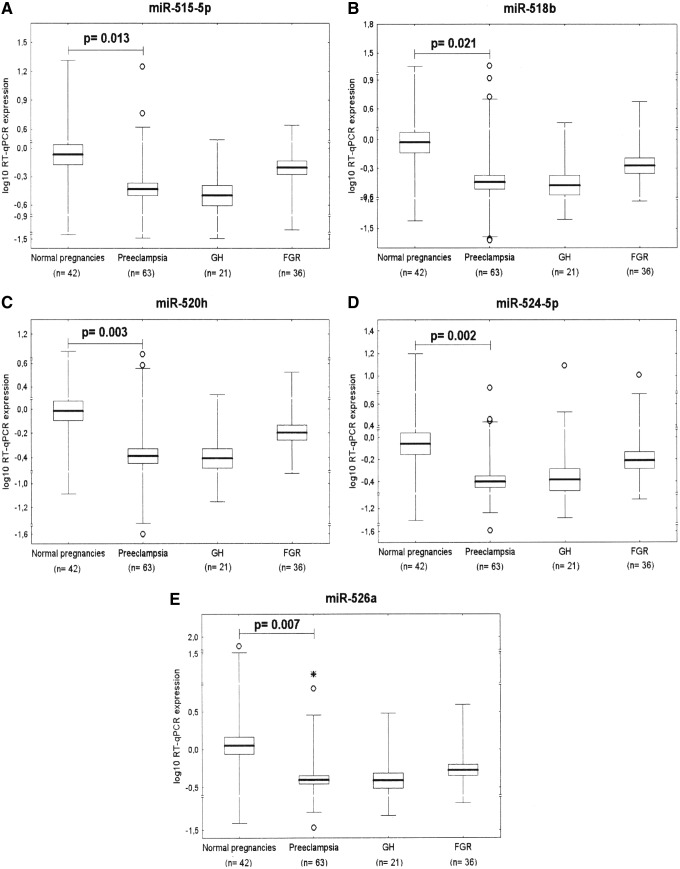
Downregulation of miR-515-5p, miR-518b, miR-520h, miR-524-5p, and miR-526a represents a unique feature of preeclampsia
After FDR correction (q*=0.022), statistical analysis revealed that placental expression of miR-515-5p (0.923±2.317 vs. 2.581±3.981, p=0.013), miR-518b (1.096±3.087 vs. 3.235±5.757, p=0.021), miR-520h (0.798±1.449 vs. 1.943±2.366, p=0.003), miR-524-5p (0.672±1.055 vs. 2.434±3.784, p=0.002), and miR-526a (0.832±2.122 vs. 5.939±14.978, p=0.007) differed significantly between preeclamptic and normal pregnancy groups (Fig. 3).
FIG. 3.
Downregulation of (A) miR-515-5p, (B) miR-518b, (C) miR-520h, (D) miR-524-5p, and (E) miR-526a represents a unique feature of preeclampsia.
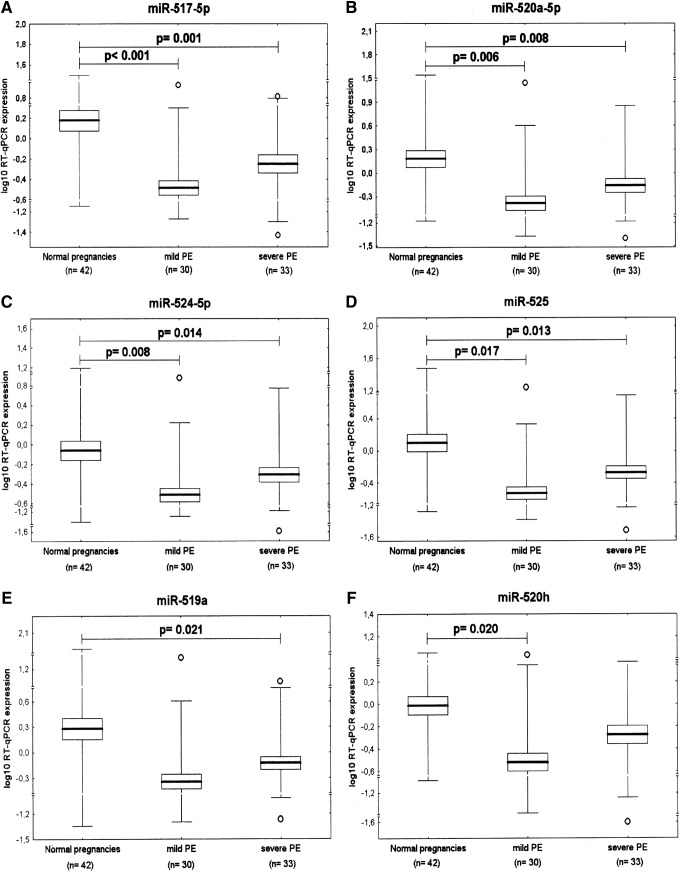
The association study of microRNA expression and the severity of preeclampsia with respect to clinical signs and delivery date
Gene expression of those C19MC microRNAs that were found to be downregulated in preeclampsia were analyzed in relation to the severity of the disease with respect to the degree of clinical signs (mild vs. severe preeclampsia) and delivery dates (before or after 34 weeks of gestation). When compared with normal pregnancies, using FDR analysis (q*=0.022), significant downregulation of four microRNAs was observed in both mild (miR-517-5p: 0.636±1.495 vs. 3.821±4.938, p<0.001; miR-520a-5p: 0.963±2.421 vs. 4.320±6.586, p=0.006, miR-524-5p: 0.572±1.343 vs. 2.434±3.784, p=0.008 and miR-525: 0.876±3.071 vs. 4.089±6.931, p=0.017) and severe preeclampsia (miR-517-5p: 1.076±1.383 vs. 3.821±4.938, p=0.001; miR-520a-5p: 1.205±1.192 vs. 4.320±6.586, p=0.008, miR-524-5p: 0.757±0.712 vs. 2.434±3.784, p=0.014 and miR-525: 0.929±1.094 vs. 4.089±6.931, p=0.013). Expression of miR-519a was significantly lower (1.266±1.866 vs. 9.992±21.452, p=0.021), but only in patients with severe preeclampsia. Similarly, decreased expression of miR-520h (0.690±1.887 vs. 1.943±2.366, p=0.020) was observed exclusively in patients with mild preeclampsia (Fig. 4).
FIG. 4.
The association study of microRNA expression and the severity of preeclampsia with respect to clinical signs shows downregulation of (A) miR-517-5p, (B) miR-520a-5p, (C) miR-524-5p, and (D) miR-525 in both, mild and severe PE, and significantly lower expression of (E) miR-519a in mild and (F) miR-520h in severe PE. PE, preeclampsia.
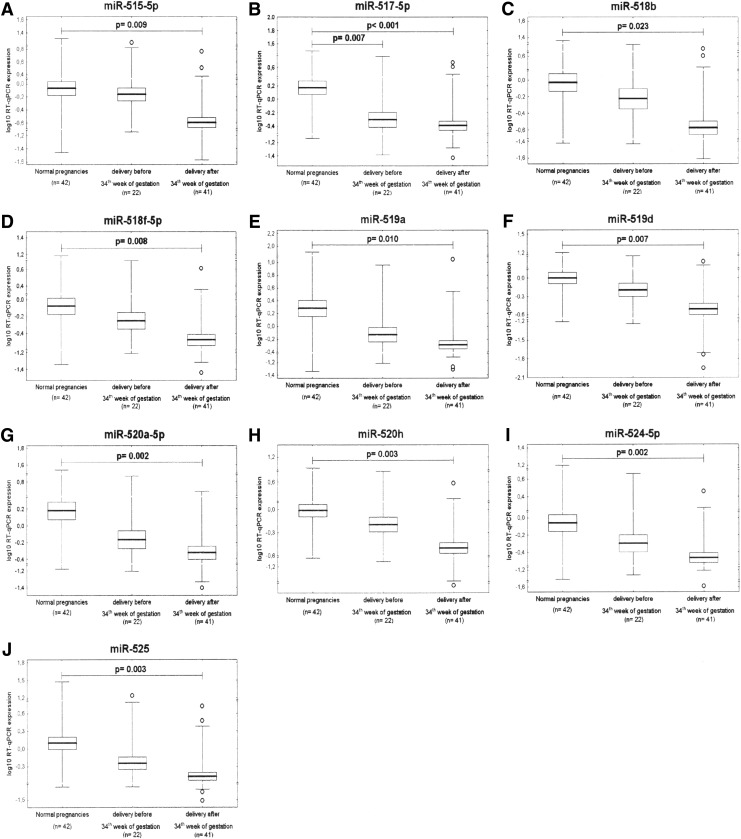
After FDR analysis (q*=0.025), while gene expression of C19MC microRNAs differed significantly between preeclamptic pregnancies delivering after 34 weeks of gestation and normal pregnancies (miR-515-5p: 1.613±3.483 vs. 2.581±3.981, p=0.009; miR-517-5p: 0.694±0.935 vs. 3.821±4.938, p<0.001; miR-518b: 0.622±1.373 vs. 3.235±5.757, p=0.023; miR-518f-5p: 0.547±0.710 vs. 2.262±3.475, p=0.008; miR-519a: 0.902±1.702 vs. 9.992±21.452, p=0.010; miR-519d: 0.637±0.941 vs. 2.423±3.454, p=0.007; miR-520a-5p: 0.856±0.995 vs. 4.320±6.586, p=0.002; miR-520h: 0.540±0.728 vs. 1.943±2.366, p=0.003; miR-524-5p: 0.488±0.503 vs. 2.434±3.784, p=0.002 and miR-525: 0.571±0.884 vs. 4.089±6.931, p=0.003), the difference between preeclamptic pregnancies requiring delivery before 34 weeks of gestation and controls was only observed for miR-517-5p (1.179±2.017 vs. 3.821±4.938, p=0.007) (Fig. 5).
