Abstract
Aortic stenosis (AS) occurs in almost 10% of adults over age 80 years with a mortality about 50% at 2 years unless outflow obstruction is relieved by aortic valve replacement (AVR). Development of AS is associated with anatomic, clinical and genetic risk factors including a bicuspid valve in 50%; clinical factors that include older age, hypertension, smoking, diabetes and elevated serum lipoprotein(a) [Lp(a)] levels; and genetic factors such as a polymorphism in the Lp(a) locus. Early stages of AS are characterized by focal areas of leaflet thickening and calcification. The rate of hemodynamic progression is variable but eventual severe AS is inevitable once even mild valve obstruction is present. There is no specific medical therapy to prevent leaflet calcification. Basic principles of medical therapy for asymptomatic AS are patient education, periodic echocardiographic and clinical monitoring, standard cardiac risk factor evaluation and modification and treatment of hypertension or other comorbid conditions. When severe AS is present, a careful evaluation for symptoms is needed, often with an exercise test to document symptom status and cardiac reserve. In symptomatic patients with severe AS, AVR improves survival and relieves symptoms. In asymptomatic patients with severe AS, AVR also is appropriate if ejection fraction is < 50%, disease progression is rapid or AS is very severe (aortic velocity > 5 m/s). The choice of surgical or transcatheter AVR depends on the estimated surgical risk plus other factors such as frailty, other organ system disease and procedural specific impediments.
Keywords: Aortic stenosis, Valve replacement, Echocardiography
Introduction
Aortic stenosis (AS) is the most common type of valvular heart disease. Aortic valve disease spans a disease spectrum that begins with mild fibrocalcific leaflet changes, termed aortic sclerosis, which is associated with a 50% increased risk of adverse cardiovascular events, even with normal leaflet opening.1) Aortic sclerosis often progresses to more severe leaflet calcification with the end stage of disease characterized by obstruction of left ventricular (LV) outflow resulting in the inability to adequately increase cardiac output with exertion. Once even mild symptoms are present, failure to relieve LV outflow obstruction leads to heart failure and death, with a mortality as high as 50% over 2 years.2) Although the prevalence of AS is only about 0.2% among adults aged 50 and 59 years, it is as high as 9.8% in those 80 years or older, with an overall prevalence of 2.8% in adults over 75 years of age.3),4) Several population-based studies have shown a significant association between age and calcific aortic valve disease. Therefore it is likely that the prevalence of aortic valve stenosis will increase even further with the demographic change toward an elderly population in developed countries.
The 2014 American Heart Association and American College of Cardiology (AHA/ACC) Valve Guidelines introduced a new classification of AS with four stages, defined by combining information about patient symptoms, leaflet anatomy, valve hemodynamics, and LV function (Table 1). This patient centered approach allows close alignment between disease stage and treatment recommendations.5) In addition, these guidelines emphasize the Heart Valve Team approach, with an integrative decision making approach by valve experts, imaging specialists, cardiothoracic surgeons and interventional cardiologists. Valve centers of excellence with high surgical/interventional volumes and low complication rates also participate in outcomes registries, implement quality improvement processes and ensure adherence to guidelines.6)
Table 1. Stage of aortic valve stenosis.

ΔP: mean gradient, AS: aortic stenosis, AVA: aortic valve area, DSE: dobutamine stress echocardiography (maximum dose 20 mcg/mg/min), EF: ejection fraction, LV: left ventricular, SV: stroke volume, Vmax: maximum aortic velocity
Stages of disease
Stage A disease is defined as the patient at risk of developing AS who is currently asymptomatic without valve obstruction; for example a young patient with a normally functioning bicuspid valve or an older adult with aortic valve sclerosis. Stage B disease is present if there is evidence of progressive leaflet calcification and thickening with reduced leaflet motion resulting mild to moderate valve obstruction. In patients with stage A and stage B disease, standard cardiac risk factor evaluation and reduction, including treatment of hypertension, is particularly important although these patients do not benefit from aortic valve replacement (AVR).6) In addition, periodic monitoring is recommended for evaluation of progressive valve obstruction and patient symptoms. When severe valve obstruction is present, the most important distinction is between asymptomatic (stage C) and symptomatic (stage D) disease with sub-classifications for LV dysfunction (stage C2) and for low-gradient severe AS (stage D2 or D3).
Severe AS is defined as calcified and thickened valve leaflets with reduced systolic opening and an antegrade velocity across the valve of 4.0 m/s or higher, equivalent to a mean systolic transaortic pressure gradient of 40 mm Hg or higher.7),8) Typically, aortic valve area (AVA) is 1.0 cm2 or less although this is not required for diagnosis of severe AS because a high velocity or gradient alone is predictive of clinical outcome regardless of valve area. Asymptomatic patients with severe AS and an LV ejection fraction of 50% or higher (stage C1) are distinguished from those with LV systolic dysfunction (stage C2) because outcomes and treatment recommendations are different for these subsets of disease.
In the symptomatic patient with AS, a velocity of 4.0 m/s or higher or mean gradient of 40 mm Hg or higher is consistent with high-gradient severe AS (stage D1). The goal is simply to establish whether AS is severe enough to cause symptoms. Again, valve area typically is 1.0 cm2 or less but may be larger in patients with mixed stenosis and regurgitation or a large body size; in any case, severe aortic valve disease is present and relief of valve obstruction will improve clinical outcome.
