Abstract
Acute myeloid leukemia is the most common indication for an allogeneic hematopoietic cell transplant. The introduction of reduced intensity conditioning has expanded the recipient pool for transplantation, which has importantly made transplant an option for the more commonly affected older age groups. Reduced intensity conditioning allogeneic transplantation is currently the standard of care for patients with intermediate or high-risk acute myeloid leukemia and is now most often employed in older patients and those with medical comorbidities. Despite being curative for a significant proportion of patients, post-transplant relapse remains a challenge in the reduced intensity conditioning setting. Herein we discuss the studies that demonstrate the feasibility of reduced intensity conditioning allogeneic transplants, compare the outcomes of reduced intensity conditioning versus chemotherapy and conventional myeloablative conditioning regimens, describe the optimal donor and stem cell source, and consider the impact of post-remission consolidation, comorbidities, center experience, and more intensive (reduced toxicity conditioning) regimens on outcomes. Additionally, we discuss the need for further prospective studies to optimize transplant outcomes.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an established treatment modality that is potentially curative for many patients with acute myeloid leukemia (AML).1–5 AML itself is the most common indication for adults undergoing HCT today. For patients with high-risk disease, HCT is perhaps the most effective curative treatment and is considered the standard post-remission therapy in first complete remission (CR).6–8 In the early years of HCT, only younger patients with AML were considered eligible for transplantation due to the toxicity inherent in the conventional myeloablative conditioning (MAC) regimens; thus, in previous times, cure for the disease was often available only to the young and the fit.
In the USA, the Surveillance, Epidemiology, and End Results (SEER) data show that the average age at diagnosis of AML is 66 years with more than 60% of cases occurring in patients over the age of 55 (SEER Database, http://seer.cancer.gov). Clearly, AML is a disease of older patients, and over the years, recognition of the need to offer transplantation to older adults and/or patients with comorbid disease has spurred the development of less toxic, more tolerable preparative regimens – the so-called reduced intensity conditioning (RIC) regimens. The hazard of death associated with HCT has improved significantly over the past decade, with a reduction in non-relapse mortality (NRM) of over 50% along with better long-term survival after HCT, and this is felt to be due in large part to the introduction of RIC regimens. Considering that allogeneic transplants are being increasingly performed in older patients with higher risk disease and more comorbid illness, this reduction in NRM is remarkable.9
The pre-transplantation conditioning regimen has two primary goals: to suppress the host’s immune system sufficiently to allow adequate engraftment of the donor’s cells and to reduce the presence of any residual neoplastic cells. Historically the emphasis of the therapeutic effect of HCT was on the conditioning regimen, with the thought that the more intensive the regimen, the more effective.10 However, in recent years, the paradigm has shifted to optimization of the therapeutic impact of the graft-versus-leukemia effect as opposed to just the cytotoxic effects of the conditioning regimen alone.
Background to the development of reduced intensity conditioning
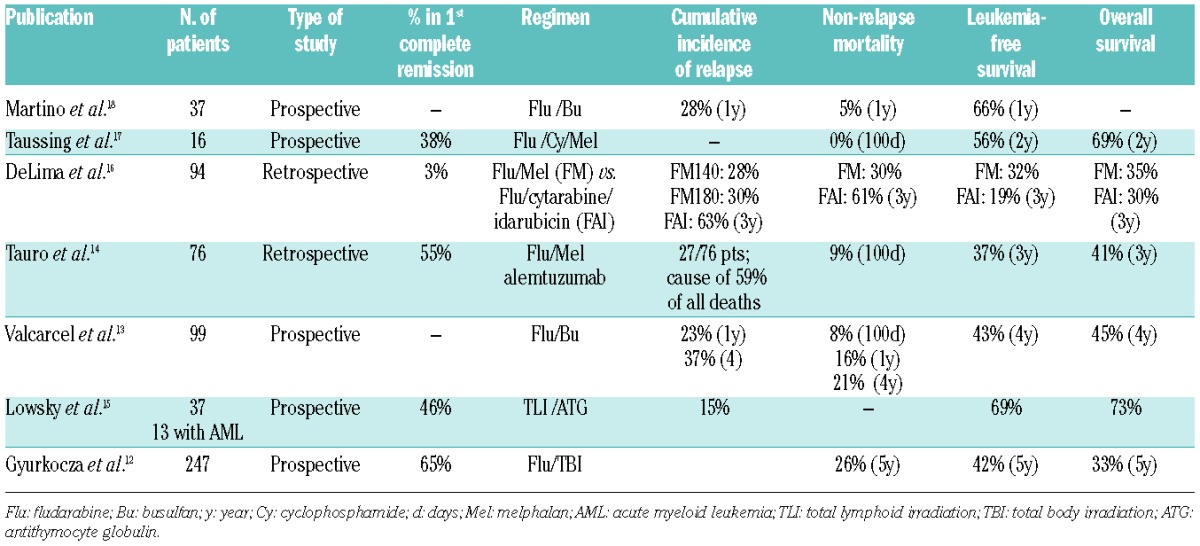
Over the past two decades, the development of RIC regimens has come in the form of: (i) the introduction of the purine analog, fludarabine; and (ii) dose reductions of alkylating agents or total body irradiation (TBI). Regimens that relied on fludarabine or lower doses of the conditioning agents were referred to as either non-myeloablative or RIC. Non-myeloablative regimens differ from RIC regimens in that the former may result in only minimal cytopenias that do not require stem cell support whereas RIC regimens do require stem cell support.11 The introduction of fludarabine revolutionized the development of RIC regimens, and it now serves as the backbone of most RIC regimens which also include either a reduced dose of an alkylating agent or a reduced dose of TBI. Fludarabine is generally well tolerated and synergizes well with alkylating agents to enhance inhibition of DNA repair mechanisms.10 Multiple RIC regimens have been developed and described12–19 (Table 1).
Table 1.
Reduced intensity conditioning studies in myeloid malignancies.
In the late 1990s and early 2000s, the feasibility and efficacy of lower intensity conditioning regimens were demonstrated in several studies that showed successful engraftment in recipients of grafts from both related19,20 and unrelated donors.21,22 These regimens were also demonstrated to be a treatment modality that can be successful in older patients with hematologic malignancies.23 McSweeney et al. described 45 patients with a median age of 56 years who had human leukocyte antigen (HLA)-identical sibling donors and relative contraindications to conventional conditioning for HCT. In these patients a conditioning regimen of TBI alone (200 cGy) produced a survival rate of >66% after a median follow-up of 417 days with a NRM rate of only 6.7%. The associated toxicities were mild, and over 50% of patients were able to have their transplant done completely in the outpatient setting.23 This set the stage for future studies focusing on the potent immunological graft-versus-leukemia effect to induce cures as opposed to just on intensive pre-transplantation marrow ablative strategies.