FIG. 5.
The association study of microRNA expression and the severity of preeclampsia with respect to delivery date reveals downregulation of (A) miR-515-5p, (B) miR-517-5p, (C) miR-518b, (D) miR-518f-5p, (E) miR-519a, (F) miR-519d, (G) miR-520a-5p, (H) miR-520h, (I) miR-524-5p and (J) miR-525 in PE cases delivering after 34th week of gestation. Lower expression in PE cases requiring delivery before 34th week of gestation is evident only in (B) miR-517-5p.
However, microRNA gene expression levels did not differ between 17 pregnancies with severe preeclampsia that required delivery before 34 weeks of gestation and 23 mild preeclampsia patients delivering after 34 weeks of gestation (miR-515-5p: p=1.0; miR-517-5p: p=0.885; miR-518b: p=1.0; miR-518f-5p: p=1.0; miR-519a: p=1.0; miR-519d: p=1.0; miR-520a-5p: p=1.0; miR-520h: p=0.955; miR-524-5p: p=0.828 and miR-525: p=1.0). Similarly, no differences in microRNA expression were observed in 18 patients suffering from severe preeclampsia who delivered after 34 weeks of gestation compared with 23 patients with mild preeclampsia who delivered after 34 weeks of gestation (miR-517-5p: p=0.797; miR-518b: p=1.0; miR-518f-5p: p=1.0; miR-519a: p=1.0; miR-519d: p=1.0; miR-520a-5p: p=1.0; miR-520h: p=1.0; miR-524-5p: p=1.0 and miR-525: p=1.0). Since few patients with mild preeclampsia delivered before 34 weeks of gestation due to other reasons such as acute respiratory failure, they were excluded from mutual comparison.
In addition, the association between C19MC microRNA gene expression and the occurrence of previous hypertension in the group of patients with preeclampsia was determined. The statistical analyses revealed no difference between the group of preeclampsia superposed on chronic hypertension and/or GH and the group of patients with unexpected onset of preeclampsia (miR-515-5p: p=0.392, miR-517-5p: p=0.792, miR-518b: p=0.423, miR-518f-5p: p=0.348, miR-519a: p=0.336, miR-519d: p=0.370, miR-520a-5p: p=0.298, miR-520h: p=0.465, miR-524-5p: p=0.206, miR-525: p=0.437, and miR-526a: p=0.352).
The association study of microRNA expression and the severity of the disease with respect to Doppler ultrasonography monitoring
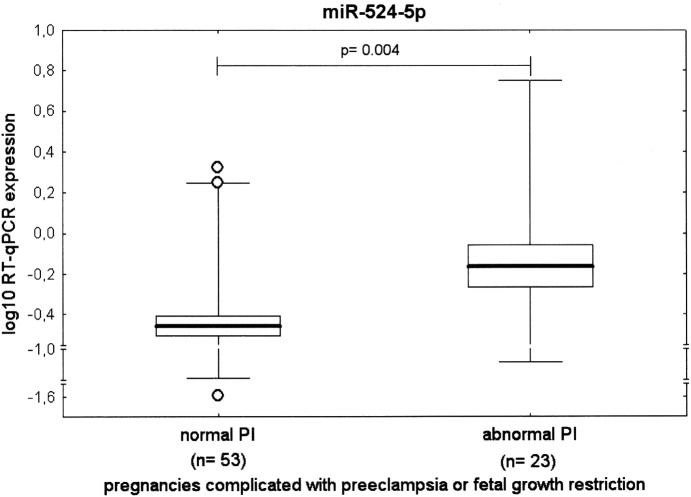
The association between gene expression of particular C19MC microRNAs (miR-515-5p, miR-517-5p, miR-518b, miR-518f-5p, miR-519a, miR-519d, miR-520a-5p, miR-520h, miR-524-5p, miR-525, and miR-526a) and Doppler ultrasonography parameters (the PI in the UA, the PI in the MCA, and the CPR) was analyzed in the cohort of pregnancies complicated with preeclampsia or FGR. The FDR approach (q*=0.0045) used to correct for multiple comparisons revealed the difference in 1 out of the 11 microRNAs tested (miR-524-5p, 0.472±0.394 vs. 1.216±1.676, p=0.0041) within the group of complicated pregnancies with normal and abnormal values of flow rate in the UA. Pregnancy-related complications with normal blood flow velocity waveforms showed significantly decreased expression of miR-524-5p compared with patients with abnormal values of flow rate in the UA (Fig. 6). Nevertheless, statistical analysis showed no effect of the PI in the MCA and the CPR on microRNA gene expression levels of any of the microRNAs identified to be downregulated in placental samples derived from patients with preeclampsia and/or FGR (miR-515-5p: p=0.661, p=0.724; miR-517-5p: p=0.721, p=0.380; miR-518b: p=0.820, p=0.522; miR-518f-5p: p=0.797, p=0.729; miR-519a: p=0.705, p=0.252; miR-519d: p=0.953, p=0.333; miR-520a-5p: p=0.836, p=0.491; miR-520h: p=0.982, p=0.841; miR-524-5p: p=0.485, p=0.653; miR-525: p=0.803, p=0.477; and miR-526a: p=0.926, p=0.290).
FIG. 6.
The association study of microRNA expression and the severity of the disease with respect to Doppler ultrasonography monitoring (the pulsatility index in the umbilical artery).
C19MC microRNA target prediction
The extensive file of predicted targets of all downregulated C19MC microRNAs in patients with established GH, preeclampsia, or FGR indicates that a large group of genes is potentially involved in the regulation of the immune system and the inflammatory response (Tables 3 and 4).
Table 3.
The List of Predicted Target Genes of Differentially Expressed C19MC microRNAs in Gestational Hypertension, Preeclampsia, and Fetal Growth Restriction in Relation to the Immune System and the Inflammatory Response
| microRNA | miR-515-5p | miR-517-5p | miR-518b | miR-518f-5p | miR-519a | miR-519d | miR-520a-5p | miR-520h | miR-524-5p | miR-525 | miR-526a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of predicted target genes | 571 | 239 | 30 | 215 | 685 | 844 | 486 | 462 | 916 | 482 | 215 |
| Unique target genes | API5 | FAS | CD5 | LILRA1 | BAG5 | BCL10 | ELF4 | DIDO1 | API5 | TOX | BCAP29 |
| CIAPIN1 | IL9R | PRDX6 | EGLN3 | CD69 | CASP2 | CCL7 | IFNAR1 | BCL10 | OLR1 | CD302 | |
| HSPA12A | IRAK3 | TOLLIP | FLT3 | CXCL12 | CD69 | SOCS3 | PIAS2 | CD276 | IGF1R | DNAJC21 | |
| HSPH1 | LILRA2 | TLR6 | DNAJB9 | CD84 | OLR1 | RFX4 | CD47 | TLR7 | HSP90AA1 | ||
| IL16 | MTDH | TNFRSF19 | FZD6 | DNAJB6 | IGF1R | TGFB2 | CD8A | ACVR2B | IGFBP1 | ||
| IL6R | PAPPA | CXCL11 | HIF1A | HSPA8 | TLR7 | THAP2 | HSPA1B | ATRN | TLR2 | ||
| TNFAIP8L1 | BCLAF1a (Yoshitomi et al.,2011) | TNFSF4 | HSPA13 | IL17RD | ACVR2B | TLR5 | IL1A | CD2 | TNFRSF19 | ||
| XIAP | IFNA1 | ADAM19 | HSPA8 | IL8 | ATRN | CD38 | IL1RL1 | CD300LB | TNFSF15 | ||
| CASP10 | IL6 | TLR2 | IER3IP1 | MCL1 | CD2 | SMAD6 | SMAD4 | CD46 | TRAF6 | ||
| CD46 | NFKB1 | ADAM9 | NFAT5 | PDCD1LG2 | CD300LB | TGFBR2 | SMURF2 | CD93 | |||
| ATRN | CASP10 | PDCD1LG2 | SMAD5 | CD46 | VEGFAa (Ye et al.,2008) | SOCS4 | HSF5 | ||||
| IL10RA | BCL2 | TNF | TGFBR2 | CD93 | HIF1A | TNFAIP1 | IL10RA | ||||
| TGFBR2 | TNFRSF10D | TNFAIP1 | HSF5 | PAFAH1B2 | CD46 | MMD2 | |||||
| BBC3 | TNFSF11 | IL10RA | BNIP3L | CD1A | PPARA | ||||||
| BCL2L2a (Huang et al.,2011) | BID | MMD2 | IRAK4 | IRAK2 | AHSA2 | ||||||
| CASP3a (Huang et al.,2011) | CPAMD8 | PPARA | SMAD2 | IRAK4 | |||||||
| CISH | PPARAa (Martinelli et al.,2010) | AHSA2 | SMAD3 | SMAD2 | |||||||
| FLT1 | TNFRSF1A | SMAD7 | SMAD3 | ||||||||
| BCL2a (Huang et al.,2011) | TP53a (Fornari et al., 2012) | ||||||||||
| BNIP3La (Ye et al.,2008) | F3 | ||||||||||
| IRAK2 | HIF1A | ||||||||||
| SMAD7 | PAFAH1B2 | ||||||||||
| TGFBR2 | BNIP3L | ||||||||||
| TNFRSF21 | IRAK4 | ||||||||||
| TOLLIP | SMAD7 | ||||||||||
| TNFRSF21 |
Experimentally verified targets according to miRWalk database.
