Abstract
Background
Hepatocellular carcinoma (HCC) is prevalent worldwide and early diagnosis of HCC is critical for effective treatment and optimal prognosis.
Methods
Serum was screened first by immunoproteomic analysis for HCC-related tumor associated antigens (TAAs). Selected TAAs were clinically evaluated retrospectively in patients with HCC, liver cirrhosis, chronic hepatitis and healthy controls. Levels of autoantibody to the selected TAAs were measured by protein microarrays containing protein antigens of the candidate TAAs. Analyses were done by using receiver operating characteristics (ROC) to calculate diagnostic accuracy.
Findings
Twenty-two candidate TAAs were assessed by protein microarray analysis in 914 participants with serum α-fetoprotein (AFP) available. Twelve candidate TAAs were statistically different in signal intensity between HCC and controls. Among them, CENPF, HSP60 and IMP-2 showed AUC (area under the curve) values of 0.826, 0.764 and 0.796 respectively for early HCC. The highest prevalence of autoantibody positivity was observed in HCC cases with BCLC tumor stage A, well-differentiated histology and Child-Pugh grade C. Specifically, 73.6% or 79.3% cases of early HCC with negative AFP were positive for autoantibody to CENPF or HSP60.
Interpretation
Tumor-associated autoimmune reactions may be triggered by early stage HCCs. Measurement of serum autoantibody to TAAs may be complementary to AFP measurements and improve diagnosis of early HCC.
Keywords: Hepatocellular carcinoma, Early diagnosis, Serological marker, Autoantibody, Tumor associated antigen
Highlights
-
•
Tumor-associated autoimmune reaction may be triggered by early stage HCCs, and TAAs may be potential marker for early HCC.
-
•
Measurement of autoantibody to TAAs may be complementary to AFP measurements and improve diagnosis of early HCC.
-
•
Generation of autoantibody to CENPF may result from autoimmune reaction in response to overexpression of CENPF in tumor cell.
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third leading cause of cancer-related death worldwide (Theise et al., 2014). Nearly half of all new cases of liver cancer (50.5%) and related deaths (51.4%) are estimated to occur in China (Theise et al., 2014). HCC accounts for 70–80% of all liver cancers. The survival rate of patients with HCC after the onset of symptoms is generally less than one year on the late presentation of HCC at least in part due to a lack of reliable tools for early diagnosis (Theise et al., 2014).
Ultrasound is recommended as a screening tool for early detection of HCC. However, ultrasound is not very sensitive and is highly operator dependent (Poon et al., 2009). Computed tomography (CT) is not recommended as a screening tool for HCC because of the attendant radiation exposure (Poon et al., 2009, Lee et al., 2012). One current focus of HCC research is the development of a blood test to aid the diagnosis of this disease. The traditional serum biomarker for HCC, α-fetoprotein (AFP) has been found to have a sensitivity of 41–65%, and a specificity of 80–90% when a cut-off value of 20 ng/ml has been used. The sensitivity was lower when AFP was used to detect early-stage HCC (Farinati et al., 2006). Many other serologic biomarkers of HCC are available, including des-gamma carboxyprothrombin (DCP) and Lens culinaris agglutinin-reactive AFP (AFP-L3) (Bertino et al., 2012), Dickkopf-1 (DKK1) (Shen et al., 2012), and squamous cell carcinoma antigen (SCCA) (Zhao et al., 2013). However, these markers are insufficient for the early diagnosis of HCC (Yau et al., 2013, Stefaniuk et al., 2010). Therefore, there is an urgent need for the identification of novel diagnostic markers for this purpose.
Recent studies have shown that the abnormal protein release by tumor cells can elicit humoral immune responses as self-antigen and are called tumor-associated antigens (TAAs). Unlike autoantibodies in autoimmune diseases, autoantibodies against the TAAs have been reported in a wide variety of tumors. Some have been reported to be present several months to years before manifestations of the clinical signs of tumor (Tan et al., 2009, Xu et al., 2014, Werner et al., 2015). Furthermore, magnified signals of the anti-TAA autoantibodies can be easier to detect than TAAs themselves, suggesting that the measurement of anti-TAA antibodies may have various advantages as immunodiagnostic markers (Tan et al., 2009, Lacombe et al., 2014). Therefore, anti-TAA autoantibodies seem to have great potential value in the screening and early diagnosis of cancer. Autoantibodies against TAAs have been reported in patients with HCC, but only by a series of studies on small cohorts (Yau et al., 2013). Therefore, the potential value of the anti-TAAs as serum biomarkers for HCC especially for early stage HCC, is still unclear.
The aim of the present study was to use a protein microarray system for high throughput analysis (Bruix et al., 2005) to screen for autoantibody-based serum markers for early HCC, and to evaluate the value and timing of those autoantibodies in the early diagnosis of HCC.
2. Material and Methods
2.1. Study Population
For screening of specific TAAs for early HCC, total protein from frozen tissues of ten cases of HCC was extracted as an antigen library for serological proteome analysis (SERPA). The tumor samples were obtained from patients with hepatitis B virus (HBV)-related HCC with Barcelona Clinic Liver Cancer staging system (BCLC) stage A. The patients underwent surgical treatment at the Beijing You-An Hospital from 2011 to 2012. There were 7 men and 3 women, aged 42–62 years with a median age of 51.5 years. Serum samples for SERPA were also obtained from 10 cases of HBV-related HCC with BCLC stage A. The demographics were 7 men and 3 women, aged 43–65 years with a median age of 52.6 years. Other sera from 10 HBV-related cirrhosis patients without HCC, and 10 healthy volunteers were used as controls.
For clinical validation, a total of 914 serum samples were applied, including 295 cases of HCC, 132 cases of liver cirrhosis, 119 cases of chronic hepatitis, 103 cases of other cancers (23 colon cancer, 31 rectal cancer, 30 gastric cancer and 19 non-gastrointestinal tumor), and 265 healthy controls. Clinical characteristics of the samples are shown in Table 1 and supplementary Tables 1 and 2. All the samples had AFP levels measured with a commercially available electrochemiluminescence immunoassay kit (Roche, USA). Samples with an AFP value of more than 20 ng/ml were defined as AFP positive. The patients were retrospectively recruited during 2010–2013 from the Liver Research Center, Departments of Oncology, and General Surgery, Beijing Friendship Hospital, Capital Medical University, Department of Minimally Invasive Interventional Radiology, Beijing Youan Hospital, Capital Medical University and the Department of Hepatopancreatobiliary and Splenic Medicine, Affiliated Hospital of Medical College of Chinese People's Armed Police Force. HCC diagnosis was based on ultrasound, CT, or Magnetic Resonance Imaging (MRI) characteristics and biochemistry (AFP serology and liver function enzymes), according to the American Association for the Study of Liver Diseases guidelines (Bruix et al., 2005). Early-stage HCC was defined according to the BCLC stage 0 + A (Llovet et al., 2008). Diagnosis of chronic hepatitis B included the presence of HBsAg more than six months, HBV DNA concentrations higher than 103 copies/ml and serum alanine aminotransferase elevation, according to the guidelines of prevention and treatment of chronic HBV infection (Lok and McMahon, 2009). Diagnosis of chronic hepatitis C was based on positivity for anti-HCV antibody along with HCV RNA (> 500 IU/ml) in patients with signs of chronic hepatitis. Alcoholic hepatitis was defined by the history of alcohol abuse more than 5 years (alcohol consumption for male ≥ 40 g/day and female ≥ 20 g/day) or alcohol consumption ≥ 80 g/day within 2 weeks, elevated ALT, AST, GGT and AST/ALT > 2 without evidence of liver dysfunction and HBV/HCV infection. Diagnosis of liver cirrhosis should meet at least one of the following three criteria: (1) Histologically confirmed of cirrhosis (Metavir F4 or Ishak 5/6); (2) clinically presentence of liver dysfunction (albumin < 35.0 g/l, or International Normalized Ratio (INR) > 1.3) and portal hypertension (endoscopy showing esophageal varices, or imaging showing liver surface nodularity, splenomegaly or hypersplenism); (3) chronic liver disease with development of ascites, variceal bleeding or encephalopathy. Non-liver cancer patients were pre-operative patients who had no history of liver disease. The healthy controls were eligible blood donors with normal liver biochemistry, no history of liver disease and no malignant disease. Patients with autoimmune liver disease such as autoimmune hepatitis, primary biliary cirrhosis, as well as other autoimmune diseases such as diabetes, rheumatoid arthritis, and systemic lupus erythematosus were excluded from the study.