In symptomatic patients with a calcified valve with reduced leaflet opening and a small valve area (≤ 1.0 cm2) but a low transvalvular velocity (< 4 m/s) and mean systolic gradient (< 40 mm Hg), the possibility of low-flow low-gradient severe AS must be considered. In the setting of LV systolic dysfunction (ejection fraction < 50%), a low-dose (maximum 20 mcg/m2/min) dobutamine stress echocardiogram showing an increase in aortic velocity to 4 m/s or higher with valve area remaining less than 1.0 cm2 is consistent with low-flow low gradient severe AS (stage D2), rather than moderate AS with concurrent primary myocardial disease.
Diagnosis is more challenging in the symptomatic patient with possible low-flow low-gradient severe AS when LV systolic function is normal. Stress echocardiography has not been shown to be useful in this subset of patients.
Typically, low-flow low gradient severe AS with normal LV ejection fraction (stage D3) is diagnosed in older patients, predominantly women, with a heavily calcified valve, an indexed AVA < 0.6 cm2 and a small hypertrophied LV resulting in a stroke volume index less than 35 mL/m2. It is critical that measurements of AS severity be confirmed when the patient is normotensive because valve hemodynamics are affected by concurrent hypertension. Stage D3 severe AS is a diagnosis of exclusion and should be made only if all other possible causes for the patient symptoms have been fully evaluated and treated.
Risk factors for calcific aortic valve disease
Pathogenesis of AS is determined by clinical factors, genetics and valve anatomy. Calcification occurs in many patients with a normal trileaflet aortic valve, however presence of bileaflet anatomy accounts for 60% of AVRs under the age of 70 and 40% of those 70 years or older.9),10) Bicuspid aortic valve disease is present in 1-2% of the United States population and nearly all will require AVR during their lifetime.11),12) Rheumatic heart disease remains prevalent in underdeveloped countries, where improvement in primary prevention is needed. Specifically prevention and treatment of streptococcal pharyngitis prevents rheumatic fever and rheumatic valve disease.
Bicuspid aortic valve disease appears to be inherited in an autosomal dominant pattern, with variable penetrance, in some families. However, a specific single gene abnormality has not yet been identified.13) Familial inheritance patterns have also been reported for calcific valve disease in patients without a congenital bicuspid valve.14) In a large genome wide association study a specific polymorphism in lipoprotein(a) [Lp(a) locus rs10455872] has been shown to be associated with elevated serum levels of Lp(a) as well with incident AS [hazard ratio (HR) per allele, 1.68; 95% confidence interval (CI), 1.32 to 2.15] and AVR (HR, 1.54; 95% CI, 1.05 to 2.27).15) In a separate cohort, the same Lp(a) polymorphism was associated with elevated serum Lp(a) levels and with incident AS, further supporting a genetic component in risk of AS.16) In a prospective validation study the Lp(a) risk genotypes also were associated with elevated serum Lp(a) levels and increased risk of AS, with a serum Lp(a) level > 90 mg/dL predicting a threefold increased risk of AS.17)
Clinical factors associated with the development of calcific valve disease are similar to those associated with atherosclerosis and coronary artery disease (Table 2). In population-based studies prevalent calcific valve disease was associated with older age, male gender, elevated serum low-density lipoprotein (LDL) and Lp(a) levels, hypertension, smoking, diabetes and metabolic syndrome.4),18),19) A Mendelian randomization study design using community-based cohorts confirmed that serum LDL levels are associated with aortic valve calcification and AS (HR per mmol/L, 1.51; 95% CI, 1.07-2.14; p = 0.02).20) Patients with a history of disorders of calcium metabolism, renal failure, or mediastinal radiation also are at increased risk of AS.21)
Table 2. General summary of strength of associations seen in observational and epidemiologic studies of clinical risk factors and calcific aortic valve disease.
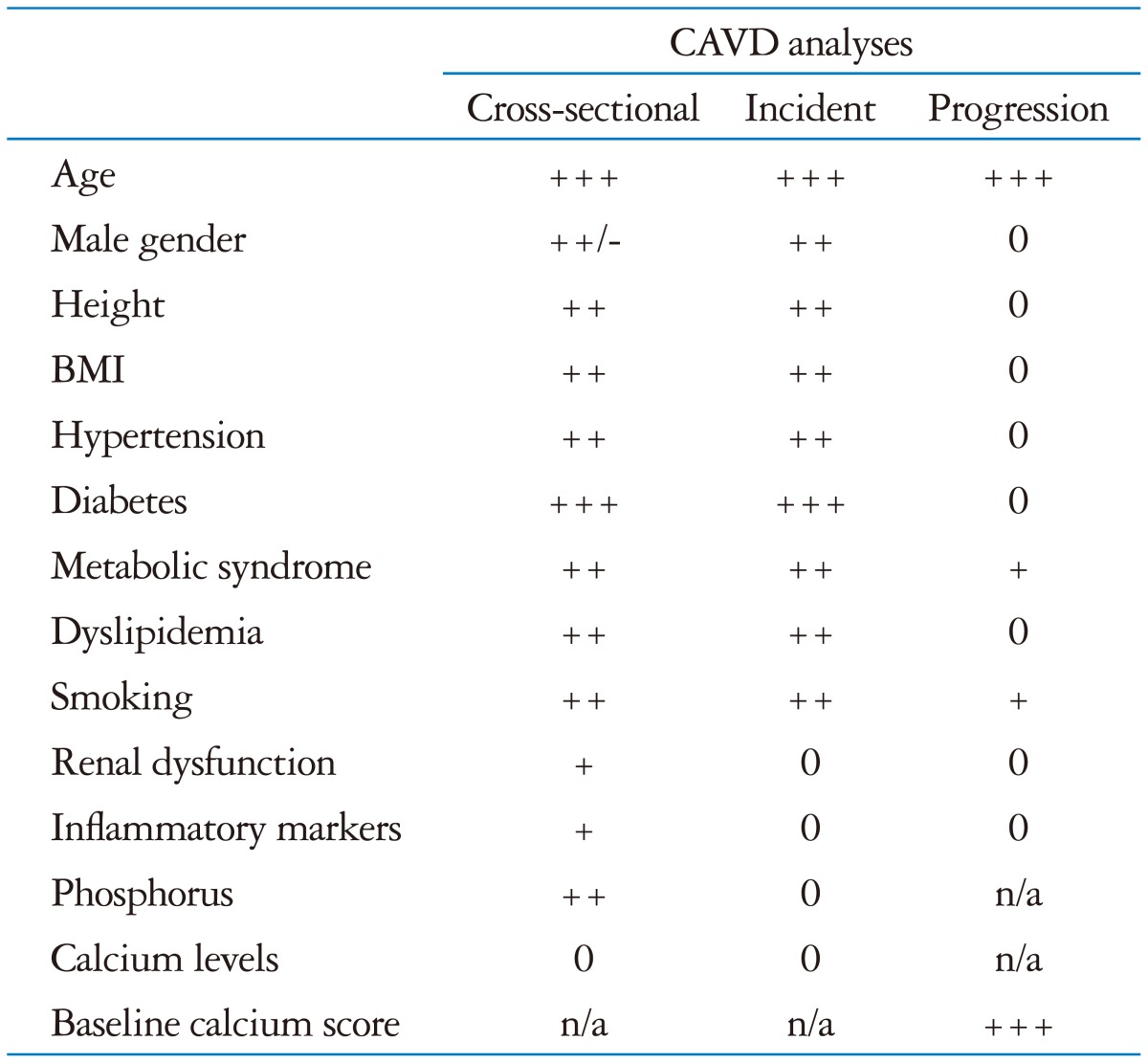
BMI: body mass index, CAVD: calcific aortic valve disease, +: weak positive association, ++: modest positive association, +++: strong positive association, -: weak negative association, 0: no association seen, n/a: insufficient data available. From Owens DS, O'Brien KD. Clinical and genetic risk factors for calcific valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald's heart disease. 4th ed. Philadelphia: Elsevier;2014. Chapter 4, page 54, Table 4-1