Table 4.
The Role of Predicted Target Genes of Differentially Expressed C19MC microRNAs in the Pathogenesis of Preeclampsia, Fetal Growth Restriction, and Gestational Hypertension
| Gene official symbol | Gene full name | The role in the pathogenesis of pregnancy-related complications |
|---|---|---|
| VEGFA | Vascular endothelial growth factor A | VEGFA, a major factor in angiogenesis, binds to two tyrosine kinase receptors, VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1), and regulates endothelial cell proliferation, migration, vascular permeability, secretion, and other endothelial functions. Immunohistochemical studies have shown high expression of VEGFA in the placental villous structures of patients with preeclampsia (Dubova et al., 2013). |
| FLT1 | fms-related tyrosine kinase-1 (Flt-1), Vascular endothelial growth factor receptor 1 (VEGFR-1) | Placental overexpression of soluble fms-related tyrosine kinase-1 (sFLT1), an alternatively spliced form of VEGFR-1, specifically in the fetal-derived trophoblast cells, has been implicated as the underlying cause of preeclampsia (Maynard et al., 2003; Fan et al., 2014). Immunohistochemical studies show high expression of VEGFR-1 in the placental villous structures of patients with preeclampsia (Dubova et al.,2013). |
| PPARA | Peroxisome proliferator-activated receptor alpha | PPARs are involved in trophoblast invasion, placental development, parturition, and pregnancy-specific diseases. In clinical pregnancy-specific disorders, including preeclampsia, gestational diabetes, and intrauterine growth restriction, aberrant regulation of components of the PPAR system parallels dysregulation of metabolism, inflammation, and angiogenesis. The reduction of transcriptional activity observed in the sera of preeclamptic women has been seen with PPARα. The reduction in potential circulating PPAR activators has been observed weeks and sometimes months before the onset of maternal symptoms and a clinical diagnosis of PE. Partially characterized inflammatory, angiogenic, and metabolic disturbances in pregnancy-related diseases suggest that synthetic PPAR agonists may be of potential use in these conditions (Wieser et al., 2008). |
| HIF1A | Hypoxia-inducible factor 1, alpha subunit (basic helix-loop-helix transcription factor) | HIF-1 functions as a master regulator of cellular and systemic homeostatic response to hypoxia by activating transcription of many genes, including those involved in energy metabolism, angiogenesis, and apoptosis. Levels of HIF-1α in the placentas of preeclamptic patients have been found to be higher than the levels in placentas of healthy pregnant women (Tal, 2012; Sezer et al., 2013). |
| F3 | Coagulation factor III (thromboplastin, tissue factor) | Higher expression and/or release of plasma tissue factor (F3) from the placenta may contribute toward a pathological hypercoagulable state in preeclampsia patients (Teng et al., 2010). The F3 level in the preeclampsia group was significantly higher than that in the control group. |
| PAFAH1B2 | Platelet-activating factor acetylhydrolase IB, regulatory subunit 1 (45 kDa) | The PAFAH1B2 gene encodes the beta subunit of PAF-AH that inactivates PAF. Increased PAF-AH activity in trophoblasts from preeclamptic placenta has been detected (Gu et al., 2006). |
| OLR1 | Oxidized low-density lipoprotein (lectin-like) receptor 1 | The expression levels of oxidized low-density lipoprotein (lectin-like) receptor 1 (LOX-1 protein encoded by OLR1 gene) were significantly higher in the placental tissues of the early-onset preeclampsia than in normal pregnancies (Meng et al., 2008). |
| TP53 | Tumor protein p53 (p53) | Preeclampsia is characterized by exaggerated apoptosis of villous trophoblast of placental villi. p53, encoded by TP53 gene, and FAS play a central role in regulation of programmed cell death. Protein expression of p53 was significantly increased in pregnancies complicated by PE (Sharp et al., 2014). |
| TNFRSF6 | FAS receptor (FasR), also known as apoptosis antigen 1 (APO-1 or APT), cluster of differentiation 95 (CD95), or tumor necrosis factor receptor superfamily member 6 | Preeclampsia is characterized by exaggerated apoptosis of villous trophoblast of placental villi. Fas-mediated apoptosis may play a role in conditions related to abnormal placentation, such as preeclampsia (Neale and Mor, 2005). |
| CASP10 | Caspase-10, apoptosis-related cysteine peptidase | Caspase-10 is involved in apoptosis in the preeclamptic placenta.The expression of caspase-10 has been found to be significantly increased in full-term preeclamptic placentas (Han et al., 2006). |
| CASP3 | Caspase-3, apoptosis-related cysteine peptidase | Apoptotic marker caspase-3 is significantly increased in villous trophoblasts of patients with preeclampsia, HELLP syndrome, and IUGR, indicating increased placental apoptosis (Cali et al., 2013). Hypoxia induces apoptosis in trophoblastic cells accompanied by increased expression of Caspase-3 (Ishioka et al., 2006). |
| BCL2 | B-cell CLL/lymphoma 2 | This gene encodes an integral outer mitochondrial membrane protein that blocks the apoptotic death of some cells. Severe preterm FGR, with or without PE, are associated with increased expression of BCL2 in both the placenta and maternal blood. In preterm PE, but not FGR, there is increased placental expression of BCL-XL and BCL2, but only BCL2 is significantly increased in the maternal blood. Increased expression of genes of the intrinsic apoptosis pathway reflects the severity of FGR as determined by deteriorations in umbilical artery Doppler velocimetry. In severe early-onset FGR, there is increased expression of genes regulating intrinsic apoptosis in both the placenta and maternal blood (Whitehead et al., 2013). |
| BNIP3L | BCL2/adenovirus E1B 19 kDa interacting protein 3-like | A member of the BCL2/adenovirus E1B 19 kd-interacting protein (BNIP) family protects cells from virally induced cell death and interacts with E1B 19 kDa-like sequences of BCL2, another apoptotic protector. The pro-apoptotic proteins BNip3 and Nix are expressed in the human placenta. Pregnancies with placental dysfunction and hypertensive pregnancy disorders with different clinical manifestations are characterized by a significantly decreased placental expression of BNip3 and Nix proteins (Stepan et al., 2005). |
| AHSA2 | AHA1, activator of heat shock 90 kDa protein ATPase homolog 2 | The upregulation of Hsp90, found in placental tissues affected with mild preeclampsia (Hromadnikova et al., 2015), may be associated with downregulation of miR-520a* and miR-525. Both microRNAs have been predicted to regulate the expression of the AHSA2 gene, which is an activator of Hsp90 protein ATPase homolog 2. |
| HSF5 | Heat shock transcription factory family member 5 | The upregulation of Hsp27, Hsp90, and HspBP1, found in placental tissues affected with mild preeclampsia (Hromadnikova et al., 2015), may be associated with downregulation of miR-520a* and miR-525. Both microRNAs have been predicted to regulate the expression of HSF5 gene, a transcriptional activator of heat shock genes. |
| HSP90AA1 | Heat shock protein 90 kDa alpha (cytosolic), class A member 1 (Hsp90) | The upregulation of Hsp90, found in placental tissues affected with mild preeclampsia (Hromadnikova et al., 2015), may be associated with downregulation of miR-526a, which has been predicted to regulate expression of the HSP90AA1 gene encoding heat shock protein 90 (Hsp90), a molecular chaperon essential for activating many signaling proteins in the eukaryotic cells. |
| IGFBP1 | Insulin-like growth factor-binding protein 1 (IBP-1), also known as placental protein 12 (PP12) | The expression of IGFBP-1 in hypertensive disorder complicating pregnancy group was higher than that in the control group. Its expression in mild and severe preeclampsia patients was higher than that in control group (Shang et al., 2005). |
| TLR2 | Toll-like receptor 2 | TLRs may modulate the innate immune response of the maternal syndrome of preeclampsia and might trigger the differential inflammatory response existing between early-onset preeclampsia and normotensive IUGR. Women with preclampsia had increased TLR2 mRNA and protein expressions in peripheral blood compared with other groups (Xie et al., 2010). Another study demonstrated the associations between the presence of periodontal pathogens and the increased expression of TLR-2 in the placental tissue of patients with hypertensive disorders compared with the placentas of healthy normotensive patients (Chaparro et al., 2013). TLR2 engagement by the synthetic lipoprotein FSL-1 enhances pro-inflammatory and pro-labor mediators in human fetal membranes and myometrium via MyD88/TRAF6/NF-κB (Lim et al., 2014). |