Table 1.
Clinical characteristics of research subjects for SERPA analysis and microarray detection.
| Variables | SERPA analysis (n = 30) |
Microarray detection (n = 914) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HCC (n = 10) |
Liver cirrhosis (n = 10) |
Healthy control (n = 10) |
HCC (n = 295) |
Liver cirrhosis (n = 132) |
Chronic hepatitis (n = 119) |
Non-liver cancers (n = 103) |
Healthy controls (n = 265) |
||
| AFP (ng/ml) | < 20 | 6 | 8 | 10 | 122 | 116 | 101 | 101 | 265 |
| 20–400 | 4 | 2 | 0 | 119 | 15 | 17 | 2 | 0 | |
| > 400 | 0 | 0 | 0 | 54 | 1 | 1 | 0 | 0 | |
| Age (years) | Range | 43–65 | 36–62 | 28–60 | 24–88 | 31–76 | 25–66 | 25–90 | 20–66 |
| (Mean) | (52.6) | (48.3) | (43.5) | (56.3) | (50.2) | (43.2) | (64.0) | (45.8) | |
| Gender | Male | 7 | 7 | 7 | 239 | 101 | 96 | 58 | 166 |
| Female | 3 | 3 | 3 | 56 | 31 | 23 | 45 | 99 | |
| HBV/HCV infection | HBV + | 10 | 10 | 0 | 260 | 108 | 96 | 1 | 0 |
| HCV + | 0 | 0 | 0 | 17 | 15 | 13 | 2 | 0 | |
| HBV −/HCV − | 0 | 0 | 10 | 18 | 9a | 10a | 89b | 265 | |
| HBsAg | Positive | 10 | 10 | 0 | 260 | 108 | 96 | 1 | 0 |
| Negative | 0 | 0 | 10 | 35 | 24 | 23 | 91b | 265 | |
| ALT (U/L) | ≤ 75 | 8 | 8 | 10 | 255 | 107 | 89 | – | 265 |
| > 75 | 2 | 2 | 0 | 40 | 25 | 30 | – | 0 | |
| Child-Pugh | A | 8 | 8 | – | 239 | 81 | – | – | – |
| B | 2 | 2 | – | 42 | 37 | – | – | – | |
| C | 0 | 0 | – | 14 | 11 | – | – | – | |
| Missing | 0 | 0 | – | 0 | 3 | – | – | – | |
| BCLC stage | A | 10 | – | – | 106 | – | – | – | – |
| B | 0 | – | – | 135 | – | – | – | – | |
| C | 0 | – | – | 39 | – | – | – | – | |
| D | 0 | – | – | 15 | – | – | – | – | |
| Tumor differentiation | Well | 4 | – | – | 32 | – | – | – | – |
| Moderate | 6 | – | – | 77 | – | – | – | – | |
| Poor | 0 | – | – | 37 | – | – | – | – | |
| Missing | 0 | – | – | 149 | – | – | – | – | |
| Tumor size (cm) | ≤ 5 | 10 | – | – | 109 | – | – | – | – |
| > 5 | 0 | – | – | 184 | – | – | – | – | |
| Missing | 0 | – | – | 2 | – | – | – | – | |
| Vascular invasion/metastasis | Yes | 0 | – | – | 46 | – | – | – | – |
| No | 10 | – | – | 247 | – | – | – | – | |
| Missing | 0 | – | – | 2 | – | – | – | – | |
HCC, hepatocellular carcinoma; BCLC: Barcelona Clinic Liver Cancer staging system; HBV +: with HBV infection; HCV +: with HCV infection; HBV −/HCV −: without HBV and HCV infection. SERPA: serological proteome analysis. –: data not available.
Alcoholic.
11 non-liver cancer cases miss the data of HBV/HCV infection.
All the samples were stored at − 80 °C until testing. The study protocol was approved by the Clinical Research Ethics Committee of Beijing Friendship Hospital, Capital Medical University. All participants provided their written informed consent to participate in this study.
2.2. SERPA Analysis for the Screening of HCC-related TAAs
Total protein from tumor tissue was extracted as described previously (Wang et al., 2014). Briefly, frozen tissue was lysed in 5 μl lysis buffer (7 mol/L urea, 2 mol/L thiourea, 4% 3-[(3-cholamidopropyl)dimethylamino]-1-propanesulfonate (CHAPS), 1% dithiothreitol (DTT), 2% immobilized pH gradient (IPG) buffer (pH 3–10), protease inhibitor cocktail) per mg tissue, and clarified by centrifugation. After measurement of protein concentration with a 2-D Quant kit (GE Healthcare), the extracted protein was purified with a 2-D Clean-up kit (GE Healthcare). Equal parts of total proteins extracted from tumor tissue of HCC cases were mixed before use.
SERPA analyses were performed as described previously (Li et al., 2008). Briefly, the first dimensional isoelectric focusing (IEF) was performed on pre-cast 18 cm immobilized pH 3–10 gradient (IPG) strips (GE Healthcare) with a total of 800 μg protein. Following IEF, the second dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed and followed by Western Blot analysis with serum samples at 1:100 dilution as the source of primary antibodies. After incubation with horseradish peroxidase (HRP) conjugated goat anti-human IgG (Invitrogen) at 1:5000 dilution, immunoreactive spots were detected using the ECL kit (Millipore) according to the manufacturer's instructions. The proteomic profile of proteins from the HCC tissue was used as a reference map for spot analysis. Spots on immunoblotting maps were matched to the reference map, and those detected in early HCC serum but not in liver cirrhotic or normal sera were excised for protein identification. Matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometer (Bruker Daltonics, Bremen, Germany) was used for identification of the selected proteins. Mascot (Matrix Science, London, UK) was used for protein identification by searching the peak lists against the International Protein Index (IPI) Human database. A protein score with a p-value less than 0.05 was considered significant.