Progression from aortic sclerosis, defined as focal areas of valve calcification and leaflet thickening, to hemodynamically significant valve obstruction occurs in 10 to 15% of patients over 2 to 5 years. However, factors associated with disease progression differ from those associated with initiation of disease suggesting differences in the disease process at the tissue level. In contrast to prevalent aortic valve disease, the only identified factors associated with disease progression are older age, male gender, stenosis severity, and the degree of leaflet calcification.19),22)
Hemodynamic progression of AS
Once even mild AS is present, defined as an aortic velocity of 2 m/s or higher, progressive stenosis occurs in nearly all patients and most will require AVR over their lifetime. Overall the average rate of hemodynamic progression in adults with mild to moderate AS is an increase in trans-aortic velocity of 0.3 m/s per year, an increase in mean gradient 7 mm Hg per year and a decrease in valve area of 0.1 cm2 per year.7) However, the rate of hemodynamic progression varies significantly between patients with progression tending to be more rapid in older patients and those with more severe valve calcification. Interestingly, the degree of valve obstruction leading to onset of symptoms also varies significantly among patients. Thus, these two factors-heterogeneity in the rate of hemodynamic progression and variability in onset of clinical symptoms mandate periodic clinical and echocardiographic evaluation for patients with asymptomatic mild to moderate AS.
Once severe valve obstruction is present, the most important distinction is between asymptomatic (stage C) and symptomatic (stage D) disease. Most patients who are educated about the disease course will recognize and report early symptoms of AS, most often dyspnea on exertion or simply a decline in exercise capacity. Once even mild symptoms due to severe AS are present, clinical outcomes are very poor unless outflow obstruction is relieved by AVR. The classic symptoms of angina, heart failure or syncope are typically the later manifestation of disease and now are seen only in patients who were not receiving medical care, failed to report early symptoms or had an inappropriate surgical delay. In those with symptomatic severe AS, the only effective treatment is surgical AVR (SAVR) or transcatheter AVR (TAVR), resulting in improved survival and reduced symptoms. If symptom status is unclear, standard treadmill exercise testing may be helpful to document exercise capacity, ensure symptoms are not provoked by exercise and to measure the blood pressure response to exertion. An elevated serum B-natriuretic peptide levels may suggest early or incipient symptoms in the patient with an equivocal clinical history.23),24)
Asymptomatic patients with hemodynamically severe AS are classified as stage C disease. The definition of "severe AS" in the guidelines was chosen to ensure that all symptomatic patients are promptly referred for valve replacement. However, many patients with numbers consistent with "severe AS" remain asymptomatic for several years and the rate of disease progression is quite variable. Survival during this asymptomatic phase is similar to age-matched control group with low risk of sudden death (< 1% per year) in retrospective series, a risk that may be even lower when patients are followed prospectively. The goals in clinical management of stage C disease are to: 1) identify any high-risk patients, 2) provide patient education and followup to ensure early symptom recognition, 3) treat comorbid conditions, and 4) optimize the timing of valve replacement. In adults with moderate to severe AS, all patients will develop symptoms due to hemodynamic progression with an event free survival of 75% to 80% at 2 years in those with velocity of less than 3.0 m/s compared to 35% to 50% in those with jet velocity of more than 4.0 m/s.7)
Medical therapy
Despite experimental models suggesting benefit with lipid-lowering therapy in preventing disease progression in calcific AS, multiple large randomized clinical trials failed to show benefit in changing hemodynamic severity or clinical outcomes.25),26) Therefore, statin therapy is not indicated for prevention of progression of AS in patients with mild to moderate calcific valve disease, unless there is concurrent coronary artery disease. Hopefully, ongoing research will identify novel disease mechanisms that might allow therapy targeted towards pathways to reduce inflammation, oxidative stress and abnormal tissue calcification.