| TLR5 | Toll-like receptor 5 | TLR5 engagement by bacterial flagellin enhances pro-inflammatory and pro-labor mediators in human fetal membranes and myometrium via MyD88/TRAF6/NF-κB (Lim et al., 2014). |
| TLR7 | Toll-like receptor 7 | RNA-mediated activation of TLR7/8 plays a key role in the development of PE. TLR7/8 activation by single-stranded RNA expressed by viruses and/or released from necrotic cells initiates a pro-inflammatory immune response. Placental immunoreactivity and mRNA levels of TLR7 and TLR8 were significantly increased in women with PE compared with normotensive women (Chatterjee et al., 2012). |
| TNFSF15 | Tumor necrosis factor (ligand) superfamily, member 15 | Inflammatory processes are present during preeclampsia and in normal pregnancies. Maternal inflammatory reactions may change toward the end of the term. The study evaluating genome signaling in peripheral blood during preeclampsia and toward the end of the term using microarrays revealed upregulation of TNFSF15 (Dahlstrøm et al., 2010). |
| IL16 | Interleukin 16 | The upregulation of the IL-16 profile in both maternal and placental systems in PE was demonstrated, suggesting that IL-16 could be an important cytokine engaged in the altered immune system and exaggerated inflammatory response in PE syndrome (Gu et al., 2008). |
| CXCL12 | Chemokine (C-X-C motif) ligand 12 | CXCL12 expression is significantly greater in the placenta of preeclamptic women compared with normal women. This may represent part of a compensatory mechanism associated with preeclampsia (Hwang et al., 2012). |
| TNF | Tumor necrosis factor | The ischemic placenta releases cytokines such as TNF-α, which causes maternal endothelial dysfunction (Lamarca, 2012). Expression of TNF-α in placental tissues and maternal sera was significantly increased in preeclamptic patients, as well as in those with IUGR. TNF-α is mainly expressed in trophoblasts, vascular endothelial cells, decidual cells, and the stroma of the stem villi. The findings suggest that an increased concentration of TNF-alpha in the maternal and umbilical serum plays a significant role in the pathogenesis of preeclampsia (Tosun et al., 2010; Zhou et al., 2012). |
| IL6 | Interleukin 6 | Decidual cells from preeclamptic patients exhibit high IL6 production (Lockwood et al., 2008). Interestingly, a study showing increased expression of IL6 mRNA together with reduced frequency of regulatory macrophages in decidual tissue of women who later progressed to develop pregnancy-induced hypertension suggests that this disturbance originates during the first trimester, before overt symptoms appear (Prins et al., 2012). One function of IL6 in influencing the progression of preeclampsia may be exerted at the level of vascular activation. IL6 production in endothelial cells is elevated after endothelial cells phagocytose necrotic trophoblast debris, and it is implicated in spreading the vascular activation response (Chen et al., 2009). Elevated circulating IL6 in preeclamptic women may exacerbate and amplify this response, by acting on trophoblasts to promote the excessive shedding of necrotic debris considered to underpin this disease (Chen et al., 2010). Other findings also suggest that increased concentrations of IL-6 in maternal and umbilical serum play a significant role in pathogenesis of preeclampsia (Tosun et al., 2010). |
| IL6R | Interleukin 6 receptor | Placentas of preeclamptic patients produce less sIL6R, but more gp130 (IL-6 receptor subunit beta) than placentas of normal pregnant patients (Wilczyński et al., 2002). |
| IL8 | Interleukin 8 | The findings suggest that an increased concentration of IL-8 in the maternal and umbilical serum plays a significant role in pathogenesis of preeclampsia (Tosun et al., 2010). IL-8 gene expression is also altered in first-trimester trophoblasts derived from women developing PE later (Farina et al., 2011). |
| CD46 | CD46 molecule, complement regulatory protein | CD46 has cofactor activity for inactivation of complement components C3b and C4b by serum factor I. The activation of neutrophils and monocytes takes place during the uteroplacental passage in preeclamptic pregnancies. Such a local inflammatory response involving enhanced leukocyte/endothelial interaction may contribute to the pathogenesis of this disorder. Increased expression of adhesion molecules and complement-related markers (CD46 and CD59) on neutrophils and monocytes has been detected (Mellembakken et al., 2002). |
| SMAD2 | SMAD family member 2 | Smad2 expression, the molecule involved in TGF-β1 signaling in the normal villous tree, is enhanced in placental tissues derived from severe fetal growth restriction—preeclampsia cases characterized by early onset and absent end diastolic velocities in the umbilical arteries (Todros et al., 2007). |
| BID | BH3-interacting domain death agonist | Hypoxia induces apoptosis in trophoblastic cells accompanied by decreased expression of Bid (Ishioka et al., 2006). |
| TRAF6 | TNF receptor-associated factor 6, E3 ubiquitin protein ligase | TLR2 engagement by the synthetic lipoprotein FSL-1 enhances pro-inflammatory and pro-labor mediators in human fetal membranes and myometrium via MyD88/TRAF6/NF-κB (Lim et al., 2014). |
| IGF1R | Insulin-like growth factor 1 receptor | A significant association between newborn birth weight and placental IGF-1R number may indicate that IGF-1R is associated with fetal growth. Indeed, the reported decrease of IGF-1R mRNA and protein in intrauterine growth retardation in rats supports this affirmation (Reid et al., 2002). On the other hand, the absence of a significant correlation between blood pressure and the IGF-1R characteristics suggests that hypertension is a pathological condition that does not significantly affect the affinity or number of placental IGF-1 receptors (Díaz et al., 2005). |
| PRDX6 | Peroxiredoxin 6 | Downregulation of Prdx6 proteins in human and rat IUGR group placentas may have led to the formation of oxidative stress that may have impaired proliferation and invasion of cytotrophoblasts (Acar et al., 2014). |
| HSPA8 | Heat shock 70 kDa protein 8, also known as heat shock cognate 71 kDa protein or Hsc70 or Hsp73 | Downregulation of Hsc70 in placentas might play an important role in the pathogenesis of PE (Gharesi-Fard et al., 2010). |
| MCL1 | Myeloid cell leukaemia 1 | MiR-29b induces apoptosis and inhibits invasion and angiogenesis of trophoblast cells. Additional studies have confirmed that miR-29b regulates the expression of MCL1 (Li et al., 2013). |
PAF-AH, platelet-activating factor acetylhydrolase; PE, preeclampsia; PPARs, peroxisome proliferator-activated receptors.
Discussion
Gene expression of C19MC microRNAs was compared between normal and complicated pregnancies. We focused on those miRNAs that were exclusively expressed in placental tissues (miR-516-5p, miR-517-5p, miR-518b, miR-519a, miR-519d, miR-525, and miR-526b) and those with high expression levels in placental tissues (miR-512-5p, miR-515-5p, miR-518f-5p, miR-519e-5p, miR-520a-5p, miR-520h, miR-524-5p, and miR-526a). These choices were based on the miRNAmap database (Hsu et al., 2008) and on the results of our previous research (Kotlabova et al., 2011; Hromadnikova et al., 2012).
Overall, the expression profile of 13 of the 15 C19MC microRNAs was different between complicated pregnancies and controls. With regard to individual pregnancy-related disorder subtypes, downregulation of C19MC microRNAs was most common in patients with preeclampsia and less common in GH.
While downregulation of only 4 out of 15 studied C19MC microRNAs was found in placental tissues derived from patients with GH (miR-517-5p, miR-519d, miR-520a-5p, and miR-525), placental tissues from pregnancies with clinically established preeclampsia showed decreased expression in 11 out of 15 C19MC microRNAs (miR-515-5p, miR-517-5p, miR-518b, miR-518f-5p, miR-519a, miR-519d, miR-520a-5p, miR-520h, miR-524-5p, miR-525, and miR-526a). In addition, 6 out of 15 placental-specific microRNAs were also identified to be associated with FGR pregnancies (miR-517-5p, miR-518f-5p, miR-519a, miR-519d, miR-520a-5p, and miR-525).