2.3. Protein Microarray Analysis for the Clinical Validation of Screened TAAs
Full length or fragments of recombinant proteins for selected TAAs which were available for enzyme-linked immunosorbent assay (ELISA) were purchased whenever possible. For two TAAs i.e. apoptosis-inducing factor (AIF) and heterogeneous nuclear ribonucleoprotein (hnRNP) A2, the full length recombinant proteins were prepared as described previously (Li et al., 2008).
The preparation of protein microarray and the microarray detection of serum samples were performed according to our previous study (Li et al., 2008). Briefly, the screened TAA proteins were diluted with an optimized individual concentration and robotically attached in ordered arrays on aldehyde-activated glass slides by a computer-controlled microchip spotting instrument (Cartesian Pixsys 3000). Human IgG (Sigma, St. Louis, MO) was used as a positive control and an internal standard for calibration of signal intensity of each test, while bovine serum albumin (Sigma) and sample liquid were used as negative controls. Microarray detection was performed with serum samples at 1:5 dilution. After incubation with rabbit anti-human IgG conjugated to HRP(sigma) at 1:8000 dilution, immunoreactive spots were detected using a chemiluminescent solution (Millipore). The signal intensities of the spots and background values were measured using array vision 7.0 (array vision, USA).
2.4. Western Blots and Immunohistochemistry Analysis for CENPF
For the analysis of levels of autoantibody to centromere protein F (CENPF), a recombinant N-terminal 120–220 amino acid fragment of CENPF protein with an N-terminal glutathione S-transferase (GST) tag was prepared (data not shown) as the antigen for Western Blot analysis, as described in our previous studies (Wang et al., 2014, Li et al., 2008). Briefly, Western Blot analysis was performed with antibody against GST (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:4000 dilution or serum samples at 1:250 dilution as primary antibodies. After incubation with HRP-conjugated goat anti-human IgG (Invitrogen) at 1:5000 dilution, immunoreactive spots were detected using the ECL kit (Millipore) according to the manufacturer's instructions.
For immunohistochemistry (IHC) analysis of CENPF, sections (4 μm thick) were cut, and after deparaffinization of the slides, endogenous peroxidase activity was blocked with 0.3% H2O2 in methanol for 30 min. Immunohistochemical staining was performed using anti-human CENPF antibody (diluted 1:100, Abcam) at 37 °C for 1 h, and visualization of antigen–antibody reactions was achieved with 3,30-diaminobenzidine (Vector SK-4100). Tissue structures were visualized by counterstaining with hematoxylin. The slides were examined separately by two independent pathologists without any prior knowledge of the patient's clinical and pathological parameters.
2.5. Statistical Analysis
Statistical analysis was performed with SPSS for Windows (version 19.0) and MedCalc (version 10.4.7.0). Differences between two independent groups were tested with the ANOVA. Receiver operating characteristic (ROC) curves were constructed to assess sensitivity, specificity, and respective areas under the curves (AUCs) with 95% CI to evaluate the diagnostic value of the serum markers. The optimum cutoff values for diagnosis were determined by calculating the Youden index, and the corresponding signal intensity number was set as a cutoff value for positivity of individual autoantibodies to TAAs. The correlations between autoantibody positivity in serum and clinicopathological characteristics were analyzed using the chi-squared (χ2) test with Yate's correction. We took p values lower than 0.05 (two sided) to indicate statistical significance.
3. Results
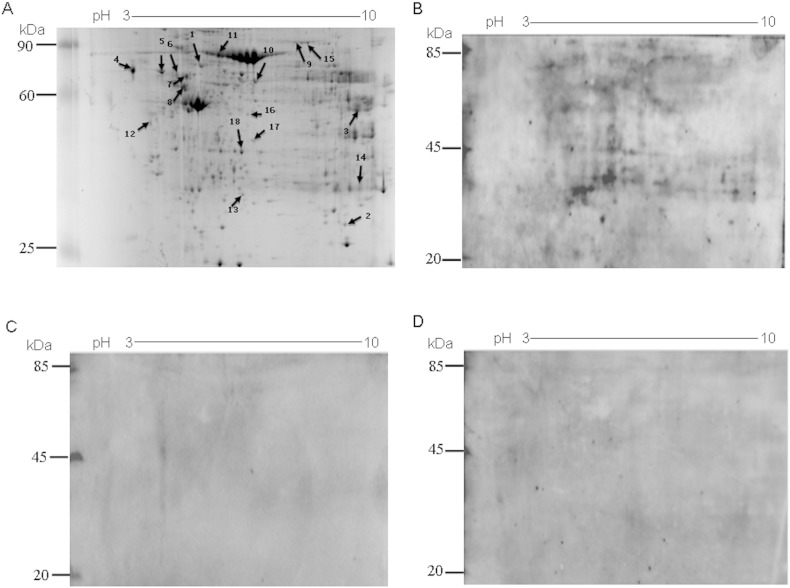
Using an antigen library of a mixture of total proteins extracted from tumor tissue of 10 cases of early HBV related-HCC, SERPA analysis was performed for the screening of HCC-related TAAs. Mixtures of serum samples of ten early HBV-related HCC cases, ten HBV-related cirrhosis cases, and ten healthy controls were used as primary antibody for Western Blot analyses. Supplementary Fig. 1A shows a representative Coomassie blue-stained 2-DE. Different patterns of reactivity were obtained by probing with HCC serum, liver cirrhosis serum, and normal control serum, and representative immunoreactive patterns with HCC, liver cirrhosis and normal control serum are shown in Supplementary Fig. 1B–D. By comparing and matching the antigenic protein profile of each 2-D immunoblot on the original 2-DE, we identified 18 protein spots that were frequently recognized by HCC serum, but not by serum from liver cirrhosis and normal controls (Supplementary Fig. 1A). By MALDI-TOF mass spectrometry analysis, the 18 immunoreactive proteins which exhibited different frequencies of recognition were identified (Supplementary Table 3).
Supplementary Fig. 1.
SERPA analysis of autoantibodies in sera from HCC, liver cirrhosis and normal serum. (A) Coomassie blue-stained 2-DE of proteins isolated from HCC tissue lysates. Arrows indicate the most immunoreactive spots recognized by serum from HCC patients. (B) HCC, (C) liver cirrhosis, or (D) normal control patient sera were used as first antibodies. EasySee Western Marker was used as a loading control.