However, cardiovascular lifestyle and pharmacologic risk factor modifications are important in adults with any degree of calcific aortic valve disease. Standard cardiovascular risk factors, including smoking, hypertension, diabetes, obesity and hyperlipidemia, should be evaluated and treated according to current guidelines. Many patients with AS will receive lipid-lowering therapy for standard indications, although not specifically to prevent AS progression. Patients with AS should be encouraged to follow a heart-healthy diet and to exercise regularly. With mild-moderate AS, exercise restrictions are not needed; asymptomatic patients with severe AS should be advised to avoid strenuous activities or competitive sports.
Hypertension is common in patients with AS. This adds to the total LV pressure overload in combination with the valve obstruction. Despite the concerns that antihypertensive medications may cause excessive peripheral vasodilation resulting in hypotension, this concern has not been supported in clinical practice. In fact, hypertension was associated with 56% higher rate of ischemic cardiovascular events and 2 fold increase in mortality over 4.3 years in a prospective study of 1616 adults with mild to moderate AS.27) Standard therapy for hypertension is appropriate in adults with AS although medications should be started at low doses with slow titration to achieve blood pressure control. It may be prudent to avoid diuretics when LV size is small. In theory, angiotensin pathway inhibitors might have beneficial for LV remodeling effects and beta blockers might be more beneficial in patients with concurrent coronary artery disease. However, there is no data on clinical outcomes with specific antihypertensive regimens in AS patients.
In symptomatic severe AS, medical therapy does not prolong life and AVR is the only effective treatment. In patients presenting with cardiogenic shock due to decompensated severe AS, there has been limited experience using vasodilator therapy with close hemodynamic monitoring to stabilize patients prior to SAVR or TAVR.28) In symptomatic patients with severe AS who refuse AVR or in whom AVR would be futile, palliative therapy with standard medical therapy to relieve symptoms of heart failure or angina is appropriate.
Indications for AVR
The new staging system (stage A to D) emphasizes a patient centered approach, where defining disease severity allows alignment of treatment recommendations with disease stages (Table 3). A constellation of symptoms, valve anatomy and hemodynamics should be considered when a patient is being considered for valve replacement. Recommendations for AVR are classified as a strong recommendation termed Class I, or a weaker recommendation classified as Class IIa (AVR is reasonable) or IIb (AVR may be considered) in the American Heart Association/American College of Cardiology and in the European Society of Cardiology guidelines (Table 3).
Table 3. AHA/ACC recommendations for timing and choice of valve replacement for aortic valve stenosis.

AHA/ACC: 2014 American Heart Association and American College of Cardiology Guidelines, AS: aortic stenosis, AVR: aortic valve replacement, either surgical or transcatheter, BP: blood pressure, ESC: 2012 European Society of Cardiology guidelines, LOE: level of evidence, LVEF: left ventricular ejection fraction, TAVR: transcatheter AVR. Adapted from Nishimura et al.6)
Symptomatic patients with severe AS
Stage D1
In patients with severe AS, the key factor in clinical decision-making is development of symptoms. Once symptoms occur, the rate of death is more than 50% at 2 years for patients unless aortic valve is performed promptly. In the absence of serious comorbid conditions that may limit life expectancy, AVR is indicated in all symptomatic patients with severe AS and should be promptly performed at the onset of symptoms.2),6),29),30),31)
Stage D2
Low-gradient AS with LV dysfunction has poorer outcomes after AVR compared to high gradient severe AS with normal LV systolic function.32) However, survival is higher in patients undergoing AVR compared to those treated medically.33) Thus, AVR is reasonable for symptomatic patients with low-flow low gradient severe AS with reduced LV ejection fraction, after confirmation of the diagnosis by low dose dobutamine stress echocardiography (Fig. 1). If LV systolic dysfunction is due to outflow obstruction and afterload mismatch, LV systolic function should normalize after AVR. However, even if the low ejection fraction is related to a primary cardiomyopathy or coronary disease, ejection fraction still should improve after AVR due to the decreased total LV afterload, if significant valve obstruction was present. On average, LV ejection fraction increases by 10 points after relief of severe AS.
Fig. 1. Evaluation and management of the patient with low-flow low-gradient aortic stenosis. The AHA/ACC class recommendation (I, IIa, or IIb) is shown in parentheses corresponding to Table 3. AHA/ACC: 2014 American Heart Association and American College of Cardiology Guidelines, AS: aortic stenosis, AVA: aortic valve area, AVR: aortic valve replacement which may be either surgical or transcatheter, DSE: dobutamine stress echocardiography (low dose), EF: ejection fraction, SVI: stroke volume indexed to body size, Vmax: maximum aortic velocity.