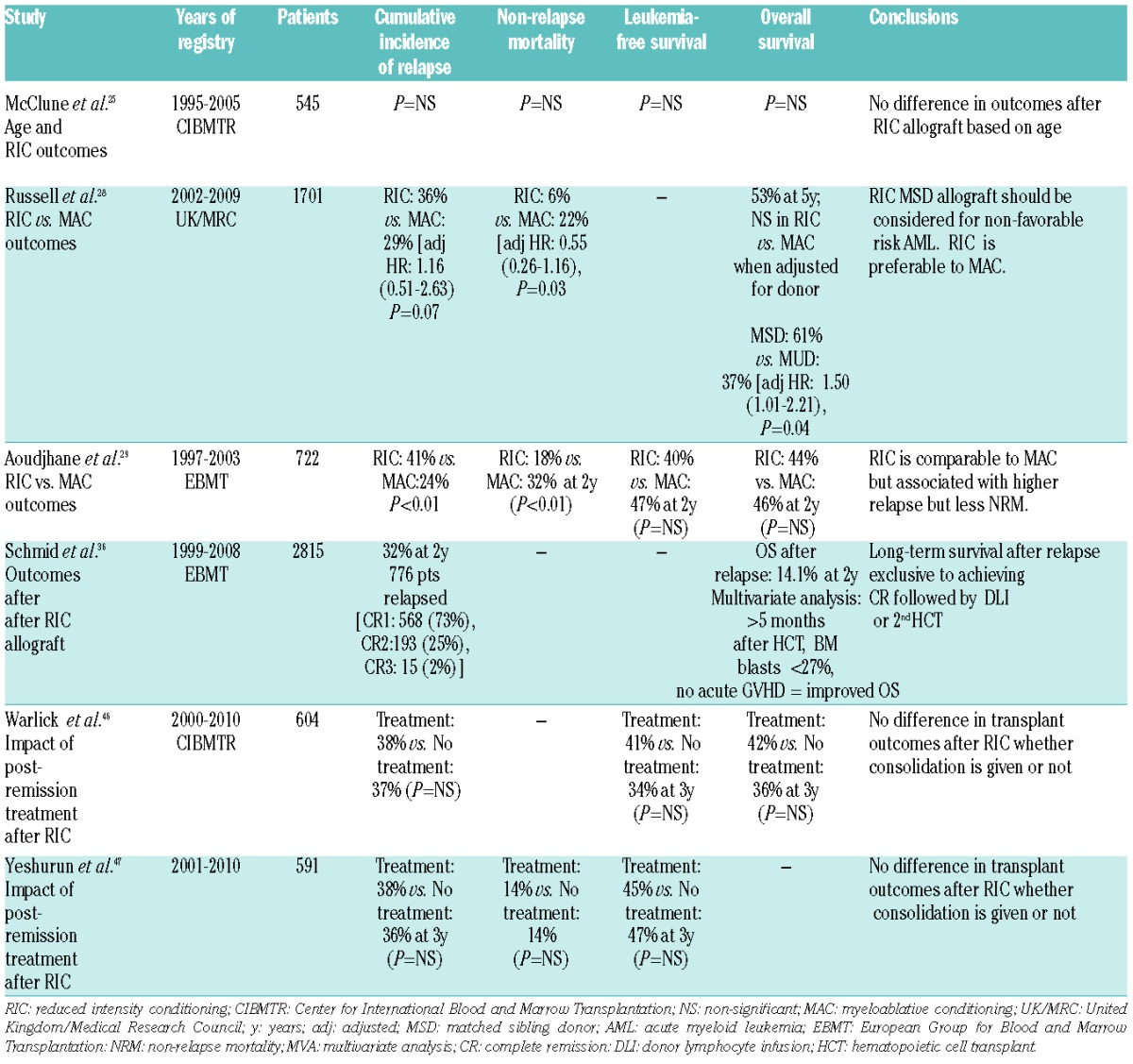
As the number of efficacious regimens grew, so did the number of patients for whom transplantation became a therapeutic option. Much enthusiasm has led to the widespread adoption of RIC HCT as a potentially curative option for older patients or those with comorbid disease, despite lack of supportive, prospective, randomized data. Nevertheless, studies have shown that age itself does not significantly affect outcomes,24,25 and RIC regimens are tolerated well allowing transplants to be offered to patients up to the age of 70. McClune et al. reported a large retrospective study of the Center for International Blood and Marrow Transplant (CIBMTR) registry investigating outcomes of 1080 patients over the age of 40 and showed that neither age nor type of conditioning regimen had any impact on survival25 (Table 2). The results of this study led to the conclusion that older age alone should not be a contraindication to HCT as similar outcomes are seen in both younger and older age groups. This has been very important for AML, a disease that typically affects a more elderly population. In this sense, the development of RIC HCT has revolutionized the therapeutic landscape for older patients with AML.
Table 2.
Registry studies of reduced intensity conditioning allografts.
Is reduced intensity conditioning better than standard chemotherapy?
There are few studies that directly address the question of whether RIC HCT is better than standard chemotherapy. Mohty et al. reported a donor versus no donor comparison study that described the utility of RIC HCT in patients over the age of 50 years with high-risk AML in CR. This study included 95 patients of whom 35 (37%) with a sibling donor would go on to have a RIC HCT, while the remainder without a donor went on to receive standard chemotherapy. In their intention-to-treat analysis, the patients with donors who underwent RIC HCT with a regimen of fludarabine, busulfan, and anti-thymocyte globulin had a significantly higher 4-year leukemia-free survival (LFS) rate of 54% than the 30% in the no donor group (P=0.01). Overall survival (OS) was also improved (P=0.01), and in their multivariate analysis, the actual performance of a RIC HCT was the strongest predictor of improved LFS [relative risk (RR)=4.0; 95% confidence interval (CI): 1.7–9.6].26 Kurosawa et al. similarly compared a small series of patients who underwent RIC HCT between the ages of 50–70 and patients who underwent standard chemotherapy; the outcomes of the former were superior, with a reduced cumulative incidence of relapse (22% versus 62%), and improved LFS and OS.27
Similarly, Russell et al. evaluated patients who were enrolled in the United Kingdom Medical Research Council AML15 (UK MRC AML15) trial and in their comparative analysis of patients who underwent RIC HCT versus standard chemotherapy alone, RIC transplantation significantly reduced the risk of relapse. However, there was no difference in OS at 5 years, and no evidence of benefit when stratified by risk groups. There was also significant heterogeneity of outcome when RIC transplants were split by donor type, with an OS benefit for those patients who received RIC matched related donor allografts. When RIC and MAC were compared, no significant difference in cumulative incidence of relapse was detected, and RIC transplants were associated with a lower risk of NRM due to death from infection and organ toxicity. The conclusions from this study were that patients between 35 and 60 years old who do not have favorable risk AML should be considered for a RIC allograft if a sibling donor is available, and that a RIC regimen is preferable to a myeloablative approach28 (Table 2).