Our finding that miR-518b expression was decreased in preeclampsia is consistent with the observation of Guo et al. (2011), who also independently demonstrated significantly decreased miR-518b expression levels in preeclampsia compared with controls. A study by Mayor-Lynn et al. (2011) showed a 0.74-fold change, but without a significant difference between preeclampsia and normal pregnancies. On the other hand, studies by Zhu et al. (2009) and Xu et al. (2014) demonstrated upregulation of miR-518b in severe preeclampsia compared with pregnancies with normal gestation. The difference in the expression levels of placenta specific miR-518b might be explained by different experimental approaches. Zhu et al. (2009) performed the study mainly using tissue from the decidual side of the placenta (a collection of pooled tissue fragments derived from 10 randomly selected sites on the placenta) using microarray and real-time RT-PCR, with the experimental data normalized to U6snRNA. Nevertheless, Xu et al. (2014) observed upregulated expression of miR-518b in basal plates of severe preeclampsia placentas using qRT-PCR. However, our group focused on the analysis of microRNA gene expression in the area of the central cotyledon and experimental real-time qRT-PCR data were normalized to synthetic C. elegans microRNA (cel-miR-39). The placenta is divided into cotyledons, each supplied by a major branch of the UA, and drained by a major tributary of the umbilical vein. These vessels enter stem villi, which branch and re-branch, similar to a tree, to form microscopic terminal villi suspended within the intervillous space. Each cotyledon has several anchoring villi that extend into the decidua basalis and are anchored to it by syncytial cells and fibrin.
With regard to miR-525, our study produced similar findings to Guo et al. (2011), in which they reported significantly lower levels in preeclamptic placentas. Nevertheless, our data are inconsistent with the study of Ishibashi et al. (2012), who found miR-525 to be significantly upregulated in preeclamptic placentas. While Ishibashi et al. (2012) also revealed upregulation of miR-518f-5p, miR-526b, and miR-519e-5p in preeclamptic placentas, our study found downregulation of miR-518f-5p and no statistically significant change in miR-519e-5p and miR-526b gene expression levels. Ishibashi et al. combined large-scale profiling of miRNA expression using high-throughput sequencing with comprehensive quantitative analysis of miRNA expression with real-time PCR-based array analysis. However, they did not provide details regarding the location of placental tissue sampling.
Our data confirmed the preliminary data of Mayor-Lynn et al. (2011), who also found no difference in miR-526b expression between patients with preeclampsia and normal pregnancies. Mayor-Lynn et al. (2011) used microarray miRNA profiling followed by validation analysis using real-time PCR with normalization to RNU6B and 18SrRNA.
A unique study by Higashijima et al. (2013) identified significantly decreased expression of miR-515-5p, miR-518b, miR-519d, miR-520h, and miR-526b in idiopathic FGR pregnancies with an undefined cause of FGR. In conformity with Higashijima et al. (2013), our independent study also showed reduced expression of miR-519d in placentas affected with FGR; however, this downregulation was also present in preeclampsia and GH. Nevertheless, in our setting, miR-515-5p, miR-518b, and miR-520h were significantly lower, but just in placentas from preeclamptic pregnancies. Higashijima et al. (2013) also observed no change in gene expression of miR-520a-5p and miR-525 in placentas derived from fetal growth-restricted fetuses; however, we observed significantly decreased expression levels of miR-520a-5p and miR-525 in placentas affected by all of the currently studied pregnancy-related complications.
Higashijima et al. (2013) carried out the study on a larger group of patients by identifying aberrantly expressed microRNAs using next-generation sequencing analysis with subsequent confirmation from quantitative real-time RT-PCR. However, they performed only the quantitative microRNA measurement, and similar to most investigators, they did not specify the location of placental tissue sampling.
Based on the results of our study, we further studied the association between microRNA expression in placental tissues and the severity of the disease with respect to the degree of clinical signs, delivery date (before or after 34 weeks of gestation), and Doppler ultrasound examination. Placental-specific microRNA gene expression appeared to be linked to the sudden onset of severe clinical symptoms requiring urgent termination of pregnancy by Caesarean section, to avoid potentially serious maternal and perinatal outcomes. Our results showed that five C19MC microRNAs (miR-517-5p, miR-520a-5p, miR-524-5p, miR-525, and miR-519a) were dysregulated in severe preeclamptic pregnancies, and one C19MC microRNA (miR-517-5p) was altered in preeclampsia requiring termination before 34 weeks of gestation. These data suggest the involvement of these microRNAs in the pathogenesis of preeclampsia. Equally, mild forms of preeclampsia persisting for several weeks (carefully monitored from the onset of the disease until delivery) were observed to be associated with similar placental-specific microRNA expression profile (miR-517-5p, miR-520a-5p, miR-524-5p, miR-525, and miR-520h). Nevertheless, the longer the pregnancy-related disorder lasted, the more extensive the downregulation of placental-specific microRNAs appeared. This suggests that the dysregulation of some C19MC microRNAs (miR-515-5p, miR-518b, miR-518f-5p, and miR-519d) does not drive the pathological process itself, but could be reflective of a compensatory mechanism.
In addition, the analysis suggested that there was no relationship between microRNA expression and previous occurrence of hypertension in patients with established preeclampsia. Similarly, no substantial changes in microRNA gene expression in placental tissues, relative to Doppler ultrasonography parameters associated with poorer outcome in preeclampsia and/or FGR, were observed.
The decreased levels of C19MC microRNAs in placental tissues may lead to upregulation of relevant proteins involved in the direction of key biological pathways such as angiogenesis, stress response, apoptosis, and hemocoagulation. The occurrence of defective placental angiogenesis, inadequate uteroplacental blood perfusion, hypoxia, and ischemia can induce local stress responses, abnormal apoptosis of placental trophoblasts, and dysfunction of the blood coagulation-fibrinolysis system, and, finally, it can induce development of a generalized maternal systemic inflammatory response (Khong et al., 1986; Redman et al., 1999). Some predicted targets of C19MC microRNAs, which were a subject of interest in this study, have been previously shown to be upregulated in placental tissue samples derived from patients with pregnancy-related complications (e.g., sFLT-1, VEGFA, HIF-1α, PAF-AH, F3, LOX-1, CASP3, CASP10, p53, TLR2, TLR7, CD46, Hsp90, AHA1, IGFBP-1, TRAF6, IL-16, CXCL12, TNF-α, IL-6, IL-6R, IL-8, BCL-2, and SMAD2) (Tables 3 and 4). The role of predicted target genes of currently identified differentially expressed C19MC microRNAs in the pathogenesis of preeclampsia, FGR, and GH is discussed in detail in Table 4.
Nevertheless, the decreased levels of some proteins (e.g., Prdx6, Hsc70, MCL1, BID, IGF-1R, and BNip3) observed in placental tissues of patients with pregnancy-related complications, predicted to also be targeted by currently examined C19MC microRNAs, are contrary to the observed microRNA downregulation (Tables 3 and 4).
Although methods to comprehensively identify miRNAs that regulate individual genes of interest are currently available, pathways involving miRNAs are often complex regulatory networks, whose regulation is difficult to understand. In addition, it makes the direct interpretation of experimental data complicated. Many genes are targeted for repression by a high number of miRNAs, which seem to regulate those genes cooperatively (Schmitz et al., 2013).
We have previously identified C19MC microRNAs (miR-516-5p, miR-517-5p, miR-518b, miR-520a-5p, miR-520h, miR-525, and miR-526a) present in maternal plasma that can differentiate between normal pregnancies and nonpregnant individuals (Kotlabova et al., 2011). Our recent study also showed that the upregulation of circulating miR-516-5p, miR-517-5p, miR-520a-5p, miR-525, and miR-526a is a characteristic phenomenon of established preeclampsia (Hromadnikova et al., 2013). While plasma levels of microRNAs between the control cohort and the cohorts of patients with FGR and GH did not differ, increased levels were detected in the group of patients with established preeclampsia.
It seems that while the placental tissues are able to respond to various pregnancy-related disorders, including pregnancy-induced hypertension, FGR, and/or preeclampsia via downregulation of C19MC microRNAs (miR-517-5p, miR-520a-5p, miR-525, and miR-526a), upregulation of these particular microRNAs appears in maternal circulation, but only in cases of preeclampsia. This inconsistent finding may be explained in several ways. First, the expression of various biomarkers may vary in different placental zones as has been previously shown. For example, the expression of various biomarkers in the inner region of placenta can differ compared with the outer part of the tissue (Abdulsid et al., 2013). Furthermore, the expression may be different between a thrombus and an infarction (Wataba et al., 2004). In this study, we focused on the examination of microRNA levels in a specific area, that is, in the central cotyledon zone, where the umbilical cord is inserted into the chorionic plate. However, the placenta is being continuously remodeled during normal placental development, and extracellular nucleic acids of both fetal and placental origin, packed into either trophoblast-derived apoptotic bodies or shedding syncytiotrophoblast micro-particles, may be detected in maternal circulation during the course of normal gestation (Nelson, 1996; Oudejans et al., 2003; Huppertz and Kingdom, 2004; Orozco et al., 2006; Hromadnikova, 2012). It is obvious that the levels of circulating nucleic acids, mainly the levels of circulating placental-specific C19MC microRNAs, present in maternal circulation during gestation reflect the overall status of the placenta.
Conclusion
This study demonstrated that pregnancy-related complications were associated with alterations in placental microRNA expression. The study revealed that downregulation of some C19MC microRNAs is a common phenomenon shared between GH, preeclampsia, and FGR. On the other hand, the study pointed to the fact that some C19MC microRNAs are exclusively downregulated, but only in association with preeclampsia.
Acknowledgments
This work was exclusively supported by the Grant Agency of Czech Republic (no. 304/12/1352) and partially supported by the Charles University research program PRVOUK P32.