Stage D3
The prevalence of low-flow low-gradient severe AS with preserved LV ejection fraction is controversial but a reasonable estimate is between 5% and 25% of patients with severe AS. Many patients with apparent low-flow low-gradient AS with normal ejection fraction simply have moderate AS with clinical outcomes paralleling those for other patients with moderate AS.34) A second source of confusion arises when diagnostic data is suboptimal with measurement error accounting for an erroneous diagnosis of severe AS. In addition, it is important to measure AS severity when the patient is normotensive, to exclude other causes for AS symptoms and to take patient body size into consideration. Finally, patient-prosthesis mismatch should be avoided by comparing the expected hemodynamics after valve replacement to those of the native diseased valve.35)
In patients with a confirmed diagnosis of severe low-output low-gradient severe AS, it is likely that AVR will improve survival and decrease symptoms. In a prospective observational study of 260 patients with symptomatic low-gradient severe AS with preserved ejection fraction, medical therapy was associated with 2-fold greater all-cause mortality than AVR over mean 28 ± 24 months of followup.36) Similarly, based on an echocardiographic database of 1704 consecutive patients with severe AS, AVR was associated with a 69% mortality reduced in patients with low-flow low-gradient severe AS with normal ejection fraction.37)
Asymptomatic patients with severe AS
Stage C1
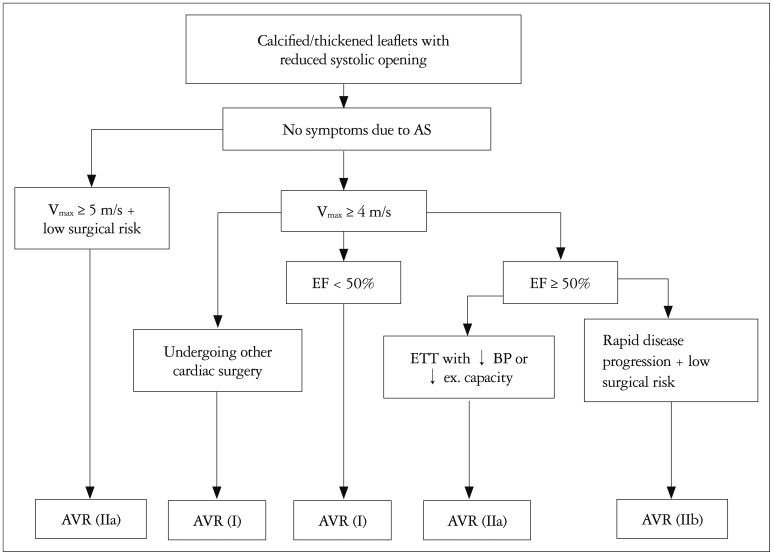
The rate of symptom onset with severe AS is higher in patients with more severe AS. In asymptomatic patients with an aortic velocity of 5.0 m/s or greater, the rate of symptom onset is 50% at 2 years.38) Therefore, if surgical risk is low (< 1%), it is reasonable to consider elective AVR in this group of patients, even in the absence of symptoms (Fig. 2).39)
Fig. 2. Timing of aortic valve replacement in adults with asymptomatic aortic stenosis. The AHA/ACC class recommendation (I, IIa, or IIb) is shown in parentheses corresponding to Table 3. AHA/ACC: 2014 American Heart Association and American College of Cardiology Guidelines, AS: aortic stenosis, AVR: aortic valve replacement which may be either surgical or transcatheter, BP: blood pressure, EF: ejection fraction, ETT: exercise treadmill test, ex: exercise, Vmax: maximum aortic velocity.
Asymptomatic patients with severe AS who demonstrate decreased exercise tolerance or a failure of systolic blood pressure to increase by at least 20 mm Hg on exercise testing have a rate of as symptom onset of 60-80% within one to two years. Patients with rapid progression of disease, defined as increase in aortic velocity of 0.3 m per second per year or greater also are at high risk of imminent symptom onset. In both these patients groups, AVR is appropriate as indicated in Table 3.
Stage C2
The presence of a low LV ejection fraction in a patient with severe AS is an indication for AVR because it is likely that severe AS is the cause of LV systolic dysfunction. In patients with severe AS and a low ejection fraction, survival is higher, symptoms are reduced and LV ejection fraction is higher in those undergoing SAVR compared to those treated with medical therapy.40),41)
Stage B
In asymptomatic adults with progressive (mild-moderate) AS, there is no evidence that AVR improves long-term outcomes. The risk of valve replacement (and the risks of a prosthetic valve) are higher than the risk of sudden death, which is estimated at < 1% per year even with severe AS in asymptomatic adults.42) An exception to this approach is the patient with moderate AS who is undergoing other cardiac surgery. In this setting, AVR is recommended when severe AS is present and is reasonable if moderate AS is present because AS is progressive, with symptom onset likely within 5 years, and the risk of reoperative SAVR is high.6) It is possible that this recommendation will be modified in the future as TAVR becomes an acceptable alternative to SAVR.
Choice of Intervention
In the patient with AS, there are two separate decisions regarding valve replacement. First, decide if AVR is indicated for relief of valve obstruction. Then, if AVR is appropriate, begin to think about the choice between SAVR and TAVR. The factors influencing the choice of AVR type include estimated surgical risk, comorbid conditions, patient frailty, technical impediments to either SAVR or TAVR, patient age and expected longevity as well as patient preferences and values.
Current procedural risk estimates for cardiac surgical procedures include the European System for Cardiac Operative Risk Evaluation (EuroSCORE) and the Society of Thoracic Surgeons Predicted Risk Of Mortality (STS-PROM) score. These scoring systems assess the patient's risk of operative mortality and morbidity based on demographic factors, clinical variables, and the planned procedure. Evaluation of patients being considered for surgical vs. transcatheter approach is challenging as these risk scores are based on surgical (not transcatheter) outcome date, are not specific for valve disease, do not include all relevant variables (such as frailty) and do not consider structural impediments to surgery or a transcatheter approach. For example, structural impediments to SAVR include chest wall adhesions or a porcelain aorta whereas structural impediments to TAVR include poor vascular access or an aortic annulus too small or too large for currently available TAVR prosthetic valves. Thus, the Heart Valve Team bases the choice of SAVR vs. TAVR on integration of all these factors.