How does reduced intensity conditioning compare to standard conditioning?
The first study to compare outcomes of conventional MAC regimens versus RIC was that by Aoudjhane et al. This European Group for Blood and Marrow Transplantation (EMBT) registry study looked at 722 patients with AML over the age of 50 who underwent HCT. Four hundred and seven patients received MAC, which consisted of TBI doses >10 Gy or busulfan doses >8 mg/kg plus other drugs, while 315 patients who underwent RIC regimens that included fludarabine in combination with low dose TBI (<2 Gy) or busulfan doses <8 mg/kg. The results showed that NRM was higher after MAC than after RIC, while RIC transplants were associated with a higher relapse risk. In multivariate analysis, relapse risk continued to be statistically significant for patients who underwent RIC transplants. There was, however, no difference in 2-year LFS or OS. The incidences of grades II-IV acute graft-versus-host disease (GVHD) and chronic GVHD were also lower after RIC HCT although GVHD remained a major cause of non-leukemic death29 (Table 2). Other studies would show similar results with regards to the lack of difference between RIC and MAC HCT on long-term outcomes while similarly detecting a higher risk of relapse after RIC. A difference was seen when comparing non-myeloablative and RIC regimens, suggesting that at least some dose intensity is required to optimize outcomes.30,31 However, a recent study investigating whether higher doses of busulfan (6.4 mg/kg versus 3.2 mg/kg) affected outcomes gave negative results.32 The explanation for the lack of difference in long-term outcomes with regards to LFS and OS is thought to be a balance between the lower overall NRM that was previously associated with predominantly MAC transplants and the higher risk of relapse now associated with RIC HCT.
Most of the studies comparing outcomes between RIC and MAC HCT in AML have been retrospective in nature. Bornhäuser et al. published the first randomized phase 3 trial that compared RIC regimens versus standard regimens and their impact on the outcomes of NRM, incidence of relapse, LFS, and OS in patients with intermediate- or high-risk AML in first CR.33 RIC regimens consisted of four doses of 2 Gy of TBI and 150 mg/m2 of fludarabine versus a standard conditioning regimen of six doses of TBI for a total of 12 Gy of TBI and 120 mg/kg of cyclophosphamide. The median ages of the patients in the study were 44 and 45 years for the RIC and MAC groups, respectively, with the majority of patients being 41 to 60 years old. All the patients received standard cyclosporine and methotrexate as GVHD prophylaxis. Although the study was concluded early due to slow accrual of patients, 195 patients were included in the analysis, and the primary endpoint of NRM did not differ significantly between the two groups nor did the secondary endpoints of cumulative incidence of relapse, LFS, or OS differ. Moreover, other effects such as severe mucositis and inhospital mortality were less frequent in the RIC group, leading to the conclusion that RIC regimens lessened the toxic effects of transplantation, and the 1-year mortality rate was lower. As time went on, the later outcomes tended to be independent of the conditioning regimen and more affected by post-transplant issues of chronic GVHD and relapse. The limitations of the study included a possible selection bias, an upper age limit of 60 years, and the investigation of a conditioning regimen that is less reminiscent of more commonly used RIC or non-myeloablative regimens.33 Nonetheless, this was the first prospective randomized trial that directly compared a MAC regimen versus a RIC regimen and demonstrated similar outcomes. These results suggest that perhaps RIC regimens should be used preferentially in patients who are younger than 60 with AML in first CR.
What about younger patients who may not be candidates for standard MAC¿ Reports on outcomes after RIC regimens for younger patients in the adult literature are scarce as this population has been conventionally treated with MAC transplants. Sebert et al. recently described their retrospective study of patients aged 35 and older who underwent allogeneic transplants from 2000 to 2010 and found similar outcomes in patients who received RIC and MAC regimens. Relapse rates did not differ and survival outcomes at 4 years were similar.34 As mentioned above, the UK MRC AML15 trial of patients aged 35 to 60 years with non-favorable-risk AML in first CR who underwent either RIC or MAC showed no significant difference in cumulative incidence of relapse according to conditioning regimen. A survival benefit was initially detected for RIC but when adjusted for donor type, was no longer significant, potentially because of the reduced NRM associated with RIC sibling allografts which provided better outcomes than sibling MAC transplants in terms of survival (69% versus 57%). It was concluded that RIC allografts, particularly from sibling donors, should be preferentially considered in patients within the 35–60 age group28 (Table 2). Further prospective studies are needed to determine whether RIC regimens can indeed be applied more widely even in younger patients (less than 35 years old) perhaps with the benefit of reducing NRM.
What are the downsides of reduced intensity conditioning regimens?
As previously mentioned, relapse risks are higher with RIC regimens than with standard conditioning regimens and thus, relapse remains the leading cause of treatment failure.14,29,35 A large retrospective registry study of the EBMT by Schmid et al. looked into relapse risk in 2815 patients, and found a cumulative incidence of relapse of 32% ± 1%. Of the 263 patients who relapsed, 32% were able to achieve another CR. Relapsed patients were treated with discontinuation of immunosuppression as well as some form of anti-leukemia therapy based on the discretion of the treating physician. Treatment consisted of mild chemotherapy (33.5%), intensive chemotherapy (18.3%), chemotherapy followed by donor lymphocyte infusion or a second HCT (18.3%), donor lymphocyte infusion alone (15.2%) or a second HCT alone (7.6%). Two-year survival after relapse was only 14%, but was comparable to that following standard conditioning. Factors that were identified to be associated with better OS included longer time of remission after HCT (>5 months), bone marrow blasts <27%, and absence of acute GVHD after HCT. The achievement of a CR after relapse was strongly associated with improved OS, and among those patients who achieved another CR, outcomes were dependent on the use of donor cells for consolidation.36
Earlier studies showed that outcomes after RIC are dependent on disease status, with patients in CR having better outcomes than patients with active disease.37 In patients transplanted with active disease, studies have shown survival rates of 0% due to the very high risk of relapse after fludarabine/busulfan conditioning.38 Because the therapeutic effect of RIC transplant relies on the graft-versus-leukemia effect as opposed to ablative pre-transplant chemotherapy, the risk of relapse is higher and it has been clearly shown that patients with active leukemia have a higher risk of relapse and worse OS after HCT.39,40 This was recently confirmed by Usten et al. in a retrospective analysis of 85 adult AML patients who underwent RIC HCT at a single center. Utilizing strict criteria for CR, defined by both morphological and flow cytometric negativity as “stringent complete remission”, they identified patients in their study who may actually have had residual active disease, as shown by the presence of a previously identified leukemic immunophenotype by flow cytometry. Diagnostic and pre-transplant bone marrow results were re-reviewed, and their results showed that patients who had evidence of immunophenotypic residual leukemia by flow cytometry, irrespective of actual blast count, had a significantly higher risk of relapse (HR: 3.7, CI, 1.3–10.3, P=0.01) and poorer OS (HR: 2.9, 95% CI: 1.3–6.4, P=0.01) compared to the 77 patients who met the stringent CR criteria. Persistent cytogenetic abnormalities did not have an impact on outcomes.41 This study suggests that optimizing disease status in the pre-transplant period is likely critical to optimizing outcomes in the post-transplant period.