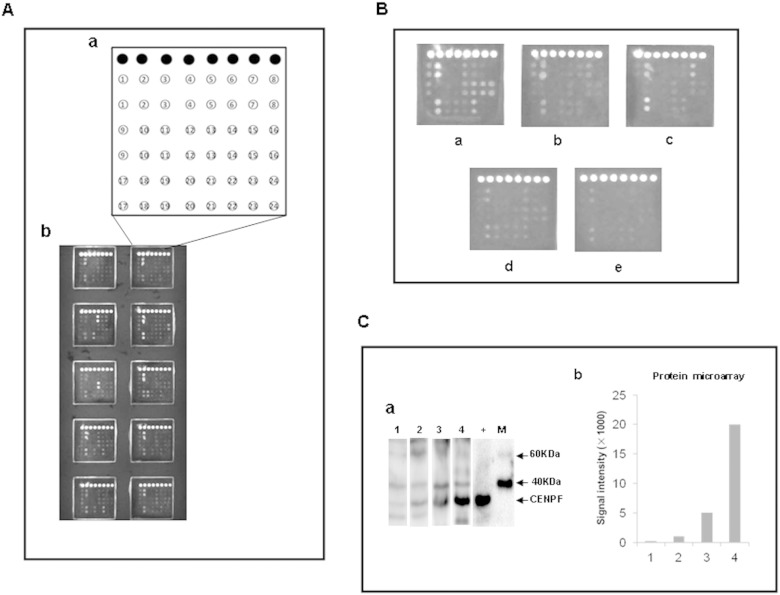
A total of 22 TAAs were used to make protein microarrays for high throughput clinical validation, including the 18 TAAs screened by the present study. For comparison of performance of the TAAs, we also included in the protein microarray two TAAs reported by other studies: insulin-like growth factor 2 mRNA-binding protein 2 (IMP-2) (Zhang and Chan, 2002) and calreticulin (CRT) (Pekarikova et al., 2010), as well as two TAAs identified by our previous study: AIF and hnRNP A2 (Li et al., 2008). The recombinant proteins of the 22 TAAs applied for preparation of the protein microarray are shown in Supplementary Table 4. A schematic representation of antigen array, and the representative scan images of the protein microarray are shown in Fig. 1A and B. Western Blot analysis of three HCC sera with various levels of autoantibody to CENPF identified by the microarray detection showed consistent serum levels of autoantibody to CENPF confirming the results obtained by the microarray detection (Fig. 1C).
Fig. 1.
Microarray detection and validation of autoantibodies in liver cirrhosis, chronic hepatitis, HCC, other cancers and normal serum. A. Schematic representation of the protein microarray for high-throughput clinical validation. (a) Design of the protein microarray. ● IgG, 1. IGKC, 2. HSP60, 3. A1AT, 4. IMP-2, 5. FIBB, 6. HSPA6, 7. ATPB, 8. Bovine serum albumin, 9. ALDH1A1, 10. PDIA1, 11. ENO1, 12. ANXA4, 13. CENPF, 14. SBP1, 15. ACY1, 16. hnRNP A2, 17. K2C1, 18. AIF, 19. CRT, 20. RGN, 21. PRDX3, 22. HINT1, 23. TBB4B, 24. Sample liquid (0.01 M PBS, pH 7.0); (b) scan images of a representative antigen array. B. Microarray detection of serum sample. Individual arrays were incubated with HCC serum (a), liver cirrhosis serum (b), chronic hepatitis serum (c), serum from other cancers (d), or normal serum (e). C. Western Blots showing a pattern of antibody titers to CENPF in HCC sera detected by the protein microarray. (a) Representative Western Blot with recombinant proteins CENPF showing reactivity of HCC sera with various levels of auto-antibody to CENPF detected by the protein microarray. The lane with “+” indicates antibody against GST used as a positive control; lane 1, a normal human serum used as negative control; lanes 2–4, three HCC positive sera 569, 462 and 635, respectively with signal intensities of 1025, 5068 and 20018 detected by the protein microarray; M. EasySee Western Marker (TransGen Biotech, Beijing). (b) Signal intensity of autoantibody against CENPF in HCC sera detected by the protein microarray. Lane 1: the normal serum sample; lanes 2–4: the three HCC positive sera 569, 462 and 635.
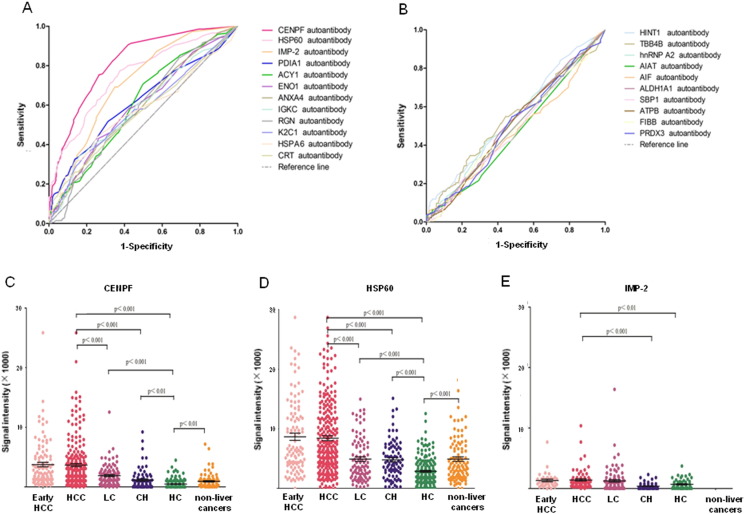
After microarray detection with the 914 serum samples, ROC curves were made for all 22 TAAs based on the individual signal intensity, and the results showed CENPF, 60 kDa heat shock protein (HSP60), IMP-2, protein disulfide-isomerase (PDIA1), aminoacylase-1 (ACY1), alpha-enolase (ENO1), annexin A4 (ANXA4), Ig kappa chain C region (IGKC), regucalcin (RGN), keratin, type II cytoskeletal 1 (K2C1), heat shock 70 kDa protein 6 (HSPA6) and CRT were significantly different (p < 0.05) between HCC and all controls (Fig. 2A–B, Supplementary Table 5). Among them, the three TAAs, CENPF, HSP60 and IMP-2 showed better diagnostic value in HCC or early HCC, with AUC (area under the curve) values of 0.816, 0.750 and 0.708, or 0.826, 0.764 and 0.796 respectively (Table 2), as well as significantly different signal intensities among HCC, liver cirrhosis, chronic hepatitis, healthy control, and other cancers (Fig. 2C–E). Comparison of prevalence of autoantibody positivity to CENPF and HSP60 between HCC and control cases showed significant difference in the number of case with autoantibody positivity to CENPF and HSP60 between HCC and liver cirrhosis or chronic hepatitis or healthy control (Supplementary Table 6). Analysis of the clinicopathological association showed that the prevalence of autoantibody positivity to CENPF and HSP60 was higher in HCC patients who were younger than 50 (p < 0.05), and the prevalence of autoantibody positivity to CENPF was higher in patients with well-differentiated HCC or with Child-Pugh grade C (p < 0.05, Table 3). Notably, for all three TAAs, CENPF, HSP60 and IMP-2, the highest prevalence of autoantibody positivity was observed in HCC cases with tumor stage BCLC A, well-differentiated histology and Child-Pugh grade C (Table 3), suggesting that the TAAs may be a good marker for surveillance and diagnosis of early HCC.
Fig. 2.
ROC curves of the 22 TAAs in discriminating between HCC and controls and representative scatter diagram of signal intensity of CENPF, HSP60 and IMP-2. Upper panel: ROC curve for the 22 TAAs. A. 12 of the 22 TAAs with p-values < 0.05. B. 10 of the 22 TAAs with p-values > 0.05. Lower panel, scatter diagrams: signal intensity in early-HCC, HCC, LC, CH, HC and other cancers. C. CENPF. D. HSP60. E. IMP-2. Black horizontal lines indicate means, and error bars are SEs. ROC, receiver operating characteristic. HCC, hepatocellular carcinoma; LC, liver cirrhosis; CH, chronic hepatitis; HC, healthy controls.
Table 2.