SAVR
SAVR remains the standard approach for patients with a low to intermediate surgical risk (STS-PROM score < 8%). SAVR benefits include survival advantage, improvement in symptoms and LV systolic function. Overall 30 day surgical mortality post aortic-valve replacement is 3% for isolated AVR. After recovery from successful AVR, the rate of overall survival is similar to that among age-matched adults without AS.43)
TAVR
TAVR is the procedure of choice risk when the risk of surgery is prohibitive and expected survival after the procedure is at least one year (Table 4). These patients have a very high mortality at 5 years even with TAVR (73%) but survival, symptoms and quality of life all were improved in the PARTNER cohort B randomized controlled clinical trial of TAVR (2 year mortality 43.4%) compared to medical therapy (2 year survival 68%).30),31),44)
Table 4. Randomized controlled clinical trials of aortic valve replacement for aortic stenosis.

AF: atrial fibrillation, AMI: acute myocardial infarction, AR: aortic regurgitation, AS: aortic stenosis, AVA: aortic valve area, CAD: coronary artery disease, CI: confidence interval, HR: hazard ratio, LVEF: left ventricular ejection fraction, MI: myocardial infarction, MR: mitral regurgitation, NYHA: New York Heart Association, ΔP: mean transaortic pressure gradient, STS-PROM: Society of Thoracic Surgeons-Predicted Risk Of Mortality, pt(s): patient(s), SAVR: surgical aortic valve replacement, STS: Society of Thoracic Surgeons, TAVR: transcatheter aortic valve replacement, TIA: transient ischemic attack, Vmax: aortic valve maximum velocity, BAV: balloon aortic valvotomy
TAVR also is a reasonable alternative to SAVR in high risk patients. Currently, high surgical risk is defined as STS-PROM score of more than 8% or moderate to severe frailty or irreversible disease of more than 2 other organ systems not likely to improve after intervention. In the Placement of Aortic Transcatheter Valves (PARTNER) cohort A study; high risk patients with severe symptomatic AS were randomized to TAVR vs. SAVR. Long-term results of the PARTNER cohort A study showed similar long-term clinical outcomes between TAVR and SAVR. This study utilized the balloon expandable Sapien-Edwards valve. At 5 years, the mortality rate was 67.8% in the TAVR arm compared with 62.4% in the surgical arm (HR 1.04, 95% CI 0.86-1.24, p = 0.76). Echocardiography after TAVR showed durable hemodynamic benefit (AVA 1.52 cm2 at 5 years, mean gradient 10.6 mm Hg at 5 years) with no evidence of structural valve deterioration.2),45),46)
In the randomized controlled clinical trial of the self-expanding Core Valve, the primary endpoint of death at one year was no different in the 390 patients randomized to TAVR (14.2%) compared to those randomized to SAVR (19.1%, p < 0.001 for non-inferiority) providing further confirmation that TAVR is a reasonable alternative to SAVR in high risk patients.47)
Complications of TAVR differ from those with SAVR. The risk of stroke was higher with TAVR compared to SAVR in early studies but now ranges from 2% to 6% with either approach. As might be expected, major vascular complications at 30 days are more common with TAVR (5.9-11%) compared to SAVR (1.7% to 3.2%). Paravalvular aortic regurgitation after TAVR has been associated with poor outcomes and it is hoped that refinements in valve design will reduce or eliminate this technical problem. Finally, the need for permanent pacer implantation after TAVR (3.8-19.8% with TAVR vs. 3.6-7.1% with SAVR) continues to be an issue, particularly with the self-expanding transcatheter valve.47)
Most recently the Nordic Aortic Valve Intervention (NOTION) study,48) suggests that TAVR might be extended to lower-risk older patients who require AVR for severe AS. In a prospective randomized clinical trial, TAVR with the self-expanding prosthesis was compared to SAVR in patients over age 70 years (n = 280) with 81% of patients with a low surgical risk score (STSPROM < 4). The first report from this study showed no significant difference in the primary composite endpoint that was death from any cause, stroke, or myocardial infarction at 1 year (13.1% for TAVR vs. 16.3% for SAVR, p = 0.43 for superiority).48)
The choice of TAVR vs. SAVR will undergo continued change over the next decade as further data is published as the long-term durability of these valves is established. The 5-year results are encouraging but the higher risk period for bioprosthetic valves is in the 10 to 20 year time frame, data we will not have for some time to come.
Palliative care
The task of balancing the risks and benefits of TAVR depends on an accurate assessment of prognosis of survival, morbidity and expected quality of life. Baseline clinical risk factors associated with poor outcomes after TAVR include advanced age, frailty, smoking, chronic obstructive pulmonary disease, liver disease, prior stroke or other systemic conditions. Ideally a validated model that predicts long-term outcomes after TAVR would help guide this analysis. However until development of such ideal model, surgical risk scores can be used to evaluate those who may benefit from TAVR (Table 4). TAVR is not recommended in patients with a life expectancy < 1 year even with a successful procedure or in patients with comorbidities that preclude significant symptomatic benefit from relief of AS.2) In this setting, appropriate palliative care should be offered as it provides the best quality of life for these patients.