What is the optimal timing of transplantation?
Studies indicate that patients with intermediate- or high-risk disease should be transplanted in first CR.6 Accordingly, the discussion regarding allogeneic transplant often begins at diagnosis, at which time a search for a HLA-matched donor (whether sibling or unrelated) should commence. Most patients with newly diagnosed AML will need to be considered for allogeneic transplant in first CR unless they are part of a specific subset of patients with good cytogenetic or molecular risk, such as patients with translocation (8;21), inversion 16, or normal cytogenetics with a mutated nucleophosmin 1 gene (NPM1) without the FMS-like tyrosine kinase - internal tandem duplication (FLT3-ITD). In older patients, age may be a more important factor than the standard cytogenetic risk groups, suggesting that perhaps older patients with even “good risk” cytogenetics should be considered for HCT upfront.42 Some authors feel that most patients with AML, should be considered for HCT upfront because the 5-year survival for relapsed AML is dismal (in the range of only 10%) and that published literature regarding long-term outcomes of AML in second CR may be overestimating outcomes after first relapse because of an inherent selection bias.43
One of the key factors dictating the timing of HCT is donor availability. For patients with an HLA-matched sibling donor (MSD) or a readily identified HLA-matched unrelated donor (MUD), the consensus is to move forward with the transplant once a remission is achieved. However, only about 35% of patients will have an HLA-matched sibling, and for older patients, this percentage is often lower because of the siblings’ prohibitive advanced age or comorbidities. For most patients, MUD are considered second but the search time is a limiting factor, making this option unfeasible for patients in need of rapid transplantation. For patients belonging to ethnic minorities, donor pools are further limited. For these patients, umbilical cord blood transplantation or haploidentical options may need to be considered and may provide more timely options for patients without a readily available HLA-MSD or MUD. The optimal donor source is discussed below.
Impact of consolidation therapy
Consolidation chemotherapy after obtaining a first CR is well established for patients with AML, particularly in non-transplant settings. Additionally, many patients require some form of post-remission therapy as a bridge to transplant, and typically this in the form of high-dose cytarabine. In practice, many patients with plans for a HCT will undergo an abbreviated course of consolidation therapy prior to transplantation. In the MAC setting, pre-transplant consolidation therapy has not shown to provide a beneficial effect on OS, LFS, or relapse incidence as demonstrated by two large registry studies,44,45 and consequently, patients with an identified donor with plans for a myeloablative transplant could forego consolidation therapy without negative impact on their post-transplant outcomes. In the RIC setting, this issue is less clear and is seemingly more pertinent in a situation in which relapse risk may be heightened by a less intensive conditioning regimen. This was addressed in a recent CIBMTR analysis of 604 patients with AML in first CR who underwent a RIC or non-myeloablative transplant. No differences were seen in 3-year cumulative incidence of relapse, LFS, or OS. Multivariate analysis confirmed the lack of effect of consolidation on outcomes.46 Similarly, Yeshurun et al. reported the impact of consolidation therapy on outcomes in a retrospective EBMT registry study of 789 patients with AML in first CR who underwent RIC HCT. They found a 3-year relapse incidence of 36%±4% for patients treated without consolidation therapy versus 38%±3% in patients who received such therapy (P=0.24). Multivariate analysis showed no impact of the consolidation therapy on relapse incidence or LFS, leading to the conclusion that there is no apparent advantage from post-remission consolidation chemotherapy before RIC HCT provided a donor is available.47 These large registry studies suggest that moving forward with a transplant once a donor is identified is reasonable, and in practice may reduce the inherent risks associated with multiple cycles of consolidation chemotherapy.
What is the optimal donor source?
As the pool of transplant-eligible patients has expanded, the need for potential donors has as well. Approximately 30–40% of patients will have an HLA-MSD48 and these are typically the first choice of donor. However, for the remaining patients in need of an HCT, alternative donors are required and are available in the form of an HLA-MUD, HLA-mismatched related donor, HLA-mismatched unrelated donor, umbilical cord blood (UCB), or haploidentical transplants.