Disclosure Statement
No competing financial interests exist.
References
- Abdulsid A., Hanretty K., and Lyall F. (2013). Heat shock protein 70 expression is spatially distributed in human placenta and selectively upregulated during labor and preeclampsia. PLoS One 8, e54540. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Acar N., Soylu H., Edizer I., Ozbey O., Er H., Akkoyunlu G., Gemici B., and Ustunel I. (2014). Expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and peroxiredoxin 6 (Prdx6) proteins in healthy and pathologic placentas of human and rat. Acta Histochem 116, 1289–300 [DOI] [PubMed] [Google Scholar]
- ACOG Committee on Practise Bulletins-Obstetrics. (2002). Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol 99, 159–167 [DOI] [PubMed] [Google Scholar]
- Akolekar R., Syngelaki A., Sarquis R., Zvanca M., and Nicolaides K.H. (2011). Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenat Diagn 31, 66–74 [DOI] [PubMed] [Google Scholar]
- Arbeille P., Berson M., Blondeau B., Durand A., Bodard S., and Locatelli A. (1995). Quantification and monitoring of vascular resistance in the lower limbs by the Doppler method (animal model). Arch Mal Coeur Vaiss 88, 1029–1034 [PubMed] [Google Scholar]
- Bamfo J.E., and Odibo A.O. (2011). Diagnosis and management of fetal growth restriction. J Pregnancy 2011, 640715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- Bentwich I., Avniel A., Karov Y., Aharonov R., Gilad S., Barad O., Barzilai A., Einat P., Einav U., Meiri E., Sharon E., Spector Y., and Bentwich Z. (2005). Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet 37, 766–770 [DOI] [PubMed] [Google Scholar]
- Bortolin-Cavaillé M.L., Dance M., Weber M., and Cavaillé J. (2009). C19MC microRNAs are processed from introns of large Pol-II, non-protein-coding transcripts. Nucleic Acids Res 37, 3464–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali U., Cavkaytar S., Sirvan L., and Danisman N. (2013). Placental apoptosis in preeclampsia, intrauterine growth retardation, and HELLP syndrome: an immunohistochemical study with caspase-3 and bcl-2. Clin Exp Obstet Gynecol 40, 45–48 [PubMed] [Google Scholar]
- Calin G.A., and Croce C.M. (2006). MicroRNAs and chromosomal abnormalities in cancer cells. Oncogene 25, 6202–6210 [DOI] [PubMed] [Google Scholar]
- Chan Y.C., Banerjee J., Choi S.Y., and Sen C.K. (2012). miR-210: the master hypoxamir. Microcirculation 19, 215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro A., Blanlot C., Ramírez V., Sanz A., Quintero A., Inostroza C., Bittner M., Navarro M., and Illanes S.E. (2013). Porphyromonas gingivalis, Treponema denticola and toll-like receptor 2 are associated with hypertensive disorders in placental tissue: a case-control study. J Periodontal Res 48, 802–809 [DOI] [PubMed] [Google Scholar]
- Chatterjee P., Weaver L.E., Doersch K.M., Kopriva S.E., Chiasson V.L., Allen S.J., Narayanan A.M., Young K.J., Jones K.A., Kuehl T.J., and Mitchell B.M. (2012). Placental Toll-like receptor 3 and Toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice. PLoS One 7, e41884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.M., Liu B., Zhao H.B., Stone P., Chen Q., and Chamley L. (2010). IL-6, TNFalpha and TGFbeta promote nonapoptotic trophoblast deportation and subsequently causes endothelial cell activation. Placenta 31, 75–80 [DOI] [PubMed] [Google Scholar]
- Chen Q., Stone P., Ching L.M., and Chamley L. (2009). A role for interleukin-6 in spreading endothelial cell activation after phagocytosis of necrotic trophoblastic material: implications for the pathogenesis of pre-eclampsia. J Pathol 217, 122–130 [DOI] [PubMed] [Google Scholar]
- Cruz-Martinez R. (2009). The role of Doppler and placental screening. Best Pract Res Clin Obstet Gynaecol 23, 845–855 [DOI] [PubMed] [Google Scholar]
- Dahlstrøm B., Esbensen Y., Vollan H., Oian P., and Bukholm G. (2010). Genome profiles in maternal blood during early onset preeclampsia and towards term. J Perinat Med 38, 601–608 [DOI] [PubMed] [Google Scholar]
- Díaz E., Cárdenas M., Ariza A.C., Larrea F., and Halhali A. (2005). Placental insulin and insulin-like growth factor I receptors in normal and preeclamptic pregnancies. Clin Biochem 38, 243–247 [DOI] [PubMed] [Google Scholar]
- Dubova E.A., Pavlov K.A., Lyapin V.M., Shchyogolev A.I., and Sukhikh G.T. (2013). Vascular endothelial growth factor and its receptors in the placental villi of pregnant patients with pre-eclampsia. Bull Exp Biol Med 154, 792–795 [DOI] [PubMed] [Google Scholar]
- Dweep H., Sticht C., Pandey P., and Gretz N. (2011). miRWalk—database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform 44, 839–847 [DOI] [PubMed] [Google Scholar]
- Enquobahrie D.A., Abetew D.F., Sorensen T.K., Willoughby D., Chidambaram K., and Williams M.A. (2011). Placental microRNA expression in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 204, 178.e112–e121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Rai A., Kambham N., Sung J.F., Singh N., Petitt M., Dhal S., Agrawal R., Sutton R.E., Druzin M.L., Gambhir S.S., Ambati B.K., Cross J.C., and Nayak N.R. (2014). Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest 124, 4941–4952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina A., Zucchini C., De Sanctis P., Morano D., Sekizawa A., Purwosunu Y., Okai T., and Rizzo N. (2011). Gene expression in chorionic villous samples at 11 weeks of gestation in women who develop pre-eclampsia later in pregnancy: implications for screening. Prenat Diagn 31, 181–185 [DOI] [PubMed] [Google Scholar]
- Fornari F., Milazzo M., Chieco P., Negrini M., Marasco E., Capranico G., Mantovani V., Marinello J., Sabbioni S., Callegari E., Cescon M., Ravaioli M., Croce C.M., Bolondi L., and Gramantieri L. (2012). In hepatocellular carcinoma miR-519d is up-regulated by p53 and DNA hypomethylation and targets CDKN1A/p21, PTEN, AKT3 and TIMP2. J Pathol 227, 275–285. Verification was performed on HepG2, Hep3B, Huh-7, SNU398, SNU449, SNU182 and SNU475 cell lines [DOI] [PubMed] [Google Scholar]
- Gharesi-Fard B., Zolghadri J., and Kamali-Sarvestani E. (2010). Proteome differences of placenta between pre-eclampsia and normal pregnancy. Placenta 31, 121–125 [DOI] [PubMed] [Google Scholar]
- Gu Y., Burlison S.A., and Wang Y. (2006). PAF levels and PAF-AH activities in placentas from normal and preeclamptic pregnancies. Placenta 27, 744–749 [DOI] [PubMed] [Google Scholar]
- Gu Y., Lewis D.F., Deere K., Groome L.J., and Wang Y. (2008). Elevated maternal IL-16 levels, enhanced IL-16 expressions in endothelium and leukocytes, and increased IL-16 production by placental trophoblasts in women with preeclampsia. J Immunol 181, 4418–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Yang Q., Lu J., Li H., Ge Q., Gu W., Bai Y., and Lu Z. (2011). A comprehensive survey of miRNA repertoire and 3′ addition events in the placentas of patients with pre-eclampsia from high-throughput sequencing. PLoS One 6, e21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.Y., Kim Y.S., Cho G.J., Roh G.S., Kim H.J., Choi W.J., Paik W.Y., Rho G.J., Kang S.S., and Choi W.S. (2006). Altered gene expression of caspase-10, death receptor-3 and IGFBP-3 in preeclamptic placentas. Mol Cells 22, 168–174 [PubMed] [Google Scholar]
- Higashijima A., Miura K., Mishima H., Kinoshita A., Jo O., Abe S., Hasegawa Y., Miura S., Yamasaki K., Yoshida A., Yoshiura K., and Masuzaki H. (2013). Characterization of placenta-specific microRNAs in fetal growth restriction pregnancy. Prenat Diagn 33, 214–222 [DOI] [PubMed] [Google Scholar]
- Hromadnikova I. (2012). Extracellular nucleic acids in maternal circulation as potential biomarkers for placental insufficiency. DNA Cell Biol 31, 1221–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromadnikova I., Dvorakova L., Kotlabova K., Kestlerova A., Hympanova L., Novotna V., Doucha J., and Krofta L. (2015). Assessment of placental and maternal stress responses in patients with pregnancy related complications via monitoring of heat shock protein mRNA levels. Mol Biol Rep 42, 625–637 [DOI] [PubMed] [Google Scholar]
- Hromadnikova I., Kotlabova K., Doucha J., Dlouha K., and Krofta L. (2012). Absolute and relative quantification of placenta-specific micrornas in maternal circulation with placental insufficiency-related complications. J Mol Diagn 14, 160–167 [DOI] [PubMed] [Google Scholar]