Balloon aortic dilation
Balloon aortic valve dilation provides modest hemodynamic benefits, which does not outweigh the risk of the procedure including stroke, aortic regurgitation and a high probability of recurrence of AS within 6 months.49) Currently this procedure is only recommended for stabilization before TAVR or surgery in patients presenting with hemodynamic instability due to severe AS.6)
Conclusions
In summary, the 2014 AHA/ACC Valve Guidelines provide a new framework of thinking about aortic valve disease, which aligns disease severity with recommendations for intervention. An integrative approach that considers clinical factors, valve anatomy, hemodynamics and LV function in addition to the patient preference and overall condition now is recommended and the importance of the Heart Valve Team in the diagnosis and treatment of disease is reaffirmed. With the publication of randomized controlled clinical trials data, refinements in valve design, and reductions in procedural complications, we will see a gradual shift towards from surgical towards transcatheter valve replacement when intervention for AS is required. In the future, intervention is likely to move even earlier in the disease course if transcatheter bioprosthetic valves demonstrate long-term durability. However until further data is available, SAVR remains the recommended treatment of severe symptomatic AS in those with low surgical risks because of the known durability of surgical prosthetic valves.
References
- 1.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 2.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 3.Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. the Tromsø study. Heart. 2013;99:396–400. doi: 10.1136/heartjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 4.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 5.Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744–756. doi: 10.1056/NEJMra1313875. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, 3rd, Thomas JD ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 7.Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft CD, Miyake-Hull CY, Schwaegler RG. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–2270. doi: 10.1161/01.cir.95.9.2262. [DOI] [PubMed] [Google Scholar]
- 8.Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 9.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925. doi: 10.1161/01.CIR.0000155623.48408.C5. [DOI] [PubMed] [Google Scholar]
- 10.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–2800. doi: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 11.Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM, Pellikka PA, Tajik AJ, Enriquez-Sarano M. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117:2776–2784. doi: 10.1161/CIRCULATIONAHA.107.740878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, Webb GD, Siu SC. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–1325. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 13.Owens DS, O'Brien KD. Clinical and genetic risk factors for calcific valve disease. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald's heart disease. 4th ed. Philadelphia: Elsevier Science; 2014. pp. 53–62. [Google Scholar]
- 14.Probst V, Le Scouarnec S, Legendre A, Jousseaume V, Jaafar P, Nguyen JM, Chaventré A, Le Marec H, Schott JJ. Familial aggregation of calcific aortic valve stenosis in the western part of France. Circulation. 2006;113:856–860. doi: 10.1161/CIRCULATIONAHA.105.569467. [DOI] [PubMed] [Google Scholar]
- 15.Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O'Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg-Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang SJ, Liu Y, Sjogren M, van der Pals J, Kälsch H, Mühleisen TW, Nöthen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Melander O, Gudnason V, O'Donnell CJ, Post WS CHARGE Extracoronary Calcium Working Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arsenault BJ, Boekholdt SM, Dubé MP, Rhéaume E, Wareham NJ, Khaw KT, Sandhu MS, Tardif JC. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7:304–310. doi: 10.1161/CIRCGENETICS.113.000400. [DOI] [PubMed] [Google Scholar]
- 17.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein (a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O'Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 19.Owens DS, Katz R, Takasu J, Kronmal R, Budoff MJ, O'Brien KD. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA) Am J Cardiol. 2010;105:701–708. doi: 10.1016/j.amjcard.2009.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JG, Luk K, Schulz CA, Engert JC, Do R, Hindy G, Rukh G, Dufresne L, Almgren P, Owens DS, Harris TB, Peloso GM, Kerr KF, Wong Q, Smith AV, Budoff MJ, Rotter JI, Cupples LA, Rich S, Kathiresan S, Orho-Melander M, Gudnason V, O'Donnell CJ, Post WS, Thanassoulis G Cohorts for Heart and Aging Research in Genetic Epidemiology (CHARGE) Extracoronary Calcium Working Group. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA. 2014;312:1764–1771. doi: 10.1001/jama.2014.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtz CE, Otto CM. Aortic stenosis: clinical aspects of diagnosis and management, with 10 illustrative case reports from a 25-year experience. Medicine (Baltimore) 2010;89:349–379. doi: 10.1097/MD.0b013e3181fe5648. [DOI] [PubMed] [Google Scholar]
- 22.Novaro GM, Katz R, Aviles RJ, Gottdiener JS, Cushman M, Psaty BM, Otto CM, Griffin BP. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;50:1992–1998. doi: 10.1016/j.jacc.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 23.Capoulade R, Magne J, Dulgheru R, Hachicha Z, Dumesnil JG, O'Connor K, Arsenault M, Bergeron S, Pierard LA, Lancellotti P, Pibarot P. Prognostic value of plasma B-type natriuretic peptide levels after exercise in patients with severe asymptomatic aortic stenosis. Heart. 2014;100:1606–1612. doi: 10.1136/heartjnl-2014-305729. [DOI] [PubMed] [Google Scholar]