Diagnostic value of autoantibodies to CENPF, HSP60 and IMP-2 for HCC or early-HCC.a
| aAb | Case number HCC/LC/CH/HC |
AUC value | 95% CI | Cutoff value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| CENPF aAb | ||||||||
| HCC | 291/105/83/200 | 0.816 | 0.785 to 0.844 | > 1152 | 75.3 | 73.7 | 68.2 | 79.9 |
| Early-HCC | 106/105/83/200 | 0.826 | 0.790 to 0.859 | > 1152 | 76.2 | 73.7 | 44.0 | 92.0 |
| HSP60 aAb | ||||||||
| HCC | 291/91/85/201 | 0.750 | 0.715 to 0.783 | > 3712 | 77.7 | 62.2 | 61.4 | 78.3 |
| Early-HCC | 106/91/85/201 | 0.764 | 0.723 to 0.801 | > 3712 | 78.1 | 62.2 | 36.6 | 91.1 |
| IMP-2 aAb | ||||||||
| HCC | 92/99/91/65 | 0.708 | 0.656 to 0.756 | > 640 | 69.2 | 65.1 | 37.8 | 87.4 |
| Early-HCC | 38/99/91/65 | 0.796 | 0.746 to 0.841 | > 640 | 81.6 | 65.1 | 25.8 | 96.0 |
Control: liver cirrhosis + chronic hepatitis + healthy controls; aAb: autoantibody; AUC, area under curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; HCC, hepatocellular carcinoma; LC, liver cirrhosis; CH, chronic hepatitis; HC, healthy controls.
Table 3.
Correlation between the prevalence of autoantibody positivity and clinicopathological parameters in HCC.
| Parameters | Prevalence of autoantibody positivity |
|||||
|---|---|---|---|---|---|---|
| CENPF aAb | p value | HSP60 aAb | p value | IMP-2 aAb | p value | |
| Sex | ||||||
| Male | 180/236 (76.3%) | 0.407 | 185/236 (78.4%) | 0.538 | 51/75 (68.0%) | 0.794 |
| Female | 39/55 (71.0%) | 41/55 (74.6%) | 11/17 (64.7%) | |||
| Age | ||||||
| < 50 | 66/78 (84.6%)a | 0.025 | 158/168 (94.1%)a | 0.000 | 20/27 (74.1%) | 0.378 |
| ≥ 50 | 153/213 (71.8%) | 68/123 (55.3%) | 42/65 (64.6%) | |||
| HBV infection | ||||||
| HBV (+) | 198/260 (76.2%) | 0.305 | 200/260 (76.9%) | 0.380 | 57/83 (68.7%) | 0.672 |
| HBV (−) | 21/31 (67.7%) | 26/31 (8.39%) | 5/9 (55.6%) | |||
| Child-Pugh | ||||||
| A | 172/237 (72.6%)a | 0.046 | 180/237 (76.0%) | 0.189 | 48/68 (70.6%) | 0.272 |
| B | 34/40 (87.0%) | 33/40 (85.2%) | 8/16 (50.0%) | |||
| C | 13/14 (92.9%) | 13/14 (92.9%) | 6/8 (75.0%) | |||
| BCLC stage | ||||||
| A | 80/105 (76.2%) | 0.782 | 82/105 (78.1%) | 0.894 | 28/38 (73.7%) | 0.280 |
| B & C & D | 139/186 (74.7%) | 144/186 (77.4%) | 34/54 (63.0%) | |||
| Histology | ||||||
| Well differentiated | 31/32 (96.7%)a | 0.003 | 27/32 (84.4%) | 0.109 | 8/11 (72.7%) | 1.000 |
| Moderately/poorly differentiated | 82/114 (71.9%) | 80/114 (70.2%) | 21/31 (67.7%) | |||
| AFP | ||||||
| ≤ 20 ng/ml | 89/122 (73.0%) | 0.173 | 101/122 (82.8%) | 0.187 | 36/53 (67.9%) | 0.768 |
| 20–400 ng/ml | 84/115 (73.0%) | 84/115 (73.0%) | 24/37 (64.9%) | |||
| > 400 ng/ml | 46/54 (85.2%) | 41/54 (7.59%) | 12/16 (75.0%) | |||
| Tumor size | ||||||
| ≤ 5 cm | 83/109 (76.2%) | 0.786 | 85/109 (78.0%) | 0.920 | 28/38 (73.7%) | 0.280 |
| > 5 cm | 136/182 (74.7%) | 141/182 (77.5%) | 34/54 (63.0%) | |||
| Vascular invasion or metastasis | ||||||
| Yes | 32/46 (69.6%) | 0.330 | 36/46 (78.3%) | 0.916 | 5/7 (71.4%) | 1.000 |
| No | 187/245 (76.3%) | 190/245 (77.6%) | 57/86 (66.3%) | |||
With statistic significance. aAb: autoantibody.
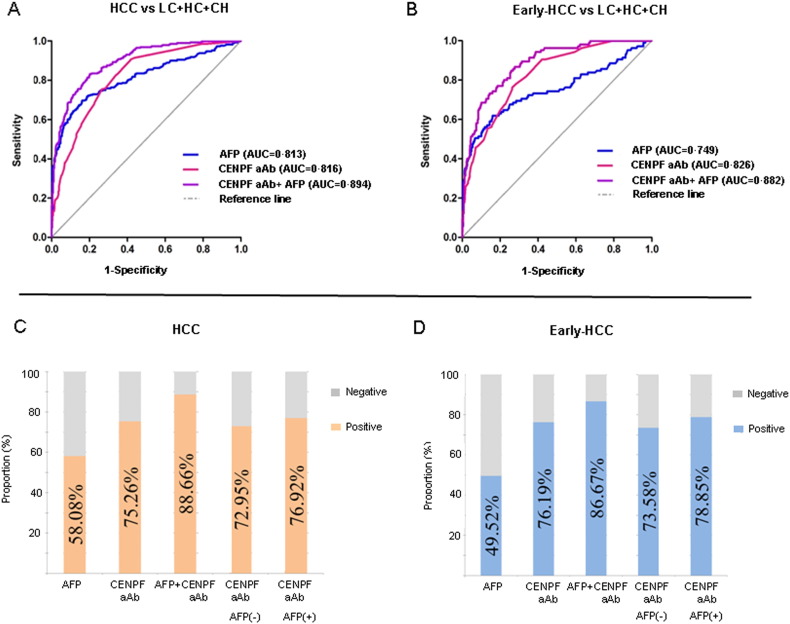
We compared only the diagnostic value of CENPF and HSP60 for early HCC with AFP, because the number of cases with results of IMP-2 autoantibody was insufficient. In contrast with total HCC, better performance of autoantibody to CENPF and HSP60 than AFP was shown when comparing early-HCC and all controls (Table 4, Fig. 3A–D), with AUC values of 0.826, 0.764 and 0.749, respectively. However, for distinguishing early-HCC from liver cirrhosis or liver cirrhosis plus chronic hepatitis, the AUC values of autoantibody to CENPF or HSP60 were similar with that of AFP (Table 4). The prevalence of autoantibody positivity to CENPF and HSP60 was significantly higher than that of AFP positivity in HCCs with tumor stage of BCLC A (p < 0.001) (Supplementary Table 7), suggesting a higher diagnostic value of autoantibody for early HCC compared with the AFP. Specifically, 73.6% and 79.3% cases of early HCC with AFP negativity were seropositive for autoantibody to CENPF or HSP60, with AUC values of 0.828 and 0.779, respectively (Table 5, Fig. 3D). These data indicated that the TAAs could be used as a complement for AFP in the diagnosis of AFP-negative early HCC to improve the detection rate. Combined autoantibody to CENPF with AFP improved the ability to distinguish HCC from all controls, with the AUC value of 0.894, or with sensitivity of 88.7% and specificity of 68.8% in the diagnosis of HCC (Fig. 3A, C); and with AUC of 0.882, or with sensitivity of 86.7% and specificity of 68.8% in the diagnosis of HCC at an early stage (Fig. 3B, D).