A large registry analysis of RIC MRD versus MUD transplant showed that RIC MUD transplants may be associated with lower relapse risk [hazard ratio (HR) 0.67, P=0.002] and superior progression-free survival (HR 0.69, P=0.002). These results suggest that perhaps the increased minor HLA disparity may improve the graft-versus-leukemia effect thus producing this lowered relapse risk for patients undergoing MUD transplants.49 A more recent update addressing the same issue identified a similar risk of relapse comparing 8/8 allele-matched MUD versus MSD and 8/8 allele-matched versus 7/8 allele-matched MUD. A moderately lower relapse risk (RR=0.78, 95% CI 0.63–0.98, P=0.03) was observed comparing the partial matched 7/8 MUD to MSD but was strongly counterbalanced by a 50% higher risk of NRM. Overall all three groups had equivalent risks of treatment failure.50
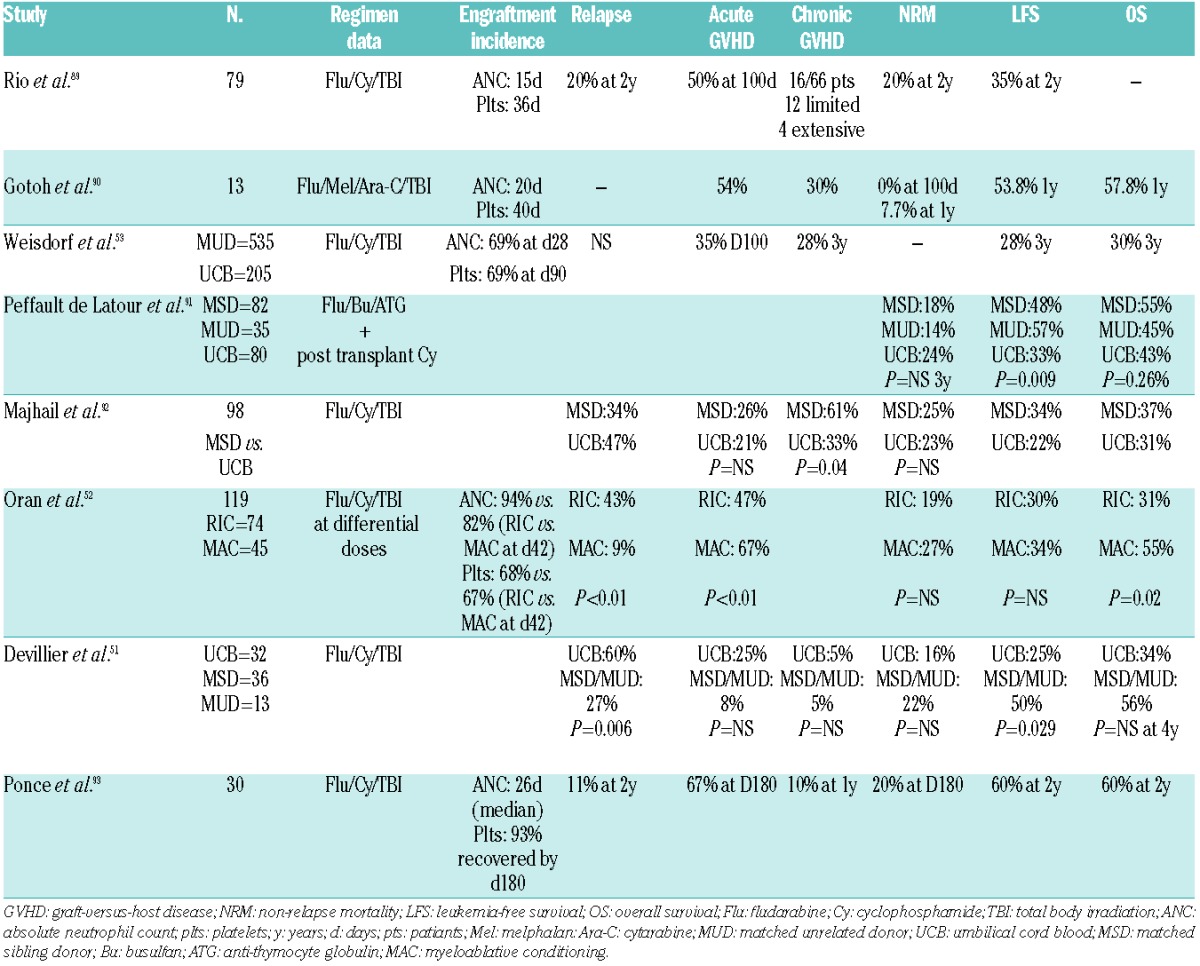
RIC UCB transplant is an option for patients without a MSD or identified MUD. Historically, patients who underwent MAC followed by UCB transplant had a higher risk of graft failure. In a recent analysis of high-risk patients with AML who underwent UCB allografting, Devillier et al. compared RIC and MAC and found that there was a high incidence of relapse after RIC-based UCB transplants. Although their cohort included high-risk patients beyond first CR, approximately half were in first CR and the cumulative incidence of relapse remained quite high, also compared to that in the MSD and MUD groups. This is in line with other studies that looked at RIC UCB allografts.51 A retrospective study showed higher relapse rates, along with decreased LFS, in patients receiving RIC UCB transplants than in those undergoing MAC UCB transplants, although such transplants were still felt to be a safe and reasonable option for those without a suitable donor.52 Another retrospective study looking at older patients over the age of 55 showed that when compared to other types of transplants e.g. MSD and MUD transplants, UCB transplants in the RIC setting are also safe and feasible. Similarly, when compared to MUD allografts, UCB transplants can extend survival in older patients in CR.53 Thus, if neither a MSD or MUD is available, it appears that RIC UCB transplantation is a reasonable option54 (Table 3).
Table 3.
Recent studies of reduced intensity umbilical cord blood transplants.
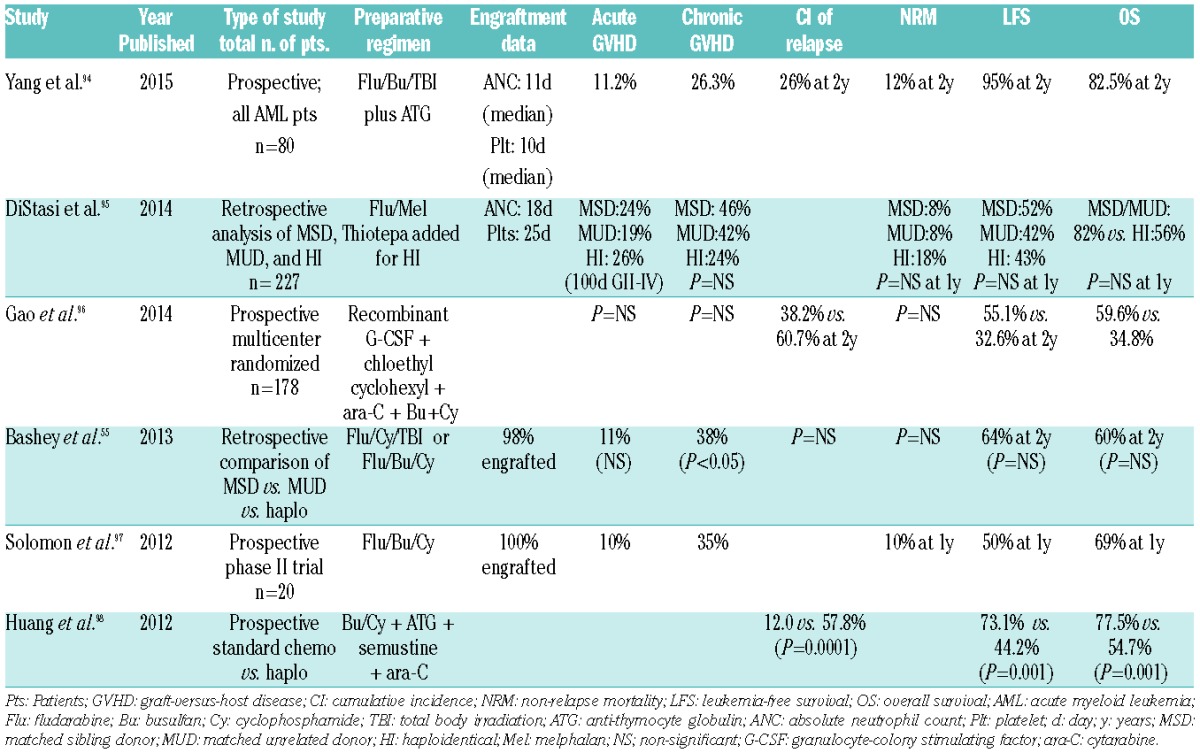
Recently, the introduction of post-transplantation cyclophosphamide has improved outcomes after T-cell-replete haploidentical transplants, and in the years to come may prove to be yet another valuable alternate donor source for patients without a fully matched sibling or unrelated donor.55 A recent study showed similar transplant outcomes in a retrospective comparison of patients with AML or myelodysplastic syndrome treated with melphalan-based conditioning and MSD versus MUD versus haploidentical transplants supporting the role of this last type of transplant in extending options for those who may not have otherwise fully matched donors.56 Several other studies have looked at RIC haploidentical transplants and are summarized in Table 4.