- 24.Lindman BR. BNP during exercise: a novel use for a familiar biomarker in aortic stenosis. Heart. 2014;100:1567–1568. doi: 10.1136/heartjnl-2014-306253. [DOI] [PubMed] [Google Scholar]
- 25.Teo KK, Corsi DJ, Tam JW, Dumesnil JG, Chan KL. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebo-controlled clinical trials on 2344 patients. Can J Cardiol. 2011;27:800–808. doi: 10.1016/j.cjca.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Bärwolf C, Holme I, Kesäniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 27.Rieck ÅE, Cramariuc D, Boman K, Gohlke-Bärwolf C, Staal EM, Lønnebakken MT, Rossebø AB, Gerdts E. Hypertension in aortic stenosis: implications for left ventricular structure and cardiovascular events. Hypertension. 2012;60:90–97. doi: 10.1161/HYPERTENSIONAHA.112.194878. [DOI] [PubMed] [Google Scholar]
- 28.Khot UN, Novaro GM, Popović ZB, Mills RM, Thomas JD, Tuzcu EM, Hammer D, Nissen SE, Francis GS. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med. 2003;348:1756–1763. doi: 10.1056/NEJMoa022021. [DOI] [PubMed] [Google Scholar]
- 29.Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS) Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 30.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 31.Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, Webb JG, Mack MJ, Douglas PS, Thourani VH, Babaliaros VC, Herrmann HC, Szeto WY, Pichard AD, Williams MR, Fontana GP, Miller DC, Anderson WN, Smith CR, Akin JJ, Davidson MJ PARTNER trial investigators. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015 Mar 15; doi: 10.1016/S0140-6736(15)60290-2. [Epub] [DOI] [PubMed] [Google Scholar]
- 32.Gotzmann M, Lindstaedt M, Bojara W, Ewers A, Mügge A. Clinical outcome of transcatheter aortic valve implantation in patients with lowflow, low gradient aortic stenosis. Catheter Cardiovasc Interv. 2012;79:693–701. doi: 10.1002/ccd.23240. [DOI] [PubMed] [Google Scholar]
- 33.Tribouilloy C, Lévy F, Rusinaru D, Guéret P, Petit-Eisenmann H, Baleynaud S, Jobic Y, Adams C, Lelong B, Pasquet A, Chauvel C, Metz D, Quéré JP, Monin JL. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol. 2009;53:1865–1873. doi: 10.1016/j.jacc.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 34.Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, Chambers JB, Egstrup K, Kesäniemi YA, Malbecq W, Nienaber CA, Ray S, Rossebø A, Pedersen TR, Skjærpe T, Willenheimer R, Wachtell K, Neumann FJ, Gohlke-Bärwolf C. Outcome of patients with low-gradient "severe" aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887–895. doi: 10.1161/CIRCULATIONAHA.110.983510. [DOI] [PubMed] [Google Scholar]
- 35.Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. 2009;119:1034–1048. doi: 10.1161/CIRCULATIONAHA.108.778886. [DOI] [PubMed] [Google Scholar]
- 36.Ozkan A, Hachamovitch R, Kapadia SR, Tuzcu EM, Marwick TH. Impact of aortic valve replacement on outcome of symptomatic patients with severe aortic stenosis with low gradient and preserved left ventricular ejection fraction. Circulation. 2013;128:622–631. doi: 10.1161/CIRCULATIONAHA.112.001094. [DOI] [PubMed] [Google Scholar]
- 37.Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation. 2013;128:1781–1789. doi: 10.1161/CIRCULATIONAHA.113.003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, Bergler-Klein J, Grimm M, Gabriel H, Maurer G. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156. doi: 10.1161/CIRCULATIONAHA.109.894170. [DOI] [PubMed] [Google Scholar]
- 39.Kang DH, Kim JH, Rim JH, Kim MJ, Yun SC, Song JM, Song H, Choi KJ, Song JK, Lee JW. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009;119:797–804. doi: 10.1161/CIRCULATIONAHA.108.802314. [DOI] [PubMed] [Google Scholar]
- 40.Connolly HM, Oh JK, Schaff HV, Roger VL, Osborn SL, Hodge DO, Tajik AJ. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation. 2000;101:1940–1946. doi: 10.1161/01.cir.101.16.1940. [DOI] [PubMed] [Google Scholar]
- 41.Clavel MA, Webb JG, Rodés-Cabau J, Masson JB, Dumont E, De Larochellière R, Doyle D, Bergeron S, Baumgartner H, Burwash IG, Dumesnil JG, Mundigler G, Moss R, Kempny A, Bagur R, Bergler-Klein J, Gurvitch R, Mathieu P, Pibarot P. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation. 2010;122:1928–1936. doi: 10.1161/CIRCULATIONAHA.109.929893. [DOI] [PubMed] [Google Scholar]
- 42.Rosenhek R, Baumgartner H. Aortic stenosis. In: Otto CM, Bonow RO, editors. Valvular heart disease: a companion to Braunwald's heart disease. 4th ed. Philadelphia: Elsevier Science; 2014. pp. 139–162. [Google Scholar]
- 43.Walther T, Blumenstein J, van Linden A, Kempfert J. Contemporary management of aortic stenosis: surgical aortic valve replacement remains the gold standard. Heart. 2012;98(Suppl 4):iv23–iv29. doi: 10.1136/heartjnl-2012-302399. [DOI] [PubMed] [Google Scholar]
- 44.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 45.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 46.Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Davidson MJ, Svensson LG, Akin J PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015 Mar 15; doi: 10.1016/S0140-6736(15)60308-7. [Epub] [DOI] [PubMed] [Google Scholar]
- 47.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Jr, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 48.Thyregod HG, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Hansen PB, Andersen LW, Olsen PS, Søndergaard L. Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Valve Stenosis: One-year Results from the All-comers Nordic Aortic Valve Intervention (NOTION) Randomized Clinical Trial. J Am Coll Cardiol. 2015 Mar 05; doi: 10.1016/j.jacc.2015.03.014. [Epub] [DOI] [PubMed] [Google Scholar]
- 49.Khawaja MZ, Williams R, Hung J, Arri S, Asrress KN, Bolter K, Wilson K, Young CP, Bapat V, Hancock J, Thomas M, Redwood S. Impact of preprocedural mitral regurgitation upon mortality after transcatheter aortic valve implantation (TAVI) for severe aortic stenosis. Heart. 2014;100:1799–1803. doi: 10.1136/heartjnl-2014-305775. [DOI] [PMC free article] [PubMed] [Google Scholar]