Table 4.
Comparison of diagnostic value of CENPF or HSP60 autoantibody with AFP for Early-HCC.a
| aAb &AFP | Cases | AUC value |
95% CI | Sensitivity (%) |
Specificity (%) |
|---|---|---|---|---|---|
| CENPF aAb | Early-HCC vs LC + HC + CH | 0.826 | 0.790 to 0.859 | 76.2 | 73.7 |
| HSP60 aAb | 0.764 | 0.723 to 0.801 | 78.1 | 62.2 | |
| AFP | 0.749 | 0.708 to 0.786 | 49.5 | 93.3 | |
| CENPF aAb | Early-HCC vs LC | 0.660 | 0.592 to 0.724 | 76.2 | 37.1 |
| HSP60 aAb | 0.690 | 0.620 to 0.754 | 78.1 | 50.6 | |
| AFP | 0.675 | 0.607 to 0.738 | 49.5 | 87.6 | |
| CENPF aAb | Early-HCC vs LC + CH | 0.727 | 0.672 to 0.777 | 76.2 | 53.7 |
| HSP60 aAb | 0.689 | 0.631 to 0.742 | 78.1 | 46.0 | |
| AFP | 0.695 | 0.638 to 0.747 | 49.5 | 86.2 |
aAb: autoantibody; AUC, area under curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; HCC, hepatocellular carcinoma; LC, liver cirrhosis; CH, chronic hepatitis; HC, healthy controls. The diagnostic cutoff values of AFP and aAb to CENPF and HSP60 were 20 ng/ml, and signal intensity of 1152 and 3712 respectively.
Fig. 3.
Comparison of the diagnostic value among autoantibodies to CENPF, AFP and combined autoantibody to CENPF and AFP for HCC or early HCC. Upper panel: ROC curve for autoantibodies to CENPF, AFP and combined autoantibody to CENPF and AFP to distinguish HCC (A) or early HCC (B) from controls (LC + CH + HC). Lower panel: comparison of positivity for the autoantibodies to CENPF, AFP and combined autoantibody to CENPF and AFP in HCC (C) or early-HCC (D). ROC, receiver operating characteristic. HCC, hepatocellular carcinoma; LC, liver cirrhosis; CH, chronic hepatitis; HC, healthy controls; aAb: autoantibody.
Table 5.
The diagnostic value of autoantibody to CENPF and HSP60 in AFP negative early-HCC.a
| aAb | Cases | AUC value | 95% CI | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| CENPF aAb | Early-HCC vs LC + HC + CH | 0.828 | 0.790 to 0.862 | 73.6 | 73.7 | 27.7 | 95.3 |
| Early-HCC vs LC | 0.659 | 0.579 to 0.732 | 73.6 | 37.1 | 37.1 | 73.6 | |
| Early-HCC vs LC + CH | 0.727 | 0.666 to 0.782 | 73.6 | 53.7 | 31.0 | 87.8 | |
| HSP60 aAb | Early-HCC vs LC + HC + CH | 0.779 | 0.737 to 0.817 | 79.3 | 62.2 | 22.8 | 95.5 |
| Early-HCC vs LC | 0.706 | 0.624 to 0.779 | 79.3 | 50.6 | 48.3 | 80.7 | |
| Early-HCC vs LC + CH | 0.706 | 0.643 to 0.765 | 79.3 | 46.0 | 30.7 | 88.0 |
aAb: autoantibody; AUC, area under curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value. The diagnostic cutoff values of aAb to CENPF and HSP60 were signal intensity of 1152 and 3712 respectively. HCC, hepatocellular carcinoma; LC, liver cirrhosis; CH, chronic hepatitis.
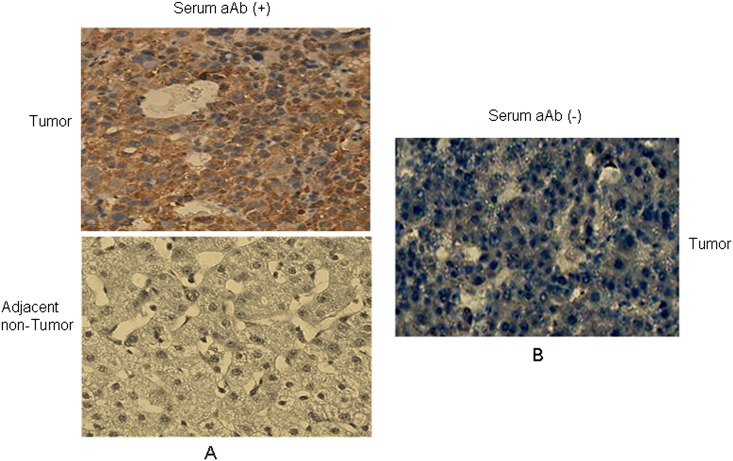
To explore the possible mechanism underlying the occurrence of autoantibody to CENPF in HCC, we analyzed the expression level and cellular localization of CENPF in HCC with various levels of autoantibody to CENPF. IHC analysis was performed on five HCC cases with high levels of serum autoantibody to CENPF (signal intensity more than 4000) and five HCC cases with negative serum autoantibody to CENPF (signal intensity less than 500). The results showed that in all HCC cases evaluated, CENPF was found in the cytoplasm or nucleus. However, overexpression of CENPF was observed in tumor tissue of all HCC cases with high levels of serum autoantibody to CENPF, while low or no CENPF expression was observed in the tumor tissue of all HCC cases with low levels of serum autoantibody to CENPF (Supplementary Fig. 2). The data suggest that the elevated CENPF autoantibody may have resulted from overexpression of CENPF protein.
Supplementary Fig. 2.
IHC analysis of expression level and cellular localization of CENPF in HCC with different levels of autoantibody to CENPF. A. A pair of representative HCC case with positive serum autoantibody to CENPF (signal intensity 4680). B. CENPF expression in a representative HCC case with negative serum autoantibody to CENPF (signal intensity 250). aAb: autoantibody. Original magnification: × 40.
4. Discussion
Although there has been an increase in the number of reports of TAAs in various types of tumor including HCC (Yau et al., 2013, Werner et al., 2015, Lacombe et al., 2014), the potential value of autoantibodies in the early diagnosis of HCC remains unclear. The major concern is that the majority of studies initially screen small sample sizes without validation by large-scale samples. In the present study, using high throughput screening and clinical validation, we reported here a series of TAAs, which might be valuable markers for early detection of HCC.