Table 4.
Recent studies on reduced intensity conditioning haploidentical transplant.
What is the optimal stem cell source?
Peripheral blood stem cells (PBSC) have been increasingly used over the past decade as the preferred stem cell source, and trials have shown that PBSC from sibling donors result in improved engraftment although increased risks of acute and chronic GVHD.57,58 A large randomized trial comparing transplantation of PBSC versus bone marrow showed no difference in survival depending on the graft source in unrelated donor transplants, although PBSC may reduce the risk of graft failure while bone marrow may be associated with a lower risk of chronic GVHD. This major study included a significant number of AML patients (n=261) although only 22% of the total group received RIC regimens. No interaction was detected between graft source and intensity of conditioning regimen. However, the stronger engraftment potential of PBSC could be advantageous in the RIC setting due to the lesser degree of immunosuppression.59
Studies directly comparing PBSC versus bone marrow for RIC HCT in AML are limited. Two large retrospective studies from the EBMT have looked directly at this question. With regards to patients who underwent HLA-identical sibling donor RIC HCT for AML in CR, an analysis of EBMT data from 1537 patients showed that engraftment was better in patients who received PBSC (99% versus 93%, P<0.0001) as was time to engraftment (15 days versus 19 days, P<0.0001). Other outcomes such as acute GVHD, severe GVHD, chronic GVHD, LFS, relapse or NRM were not statistically significantly different.60
Another similar study by the EBMT looked at the impact of stem cell source (PBSC versus bone marrow) in patients who underwent unrelated donor allografts. In this study of 602 patients with AML in CR after RIC HCT, patients who had mobilized PBSC grafts had a significantly higher incidence of acute GVHD than patients grafted with bone marrow (27.5% versus 12%, P<0.002) as well as higher risk of chronic GVHD at 2 years (43% versus 35% P=0.04). LFS survival was similar but relapse was higher in the patients who received bone marrow grafts (46% versus 32%, P=0.014). Conversely, NRM was higher in the PBSC group (28% versus 14%, P=0.004) than in patients transplanted with a bone marrow graft, suggesting that the lower NRM is due to lower incidences of acute and chronic GVHD. Engraftment rates were similar at 97% and 96%. No statistical significance was seen in LFS.61 Both these large studies, although retrospective, suggest that either graft source may be acceptable based on similar survival outcomes in patients undergoing RIC transplant for AML in remission.
Impact of comorbidities and prediction of non-relapse mortality
Multiple studies have shown that comorbidities and biological age are prognostically significant. The utility of the hematopoietic cell transplant-comorbidity index (HCT-CI) and its ability to sensitively capture the prevalence and magnitude of comorbidities and their impact pre- and post-transplant is well established.62–64 A recent study by Sorror et al. investigated whether age alone, comorbidities as assessed by the HCT-CI, or both should guide decision-making regarding eligibility for HCT as well as the conditioning regimen. In evaluating data from 3033 patients who were recipients of allografts, they found that age alone is a poor prognostic factor and when used alone as a criterion for exclusion of patients for transplant could be responsible for loss of life. They described a composite age/comorbidity index which may more accurately account for the impact of both age and comorbidity on estimating outcomes after HCT and decision-making regarding optimal regimens. This is argued to be particularly relevant for older patients over the age of 60 who may benefit most from an allograft but who may meet some resistance from clinicians who are hesitant to offer allografts based on age alone.65
Because RIC HCT is being more frequently used in older patients with more medical comorbidities, prediction of the risk of NRM is important. Two current scoring systems exist to aid in this prediction: the aforementioned HCT-CI and the EBMT score. Versluis et al. recently analyzed 812 adults with de novo or secondary AML who underwent RIC transplant consolidation and how these scores independently and collectively predicted NRM. This study showed that both the HCT-CI and the EBMT score individually demonstrated weak predictive value while the integrated score, which included 11 comorbidities, age, donor type, and positive cytomegalovirus serology, allowed identification of three distinct risk groups with 2-year NRM estimates which translated into prediction of overall survival.66 It appears that using a combined scoring system may enable better prediction of NRM than that offered by each score independently. Prospective data are need to validate the findings.
Center experience: does it matter?
Another interesting observation regarding outcomes after RIC HCT is the impact of center experience. Giebel et al. reported an EBMT study which sought to evaluate whether a center’s experience with RIC transplants had any impact on outcomes on patients with AML transplanted in first CR. In looking at 1413 RIC HCT from both MSD and MUD, outcomes were analyzed according to level of activity in the centers. It was found that patients who underwent transplants at the lowest activity centers, defined as <15 procedures over 7 years, had worse outcomes with regards to 2-year LFS (43% versus 55%, P<0.001) and NRM (24% versus 15%, P=0.004). No difference in relapse rate was detected. In multivariate analysis this continued to hold true when adjusted for other prognostic variable, thus making center experience a seemingly very important predictor of outcome.67
From reduced intensity conditioning to reduced toxicity conditioning and intermediate intensity conditioning
Relapse remains the greatest challenge after a reduced intensity allograft. Data are conflicting regarding the impact of in vivo T-cell depletion after RIC transplant with either alemtuzumab or anti-thymocyte globulin. A CIMBTR study showed an increase in relapse risk with an associated decrease in OS while a similarly large EBMT study showed no differences in transplant outcomes except for a lower risk of chronic GHVD.68,69 To achieve a reduction in relapse risk, investigators are now looking at ways to optimize dose intensity while safely minimizing NRM. Investigators previously looked at the use of 3 days of busulfan and found that the results were similar to those achieved with 4 days of the alkylating agent.70,71 A prospective, phase 2, multicenter trial recently assessed the efficacy of a RIC/reduced toxicity conditioning regimen of fludarabine plus anti-thymocyte globulin plus a higher dose of intravenous busulfan (FB3) for a total dose of 390 mg/m2 in patients with high-risk malignancies not eligible for a fully ablative MAC transplant. In a total of 80 patients aged 18 to 65 years old, high rates of engraftment, with relatively early hematopoietic recovery, were seen. At 2 years, OS and LFS rates were 62% and 50%, respectively, with a cumulative incidence of disease progression of 44% at 2 years and NRM of 11%. This study showed that increasing the anti-tumor efficacy of the reduced toxicity conditioning regimen with FB3 was effective while limiting toxicity.72 Oudin et al. also recently reported that a reduced toxicity conditioning regimen with higher doses of busulfan (390–520 mg/m2) in combination with fludarabine and anti-thymocyte globulin was associated with improved outcomes in AML/myelodysplastic syndrome, particularly with improved LFS in patients with favorable or intermediate risk cytogenetics.73 This area of investigation will likely continue to be of interest in terms of optimizing transplant outcomes.