- Hromadnikova I., Kotlabova K., Ondrackova M., Kestlerova A., Novotna V., Hympanova L., Doucha J., and Krofta L. (2013). Circulating C19MC microRNAs in preeclampsia, gestational hypertension, and fetal growth restriction. Mediators Inflamm 2013, 186041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S.D., Chu C.H., Tsou A.P., Chen S.J., Chen H.C., Hsu P.W., Wong Y.H., Chen Y.H., Chen G.H., and Huang H.D. (2008). miRNAMap 2.0: genomic maps of microRNAs in metazoan genomes. Nucleic Acids Res 36, D165–D169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Li P., Hao S., Liu L., Zhao J., and Hou Y. (2009). Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clin Chem Lab Med 47, 923–929 [DOI] [PubMed] [Google Scholar]
- Huang Y., Chuang A., Hao H., Talbot C., Sen T., Trink B., Sidransky D., and Ratovitski E. (2011). Phospho-ΔNp63α is a key regulator of the cisplatin-induced microRNAome in cancer cells. Cell Death Differ 18, 1220–1230. Verification was performed on head and neck squamous cell carcinoma cells isolated from primary tissue at the Department of Otolaryngology/Head and Neck Surgery of the Johns Hopkins University School of Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B. (2008). Placental origins of preeclampsia: challenging the current hypothesis. Hypertension 51, 970–975 [DOI] [PubMed] [Google Scholar]
- Huppertz B., and Kingdom J.C. (2004). Apoptosis in the trophoblast—role of apoptosis in placental morphogenesis. J Soc Gynecol Investig 11, 353–362 [DOI] [PubMed] [Google Scholar]
- Hwang H.S., Kwon H.S., Sohn I.S., Park Y.W., and Kim Y.H. (2012). Increased CXCL12 expression in the placentae of women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 160, 137–141 [DOI] [PubMed] [Google Scholar]
- Ishibashi O., Ohkuchi A., Ali M.M., Kurashina R., Luo S.S., Ishikawa T., Takizawa T., Hirashima C., Takahashi K., Migita M., Ishikawa G., Yoneyama K., Asakura H., Izumi A., Matsubara S., Takeshita T., and Takizawa T. (2012). Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: a novel marker for predicting preeclampsia. Hypertension 59, 265–273 [DOI] [PubMed] [Google Scholar]
- Ishioka S., Ezaka Y., Umemura K., Hayashi T., Endo T., and Saito T. (2006). Proteomic analysis of mechanisms of hypoxia-induced apoptosis in trophoblastic cells. Int J Med Sci 4, 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K.S., Wojdyla D., Say L., Gülmezoglu A.M., and van Look P.F. (2006). WHO analysis of causes of maternal death: a systematic review. Lancet 367, 1066–1074 [DOI] [PubMed] [Google Scholar]
- Khong T.Y., De Wolf F., Robertson W.B., and Brosens I. (1986). Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 93, 1049–1059 [DOI] [PubMed] [Google Scholar]
- Kotlabova K., Doucha J., and Hromadnikova I. (2011). Placental-specific microRNA in maternal circulation—identification of appropriate pregnancy-associated microRNAs with diagnostic potential. J Reprod Immunol 89, 185–191 [DOI] [PubMed] [Google Scholar]
- Lai E.C. (2002). Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet 30, 363–364 [DOI] [PubMed] [Google Scholar]
- Lamarca B. (2012). Endothelial dysfunction. An important mediator in the pathophysiology of hypertension during pre-eclampsia. Minerva Ginecol 64, 309–320 [PMC free article] [PubMed] [Google Scholar]
- Li P., Guo W., Du L., Zhao J., Wang Y., Liu L., Hu Y., and Hou Y. (2013). microRNA-29b contributes to pre-eclampsia through its effects on apoptosis, invasion and angiogenesis of trophoblast cells. Clin Sci (Lond) 124, 27–40 [DOI] [PubMed] [Google Scholar]
- Li Q.J., Chau J., Ebert P.J., Sylvester G., Min H., Liu G., Braich R., Manoharan M., Soutschek J., Skare P., Klein L.O., Davis M.M., and Chen C.Z. (2007). miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 129, 147–161 [DOI] [PubMed] [Google Scholar]
- Liang Y., Ridzon D., Wong L., and Chen C. (2007). Characterization of microRNA expression profiles in normal human tissues. BMC Genomics 8, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R., Barker G., and Lappas M. (2014). The TLR2 ligand FSL-1 and the TLR5 ligand Flagellin mediate pro-inflammatory and pro-labour response via MyD88/TRAF6/NF-κB-dependent signalling. Am J Reprod Immunol 71, 401–417 [DOI] [PubMed] [Google Scholar]
- Lin S., Cheung W.K., Chen S., Lu G., Wang Z., Xie D., Li K., Lin M.C., and Kung H.F. (2010). Computational identification and characterization of primate-specific microRNAs in human genome. Comput Biol Chem 34, 232–241 [DOI] [PubMed] [Google Scholar]
- Livak K.J., and Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Lockwood C.J., Yen C.F., Basar M., Kayisli U.A., Martel M., Buhimschi I., Buhimschi C., Huang S.J., Krikun G., and Schatz F. (2008). Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol 172, 1571–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani M.A., Padbury J.F., and Marsit C.J. (2011). miR-16 and miR-21 expression in the placenta is associated with fetal growth. PLoS One 6, e21210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli R., Nardelli C., Pilone V., Buonomo T., Liguori R., Castanò I., Buono P., Masone S., Persico G., Forestieri P., Pastore L., and Sacchetti L. (2010). miR-519d overexpression is associated with human obesity. Obesity (Silver Spring) 18, 2170–2176. Verification was performed on human embryonic kidney-293 cells and poietics primary human visceral preadipocytes [DOI] [PubMed] [Google Scholar]
- Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., Epstein F.H., Sukhatme V.P., and Karumanchi S.A. (2003). Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111, 649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor-Lynn K., Toloubeydokhti T., Cruz A.C., and Chegini N. (2011). Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reprod Sci 18, 46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellembakken J.R., Aukrust P., Olafsen M.K., Ueland T., Hestdal K., and Videm V. (2002). Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension 39, 155–160 [DOI] [PubMed] [Google Scholar]
- Meng T., Chen H.Y., Li J., and Shang T. (2008). Expression of lectin-liked oxidized low density lipoprotein receptor-1 and apoptosis associated genes in placenta and the relationship thereof with morbility of early-onset preeclampsia. Zhonghua Yi Xue Za Zhi 88, 2633–2635 [PubMed] [Google Scholar]
- Miko E., Meggyes M., Bogar B., Schmitz N., Barakonyi A., Varnagy A., Farkas B., Tamas P., Bodis J., Szekeres-Bartho J., Illes Z., and Szereday L. (2013). Involvement of Galectin-9/TIM-3 pathway in the systemic inflammatory response in early-onset preeclampsia. PLoS One 8, e71811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Prieto D.M., Ospina-Prieto S., Chaiwangyen W., Schoenleben M., and Markert U.R. (2013). Pregnancy-associated miRNA-clusters. J Reprod Immunol 97, 51–61 [DOI] [PubMed] [Google Scholar]
- Neale D.M., and Mor G. (2005).The role of Fas mediated apoptosis in preeclampsia. J Perinat Med 33, 471–477 [DOI] [PubMed] [Google Scholar]
- Nelson D.M. (1996). Apoptotic changes occur in syncytiotrophoblast of human placental villi where fibrin type fibrinoid is deposited at discontinuities in the villous trophoblast. Placenta 17, 387–391 [DOI] [PubMed] [Google Scholar]
- Noack F., Ribbat-Idel J., Thorns C., Chiriac A., Axt-Fliedner R., Diedrich K., and Feller A.C. (2011). miRNA expression profiling in formalin-fixed and paraffin-embedded placental tissue samples from pregnancies with severe preeclampsia. J Perinat Med 39, 267–271 [DOI] [PubMed] [Google Scholar]
- O'Connell R.M., Taganov K.D., Boldin M.P., Cheng G., and Baltimore D. (2007). MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A 104, 1604–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco A.F., Bischoff F.Z., Horne C., Popek E., Simpson J.L., and Lewis D.E. (2006). Hypoxia-induced membrane-bound apoptotic DNA particles: potential mechanism of fetal DNA in maternal plasma. Ann N Y Acad Sci 1075, 57–62 [DOI] [PubMed] [Google Scholar]
- Oudejans C.B., Tjoa M.L., Westerman B.A., Mulders M.A., van Wijk I.J., and van Vugt J.M. (2003). Circulating trophoblast in maternal blood. Prenatal Diagn 23, 111–116 [DOI] [PubMed] [Google Scholar]