Simultaneous clinical validation of dozens of candidate bio-markers with large-scale samples is extremely difficult. It is almost impossible to conduct large-scale screening and validation studies for specific tumor markers. The traditional techniques such as ELISA, Western Blot analysis, and radioimmunoassays which require relatively large quantities of antigen and patient samples are of limited use when performing large-scale clinical validations (Robinson et al., 2002), especially when performing numerous simultaneous validations. In the present study, we believe that we have identified for the first time, a series of TAAs in HCC using SERPA analysis with total protein extracted from tumor tissue as antigen library. We have also validated the diagnostic value of candidate TAAs compared with that of AFP in a large cohort of early HCC. Twelve of the 22 candidate TAAs were identified with significance differences between HCC and controls. Five of the 12 screened TAAs have been reported previously in HCC or other cancers, including IMP-2 (Zhang and Chan, 2002), CRT (Pekarikova et al., 2010), ENO1 (Takashima et al., 2005), CENPF (Liu et al., 2012), and HSP60 (He et al., 2007), whereas the other seven TAAs in HCC are reported here for the first time, to the best of our knowledge. Three TAAs, CENPF, HSP60, and IMP-2 showed promise for diagnosis of HCC and early HCC, with an AUC value of more than 0.7, a recognized standard for biomarkers, which have a promising diagnostic value in general (Shen et al., 2012). However, the fact that the majority of screened TAAs were identified with low value for diagnosis suggests the importance of high throughput clinical evaluation of the candidate TAAs.
As an essential nuclear protein associated with the centromere–kinetochore complex, CENPF plays a critical role in chromosome segregation during mitosis (Dai et al., 2013). Researchers have demonstrated that CENPF is overexpressed in a wide variety of human malignancies including HCC, and CENPF is an independent prognostic factor for HCC (Dai et al., 2013). A recent study has also reported detection of autoantibodies to CENPF in HCC by screening a T7 cDNA expression library (Liu et al., 2012). HSP60 is a chaperone with essential functions for cell physiology and survival, and has been reported to be involved in the pathogenesis of a number of cancers and some autoimmune disorders (Calderwood et al., 2012). HSP60 has also been shown to be present in a broad spectrum of cancers in both tissue and serum (Hamelin et al., 2011), and has been identified as a TAA in breast cancer, colorectal cancer and ovarian cancer (He et al., 2007, Bodzek et al., 2014). The autoantibodies to CENPF and HSP60 were also identified in the present study, but unlike the results of previous studies, their diagnostic value for early HCC was demonstrated through high throughput clinical evaluation. IMP-2 and CRT have been reported as TAAs of HCC and many other types of cancer by several studies (Zhang and Chan, 2002, Pekarikova et al., 2010), but not identified in the present study. However, the diagnostic value of IMP-2 for HCC was confirmed in the present study. Although there was a significant difference between autoantibody levels to CRT in HCC compared to controls, the AUC value was only 0.566, suggesting low diagnostic value for autoantibody to CRT. Another Ca-regulating protein, RGN for Ca-binding, also showed a statistically significant difference between HCC and controls, but with an AUC value of only 0.582 suggesting a similar diagnostic value as CRT.
So far, there has been less analysis concerning the clinicopathological association and comparison with AFP in the studies concerning anti-TAA autoantibodies in cancer (Yau et al., 2013, Werner et al., 2015, Lacombe et al., 2014). In the present study, clinicopathological analysis demonstrated that three TAAs, CENPF, HSP60 and IMP-2 had the highest prevalence of autoantibody positivity in HCC cases with tumor stage BCLC A, well-differentiated histology and Child-Pugh grade C. Therefore, the clinicopathological analysis in the present study implies that TAAs may have value in surveillance and diagnosis of early HCC. To date, AFP is still the main serum biomarker for HCC surveillance. However, AFP does not yield satisfactory results in the early diagnosis of HCC, particularly AFP-negative HCC. It has been reported that in small hepatic tumors, AFP expression is lower, whereas AFP expression is high in large tumors (Zhao et al., 2013). The AFP level was approximately correlated with tumor size; 80% of small HCCs did not have increased levels of AFP. The sensitivity of AFP was 52% when the tumor diameter was > 3 cm, but decreased to 25% for tumors < 3 cm (Farinati et al., 2006, Zhao et al., 2013). The present study showed that several anti-TAA autoantibodies were better than AFP for the diagnosis of early HCC when analyzed with all controls, whereas the efficacy of the TAAs was similar to AFP in distinguishing early HCC from liver cirrhosis. HCCs occur generally from liver cirrhosis. Although the specificity of CENPF (37.1%) or HSP60 (50.6%) autoantibody seemed to be relatively low for the discrimination of HCC from liver cirrhosis, with autoantibody positivity to CENPF or HSP60 in 73.6% or 79.3% of AFP negative early HCC cases (see Table 5), the TAAs could be used as a complement for AFP in the diagnosis of AFP-negative early HCC to improve the detection rate of early HCC, and the combined autoantibody to TAAs with AFP could be helpful for the detection of AFP-negative early HCC. It is worthy to note that the prevalence of the above TAAs in LC patients was relatively high, suggesting their potential value in the detection of LC, and should be investigated further.
The generation of autoantibodies to TAAs is not fully understood. TAA proteins are most likely either mutated, overexpressed, post-translationally modified, misfolded, aberrantly cleaved, or aberrantly localized in tumor cells (Casiano et al., 2006). It has been reported that HSP60 localizes mainly in the mitochondria, but in tumor cells it is also found in the cytoplasm and the cell membrane, leading to activation of autoimmune reactions (Cappello et al., 2013). In one recent study on breast cancer with serum anti-HSP60 autoantibody, it was demonstrated that the level of expression of HSP60 was significantly higher in tumor tissues suggesting that overexpression of HSP60 may be at least one of the mechanisms of developing immunogenicity against this protein in breast cancer patients (Cappello et al., 2013). Similarly, the present study also showed that the high titer of CENPF autoantibody in HCC serum may result from an auto-immune reaction in response to overexpression of CENPF, and may constitute one of the mechanisms for the generation of autoantibody to CENPF.
One limitation of our study is that the majority of sera were from patients with HBV-related disease and from China. Although HCV infections, as well as alcohol abuse are important causes of HCC, the effects of these factors were not studied. However, we plan to study the contributions of these factors in the future. Due to lack of availability of IMP-2 protein, IMP-2 was only studied in a small number of samples. Studies with more samples are needed. In addition, it is important to evaluate the data of TAAs before and after HCC occurrence, as well as before and after therapy. We plan to conduct another follow-up research in the future to evaluate further the efficacy of TAAs before and after HCC occurrence and the data of TAAs before and after therapy, as well as the evaluation for the accuracy of cut-off value of the TAAs by another independent cohort.
In conclusion, our data showed that autoantibodies against TAAs may be useful in the detection of HCC at early stages, and could be complementary to AFP as a screening test for HCC.
The following are the supplementary data related to this article.
Supplementary tables.