Another important area of investigation to optimize transplant outcomes, especially in high-risk situations, has been the sequential use of intensive chemotherapy followed by a RIC allograft. Schmid et al. previously described a regimen of fludarabine, (4 × 30 mg/m2), cytarabine (4 × 2 g/m2), and amsacrine (4 × 100 mg/m2), followed 4 days later by a RIC regimen of 4 Gy TBI, cyclophosphamide (80–100 mg/m2), and anti-thymocyte globulin. This regimen was initially developed in patients with refractory disease with promising results.74,75 It was, therefore, then evaluated in 23 patients with high-risk AML in first CR. At 4 years, OS and LFS was 72.7%, with a cumulative incidence of relapse of 4.6% at 2 years and NRM of 22.5%.76 This approach produces long-term remissions in high-risk AML, thus warranting further investigation.
Long-term complications
As more patients are becoming long-term survivors of allogeneic transplant, attention often shifts from the acute concerns of early post-transplant toxicity and relapse to long-term complications of transplant. Apart from the multi-organ effects of chronic GVHD, there are several other serious long-term complications of RIC transplants which include but are not limited to cardiovascular effects (hypertension, dyslipidemia), impaired organ function (chronic kidney disease), endocrinopathies (diabetes, hypothyroidism, hypogonadism), and bone effects (osteopenia/osteoporosis, avascular necrosis). Among the most serious side effects are secondary malignancies, which are rare but well-established complications in long-term survivors of HCT after MAC.77 They account for up to 5 to 10% of late deaths.78–81 The pathogenesis of carcinogenesis is multifactorial and based on chemotherapy and exposure to radiation as well as changes in mucosal tissue epithelium. Oncogenic viruses may contribute to carcinogenesis as well.82
As the incidence of RIC has increased, the question of whether these types of transplant increase the risk of secondary malignancies has become more pressing, particularly as related to the use of fludarabine. Shimoni et al. reported a single institutional study of 931 consecutive patients who underwent HCT with either MAC, RIC or reduced toxicity conditioning. They identified 27 patients who developed a secondary malignancy at a median of 43 months after HCT with multivariate analysis showing that fludarabine-based conditioning (HR 3.5, P=0.05) as well as moderate to severe chronic GVHD or a diagnosis of chronic myeloproliferative disorder or non-malignant disease were risk factors for a secondary malignancy. Thus the risk of secondary malignancies was not reduced and possibly even increased after fludarabine-based RIC or reduced toxicity conditioning regimens.77
However, in a recent study of the largest cohort of patients so far who have undergone RIC transplants (n=4269) for AML, myelodysplastic syndrome or lymphoma, Ringden et al. found that the cumulative incidence of all cancers was 3.35% at 10 years, which was not higher than expected in the general population. However risks were increased in patients with AML and myelodysplastic syndrome for cancers of oral sites (lip, tonsil, oropharynx), bone, soft tissue, vulva, and melanoma, with age (> 50 years) being the only independent risk factor for solid cancers (HR: 3.02, P<0.001). The conclusion from this study was that although overall cancer risk was similar, it is important to have a longer follow-up, as there was an increased risk of cancer at some sites. Longer follow-up is also needed to understand the full risks of secondary cancers after RIC regimens.83 The comparison of risks of secondary malignancy after RIC or MAC remains inconclusive but the incidence of this complication may become clearer after a longer follow-up.82 Other common, serious complications of HCT, particularly for men and women in their reproductive years, are hypogonadism and infertility. Males are likely to have post-HCT damage to the germ cell epithelium, resulting in reduced fertility despite normal levels of testosterone. However, there are reports of recovery of spermatogenesis in approximately 25% of young patients surviving more than 10 years after HCT, even among those who received TBI.84,85 Females will also have some degree of gonadal dysfunction after HCT. There are standard recommendations for endocrine replacement in the case of hypogonadism and consultation with a reproductive endocrinologist should be obtained when indicated. All patients of reproductive age should be counseled on this important complication of transplantation. Savani et al. have published comprehensively on other long-term complications of allogeneic transplantation along with recommended treatment approaches.86
Summary
RIC HCT has revolutionized the transplant landscape by allowing more patients to be eligible for transplantation. This strategy harnesses the immunological graft-versus-leukemia effect to effect its cure and has been shown in several studies not to cause major differences in long-term outcomes when compared to conventional MAC regimens. While the feasibility and effectiveness of RIC HCT have been proven, several unanswered questions remain, including the optimal conditioning regimen to reduce relapse risk, optimal donor and stem cell source, and how to continue to reduce NRM and long-term complications such as secondary malignancies. Furthermore, as increasing numbers of older patients are being offered RIC HCT, other issues that are specific to older populations must be taken into account, such as age-associated immune alterations.87 Moreover, modifications of the conditioning regimens to increase dose intensity as well as addition of novel therapies such as integration of a radiolabeled anti-CD45 antibody in the conditioning regimen88 are areas of burgeoning interest. While RIC transplants have changed for whom transplantation is an option, further work remains to improve long term outcomes.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Lazarus HM, Perez WS, Klein JP, et al. Autotransplantation versus HLA-matched unrelated donor transplantation for acute myeloid leukaemia: a retrospective analysis from the Center for International Blood and Marrow Transplant Research. Br J Haematol. 2006;132(6):755–769. [DOI] [PubMed] [Google Scholar]
- 2.Ringden O, Labopin M, Gluckman E, et al. Donor search or autografting in patients with acute leukaemia who lack an HLA-identical sibling? A matched-pair analysis. Acute Leukaemia Working Party of the European Cooperative Group for Blood and Marrow Transplantation (EBMT) and the International Marrow Unrelated Search and Transplant (IMUST) study. Bone Marrow Transplant. 1997;19(10):963–968. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum FR. Allogeneic hematopoietic stem cell transplantation for acute leukemia. Semin Oncol. 1997;24(1):114–123. [PubMed] [Google Scholar]