- Pineles B.L., Romero R., Montenegro D., Tarca A.L., Han Y.M., Kim Y.M., Draghici S., Espinoza J., Kusanovic J.P., Mittal P., Hassan S.S., and Kim C.J. (2007). Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol 196, 261.e261–e266 [DOI] [PubMed] [Google Scholar]
- Poon L.C., Akolekar R., Lachmann R., Beta J., and Nicolaides K.H. (2010). Hypertensive disorders in pregnancy: screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound Obstet Gynecol 35, 662–670 [DOI] [PubMed] [Google Scholar]
- Prins J.R., Faas M.M., Melgert B.N., Huitema S., Timmer A., Hylkema M.N., and Erwich J.J. (2012). Altered expression of immune-associated genes in first-trimester human decidua of pregnancies later complicated with hypertension or foetal growth restriction. Placenta 33, 453–455 [DOI] [PubMed] [Google Scholar]
- Redman C.W., Sacks G.P., and Sargent I.L. (1999). Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 180, 499–506 [DOI] [PubMed] [Google Scholar]
- Reid G.J., Flozak A.S., and Simmons R.A. (2002). Placental expression of insulin-like growth factor receptor-1 and insulin receptor in the growth-restricted fetal rat. J Soc Gynecol Investig 9, 210–214 [PubMed] [Google Scholar]
- Roberts J.M., and Hubel C.A. (2009). The two stage model of preeclampsia: variations on the theme. Placenta 30 Suppl A, S32–S37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld N., Aharonov R., Meiri E., Rosenwald S., Spector Y., Zepeniuk M., Benjamin H., Shabes N., Tabak S., Levy A., Lebanony D., Goren Y., Silberschein E., Targan N., Ben-Ari A., Gilad S., Sion-Vardy N., Tobar A., Feinmesser M., Kharenko O., Nativ O., Nass D., Perelman M., Yosepovich A., Shalmon B., Polak-Charcon S., Fridman E., Avniel A., Bentwich I., Bentwich Z., Cohen D., Chajut A., and Barshack I. (2008). MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol 26, 462–469 [DOI] [PubMed] [Google Scholar]
- Schmitz U., Wolkenhauer O., and Vera J. (2013). MicroRNA Cancer Regulation: Advanced Concepts, Bioinformatics and Systems Biology Tools (Springer Science & Business Media, Berlin: ), ISBN 9400755902 [Google Scholar]
- Seitz H., Royo H., Bortolin M.L., Lin S.P., Ferguson-Smith A.C., and Cavaillé J. (2004). A large imprinted microRNA gene cluster at the mouse Dlk1-Gtl2 domain. Genome Res 14, 1741–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezer S.D., Küçük M., Döger F.K., Yüksel H., Odabaşi A.R., Türkmen M.K., Cakmak B.Ç., Ömürlü I.K., and Kinaş M.G. (2013). VEGF, PIGF and HIF-1α in placentas of early- and late-onset pre-eclamptic patients. Gynecol Endocrinol 29, 797–800 [DOI] [PubMed] [Google Scholar]
- Shang L.X., Wang J., Zhang L.J., Gao H., Qu D.Y., and Wang J.H. (2005). Relationship between changes of insulin like growth factor-1 and insulin like growth factor binding protein-1 in maternal serum and placenta and pathogenesis of hypertensive disorder complicating pregnancy. Zhonghua Fu Chan Ke Za Zhi 40, 516–520 [PubMed] [Google Scholar]
- Sharp A.N., Heazell A.E., Baczyk D., Dunk C.E., Lacey H.A., Jones C.J., Perkins J.E., Kingdom J.C., Baker P.N., and Crocker I.P. (2014). Preeclampsia is associated with alterations in the p53-pathway in villous trophoblast. PLoS One 9, e87621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepan H., Leo C., Purz S., Höckel M., and Horn L.C. (2005). Placental localization and expression of the cell death factors BNip3 and Nix in preeclampsia, intrauterine growth retardation and HELLP syndrome. Eur J Obstet Gynecol Reprod Biol 122, 172–176 [DOI] [PubMed] [Google Scholar]
- Tal R. (2012). The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol Reprod 87, 134. [DOI] [PubMed] [Google Scholar]
- Teng Y., Jiang R., Lin Q., Ding C., and Ye Z. (2010). The relationship between plasma and placental tissue factor, and tissue factor pathway inhibitors in severe pre-eclampsia patients. Thromb Res 126, e41–e45 [DOI] [PubMed] [Google Scholar]
- Tili E., Michaille J.J., Cimino A., Costinean S., Dumitru C.D., Adair B., Fabbri M., Alder H., Liu C.G., Calin G.A., and Croce C.M. (2007). Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 179, 5082–5089 [DOI] [PubMed] [Google Scholar]
- Todros T., Marzioni D., Lorenzi T., Piccoli E., Capparuccia L., Perugini V., Cardaropoli S., Romagnoli R., Gesuita R., Rolfo A., Paulesu L., and Castellucci M. (2007). Evidence for a role of TGF-beta1 in the expression and regulation of alpha-SMA in fetal growth restricted placentae. Placenta 28, 1123–1132 [DOI] [PubMed] [Google Scholar]
- Tosun M., Celik H., Avci B., Yavuz E., Alper T., and Malatyalioğlu E. (2010). Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-alpha in normal pregnancies and in pregnancies complicated by preeclampsia. J Matern Fetal Neonatal Med 23, 880–886 [DOI] [PubMed] [Google Scholar]
- Valensise H., Vasapollo B., Gagliardi G., and Novelli G.P. (2008). Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension 52, 873–880 [DOI] [PubMed] [Google Scholar]
- von Dadelszen P., Magee L.A., and Roberts J.M. (2003). Subclassification of preeclampsia. Hypertens Pregnancy 22, 143–148 [DOI] [PubMed] [Google Scholar]
- Wang X., and El Naqa I.M. (2008). Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics 24, 325–332 [DOI] [PubMed] [Google Scholar]
- Wataba K., Saito T., Takeuchi M., Nakayama M., Suehara N., and Kudo R. (2004). Changed expression of heat shock proteins in various pathological findings in placentas with intrauterine fetal growth restriction. Med Electron Microsc 37, 170–176 [DOI] [PubMed] [Google Scholar]
- Whitehead C.L., Walker S.P., Lappas M., and Tong S. (2013). Circulating RNA coding genes regulating apoptosis in maternal blood in severe early onset fetal growth restriction and pre-eclampsia. J Perinatol 33, 600–604 [DOI] [PubMed] [Google Scholar]
- WHO (1988) Geographic variation in the incidence of hypertension in pregnancy. World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Am J Obstet Gynecol 158, 80–83 [PubMed] [Google Scholar]
- Wieser F., Waite L., Depoix C., and Taylor R.N. (2008). PPAR Action in Human Placental Development and Pregnancy and Its Complications. PPAR Res 2008, 527048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczyński J.R., Tchórzewski H., Głowacka E., Banasik M., Lewkowicz P., Szpakowski M., Zeman K., and Wilczyński J. (2002). Cytokine secretion by decidual lymphocytes in transient hypertension of pregnancy and pre-eclampsia. Mediators Inflamm 11, 105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F., Hu Y., Turvey S.E., Magee L.A., Brunham R.M., Choi K.C., Krajden M., Leung P.C., Money D.M., Patrick D.M., Thomas E., and von Dadelszen P. (2010). Toll-like receptors 2 and 4 and the cryopyrin inflammasome in normal pregnancy and pre-eclampsia. BJOG 117, 99–108 [DOI] [PubMed] [Google Scholar]
- Xu P., Zhao Y., Liu M., Wang Y., Wang H., Li Y.X., Zhu X., Yao Y., Wang H., Qiao J., Ji L., and Wang Y.L. (2014). Variations of microRNAs in human placentas and plasma from preeclamptic pregnancy. Hypertension 63, 1276–1284 [DOI] [PubMed] [Google Scholar]
- Ye W., Lv Q., Wong C.K., Hu S., Fu C., Hua Z., Cai G., Li G., Yang B.B., and Zhang Y. (2008). The effect of central loops in miRNA: MRE duplexes on the efficiency of miRNA-mediated gene regulation. PLoS One 3, e1719 Verification was performed on COS-7 and CNE cells obtained from DMEM (GIBCO, Carlsbad, CA, USA) and Kunming Cell Bank (Kunming, China) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi T., Kawakami K., Enokida H., Chiyomaru T., Kagara I., Tatarano S., Yoshino H., Arimura H., Nishiyama K., Seki N., and Nakagawa M. (2011). Restoration of miR-517a expression induces cell apoptosis in bladder cancer cell lines. Oncol Rep 25, 1661–1668. Verification was performed on human BC cell 25, BOY and T24 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Diao Z., Su L., Sun H., Li R., Cui H., and Hu Y. (2010). MicroRNA-155 contributes to preeclampsia by down-regulating CYR61. Am J Obstet Gynecol 202, 466.e461–e467 [DOI] [PubMed] [Google Scholar]
- Zhou P., Luo X., Qi H.B., Zong W.J., Zhang H., Liu D.D., and Li Q.S. (2012). The expression of pentraxin 3 and tumor necrosis factor-alpha is increased in preeclamptic placental tissue and maternal serum. Inflamm Res 61, 1005–1012 [DOI] [PubMed] [Google Scholar]
- Zhu X.M., Han T., Sargent I.L., Yin G.W., and Yao Y.Q. (2009). Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol 200, 661.e661–e667 [DOI] [PubMed] [Google Scholar]