Contributors
YH and JH designed the study, did the experiments, analyzed and interpreted the data, and wrote the manuscript. JL and HaL provided patients' samples and clinical data, analyzed and interpreted the data, and wrote the paper. SC, QL, XH, BZ, YW, HY and MZ did the experiments, analyzed and interpreted the data. BC, ZZ, JZ, XO and HM provided, analyzed, and interpreted patients' samples and clinical data. HoL, YL, TZ, CL, QL, ZB, JW and SZ provided patients' samples, clinical data, or both. SW, HY and JJ advised on the conception, design of the study and supervised the project. JH conceptualized and designed the study, supervised the project, and revised the paper. All authors vouch for the respective data and analysis, approved the final version and agreed to publish the manuscript.
Conflicts of Interest
We declare that we have no conflicts of interest.
Acknowledgments
The authors thank Dr Guang-Yong Chen and Dr Xiao-Yan Shi (Department of Pathology, Beijing Friendship Hospital, Capital Medical University) for critical pathological review. This study was supported by a grant from the National Natural Science Foundation of China (no. 81071973), a grant from the Scientific Research Foundation for Returned Overseas Chinese Scholars, Bureau of Human Resources and Social Security of Beijing, China (Key project, 2010, no. 20110323), and a grant from the program for National Science and Technology Major Project (2013ZX10002004), and the funders had no role in study design, data collection, data analysis, interpretation, and writing of the report.
Contributor Information
Hong You, Email: youhong30@sina.com.
Shengqi Wang, Email: sqwang@bmi.ac.cn.
Jian Huang, Email: huangj1966@hotmail.com.
References
- Bertino G., Ardiri A., Malaguarnera M. Hepatocellular carcinoma serum markers. Semin. Oncol. 2012;39:410–433. doi: 10.1053/j.seminoncol.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Bodzek P., Partyka R., Damasiewicz-Bodzek A. Antibodies against Hsp60 and Hsp65 in the sera of women with ovarian cancer. J. Ovarian Res. 2014;7:30–37. doi: 10.1186/1757-2215-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruix J., Sherman M., Practice Guidelines Committee, American Association for the study of Liver Disease Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- Calderwood S.K., Stevenson M.A., Murshid A. Heat shock proteins, autoimmunity, and cancer treatment. Autoimmune Dis. 2012:486069. doi: 10.1155/2012/486069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello F., Angileri F., de Macario E.C. Chaperonopathies and chaperonotherapy. Hsp60 as therapeutic target in cancer: potential benefits and risks. Curr. Pharm. Des. 2013;19:452–457. [PubMed] [Google Scholar]
- Casiano C.A., Mediavilla-Varela M., Tan E.M. Tumor-associated antigen arrays for the serological diagnosis of cancer. Mol. Cell. Proteomics. 2006;5:1745–1759. doi: 10.1074/mcp.R600010-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Liu L., Zeng T., Zhu Y.H., Li J., Chen L., Li Y., Yuan Y.F., Ma S., Guan X.Y. Characterization of the oncogenic function of centromere protein F in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2013;436:711–718. doi: 10.1016/j.bbrc.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Farinati F., Marino D., De Giorgio M. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am. J. Gastroenterol. 2006;101:524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- Hamelin C., Cornut E., Poirier F. Identification and verification of heat shock protein 60 as a potential serum marker for colorectal cancer. FEBS J. 2011;278:4845–4859. doi: 10.1111/j.1742-4658.2011.08385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Wu Y., Mou Z. Proteomics-based identification of HSP60 as a tumor-associated antigen in colorectal cancer. Proteomics Clin. Appl. 2007;1:336–342. doi: 10.1002/prca.200600718. [DOI] [PubMed] [Google Scholar]
- Lacombe J., Mangé A., Solassol J. Use of autoantibodies to detect the onset of breast cancer. J. Immunol. Res. 2014:574981. doi: 10.1155/2014/574981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.M., Yoon J.H., Kim K.W. Diagnosis of hepatocellular carcinoma: newer radiological tools. Semin. Oncol. 2012;39:399–409. doi: 10.1053/j.seminoncol.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Li L., Chen S.H., Yu C.H. Identification of hepatocellular carcinoma associated antigens and autoantibodies by serological proteome analysis combined with protein microarray. J. Proteome Res. 2008;7:611–620. doi: 10.1021/pr070525r. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhang J., Wang S. Screening of autoantibodies as potential biomarkers for hepatocellular carcinoma by using T7 phase display system. Cancer Epidemiol. 2012;36:82–88. doi: 10.1016/j.canep.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Llovet J.M., Di Bisceglie A.M., Bruix J. Design and endpoints of clinical trials in hepatocellular carcinoma. J. Natl. Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- Lok A.S., McMahon B.J. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- Pekarikova A., Sanchez D., Palova-Jelinkova L. Calreticulin is a B cell molecular target in some gastrointestinal malignancies. Clin. Exp. Immunol. 2010;160:215–222. doi: 10.1111/j.1365-2249.2009.04085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon D., Anderson B.O., Chen L.T. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- Robinson W.H., DiGennaro C., Hueber W. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat. Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- Shen Q., Fan J., Yang X.R. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817–826. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- Stefaniuk P., Cianciara J., Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J. Gastroenterol. 2010;16:418–424. doi: 10.3748/wjg.v16.i4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima M., Kuramitsu Y., Yokoyama Y. Overexpression of alpha enolase in hepatitis C virus-related hepatocellular carcinoma: association with tumor progression as determined by proteomic analysis. Proteomics. 2005;5:1686–1692. doi: 10.1002/pmic.200401022. [DOI] [PubMed] [Google Scholar]
- Tan H.T., Low J., Lim S.G. Serum autoantibodies as biomarkers for early cancer detection. FEBS J. 2009;276:6880–6904. doi: 10.1111/j.1742-4658.2009.07396.x. [DOI] [PubMed] [Google Scholar]
- Theise N.D., Chen C.J., Kew M.C. Liver cancer. In: Stewart B., Wild C., editors. World Cancer Report 2014. 2014. pp. 577–593. [Google Scholar]
- Wang Y., Li M., Long J. Clinical significance of increased expression of Nijmegen breakage syndrome gene (NBS1) in human primary liver cancer. Hepatol. Int. 2014;8:250–259. doi: 10.1007/s12072-013-9500-x. [DOI] [PubMed] [Google Scholar]
- Werner S., Chen H., Tao S. Systematic review: serum autoantibodies in the early detection of gastric cancer. Int. J. Cancer. 2015;136:2243–2252. doi: 10.1002/ijc.28807. [DOI] [PubMed] [Google Scholar]
- Xu Y.W., Peng Y.H., Chen B. Autoantibodies as potential biomarkers for the early detection of esophageal squamous cell carcinoma. Am. J. Gastroenterol. 2014;109:36–45. doi: 10.1038/ajg.2013.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau W.Y., Shih H.C., Tsai M.H. Autoantibody recognition of an N-terminal epitope of hnRNP L marks the risk for developing HBV-related hepatocellular carcinoma. J. Proteomics. 2013;94:346–358. doi: 10.1016/j.jprot.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chan E.K. Autoantibodies to IGF-II mRNA binding protein p62 and overexpression of p62 in human hepatocellular carcinoma. Autoimmun. Rev. 2002;1:146–153. doi: 10.1016/s1568-9972(02)00030-7. [DOI] [PubMed] [Google Scholar]
- Zhao Y.J., Ju Q., Li G.C. Tumor markers for hepatocellular carcinoma. Mol. Clin. Oncol. 2013;1:593–598. doi: 10.3892/mco.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.