- 4.Reiffers J, Stoppa AM, Attal M, et al. Allogeneic vs autologous stem cell transplantation vs chemotherapy in patients with acute myeloid leukemia in first remission: the BGMT 87 study. Leukemia. 1996;10(12):1874–1882. [PubMed] [Google Scholar]
- 5.Oliansky DM, Appelbaum F, Cassileth PA, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myelogenous leukemia in adults: an evidence-based review. Biol Blood Marrow Transplant. 2008;14(2):137–180. [DOI] [PubMed] [Google Scholar]
- 6.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and metaanalysis of prospective clinical trials. JAMA. 2009;301(22):2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanada M, Matsuo K, Emi N, Naoe T. Efficacy of allogeneic hematopoietic stem cell transplantation depends on cytogenetic risk for acute myeloid leukemia in first disease remission: a metaanalysis. Cancer. 2005;103(8):1652–1658. [DOI] [PubMed] [Google Scholar]
- 8.Cornelissen JJ, van Putten WL, Verdonck LF, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109(9): 3658–3666. [DOI] [PubMed] [Google Scholar]
- 9.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimoni A, Nagler A. Optimizing the conditioning regimen for allogeneic stem-cell transplantation in acute myeloid leukemia; dose intensity is still in need. Best Pract Res Clin Haematol. 2011;24(3):369–379. [DOI] [PubMed] [Google Scholar]
- 11.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28(17):2859–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valcarcel D, Martino R, Caballero D, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26(4):577–584. [DOI] [PubMed] [Google Scholar]
- 14.Tauro S, Craddock C, Peggs K, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005;23(36):9387–9393. [DOI] [PubMed] [Google Scholar]
- 15.Lowsky R, Takahashi T, Liu YP, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353(13):1321–1331. [DOI] [PubMed] [Google Scholar]
- 16.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104(3):865–872. [DOI] [PubMed] [Google Scholar]
- 17.Taussig DC, Davies AJ, Cavenagh JD, et al. Durable remissions of myelodysplastic syndrome and acute myeloid leukemia after reduced-intensity allografting. J Clin Oncol. 2003;21(16):3060–3065. [DOI] [PubMed] [Google Scholar]
- 18.Martino R, Caballero MD, Perez-Simon JA, et al. Evidence for a graft-versus-leukemia effect after allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning in acute myelogenous leukemia and myelodysplastic syndromes. Blood. 2002;100(6):2243–2245. [DOI] [PubMed] [Google Scholar]
- 19.Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89(12):4531–4536. [PubMed] [Google Scholar]
- 20.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91(3):756–763. [PubMed] [Google Scholar]
- 21.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and post-grafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101(4):1620–1629. [DOI] [PubMed] [Google Scholar]
- 22.Bornhauser M, Thiede C, Platzbecker U, et al. Dose-reduced conditioning and allogeneic hematopoietic stem cell transplantation from unrelated donors in 42 patients. Clin Cancer Res. 2001;7(8):2254–2262. [PubMed] [Google Scholar]
- 23.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–3400. [DOI] [PubMed] [Google Scholar]
- 24.Shimoni A, Kroger N, Zabelina T, et al. Hematopoietic stem-cell transplantation from unrelated donors in elderly patients (age >55 years) with hematologic malignancies: older age is no longer a contraindication when using reduced intensity conditioning. Leukemia. 2005;19(1):7–12. [DOI] [PubMed] [Google Scholar]
- 25.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohty M, de Lavallade H, Ladaique P, et al. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: a donor vs no donor comparison. Leukemia. 2005;19(6):916–920. [DOI] [PubMed] [Google Scholar]
- 27.Kurosawa S, Yamaguchi T, Uchida N, et al. Comparison of allogeneic hematopoietic cell transplantation and chemotherapy in elderly patients with non-M3 acute myelogenous leukemia in first complete remission. Biol Blood Marrow Transplant. 2011;17(3):401–411. [DOI] [PubMed] [Google Scholar]
- 28.Russell NH, Kjeldsen L, Craddock C, et al. A comparative assessment of the curative potential of reduced intensity allografts in acute myeloid leukaemia. Leukemia. 2014. November 7 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 29.Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia. 2005;19(12):2304–2312. [DOI] [PubMed] [Google Scholar]
- 30.Luger SM, Ringden O, Zhang MJ, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47(2):203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martino R, de Wreede L, Fiocco M, et al. Comparison of conditioning regimens of various intensities for allogeneic hematopoietic SCT using HLA-identical sibling donors in AML and MDS with <10% BM blasts: a report from EBMT. Bone Marrow Transplant. 2013;48(6):761–770. [DOI] [PubMed] [Google Scholar]
- 32.Chen YB, Coughlin E, Kennedy KF, et al. Busulfan dose intensity and outcomes in reduced-intensity allogeneic peripheral blood stem cell transplantation for myelodysplastic syndrome or acute myeloid leukemia. Biol Blood Marrow Transplant. 2013;19(6):981–987. [DOI] [PubMed] [Google Scholar]
- 33.Bornhauser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13(10):1035–1044. [DOI] [PubMed] [Google Scholar]
- 34.Sebert M, Porcher R, Robin M, et al. Equivalent outcomes using reduced intensity or conventional myeloablative conditioning transplantation for patients aged 35 years and over with AML. Bone Marrow Transplant. 2015;50(01):74–81. [DOI] [PubMed] [Google Scholar]
- 35.Al-Ali H, Cross M, Lange T, Freund M, Dolken G, Niederwieser D. Low-dose total body irradiation-based regimens as a preparative regimen for allogeneic haematopoietic cell transplantation in acute myelogenous leukaemia. Cur Opin Oncol. 2009;21(Suppl 1):S17–22. [DOI] [PubMed] [Google Scholar]
- 36.Schmid C, Labopin M, Nagler A, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012; 119(6):1599–15606. [DOI] [PubMed] [Google Scholar]
- 37.Sayer HG, Kroger M, Beyer J, et al. Reduced intensity conditioning for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia: disease status by marrow blasts is the strongest prognostic factor. Bone Marrow Transplant. 2003;31(12):1089–1095. [DOI] [PubMed] [Google Scholar]
- 38.Shimoni A, Hardan I, Shem-Tov N, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20(2):322–328. [DOI] [PubMed] [Google Scholar]
- 39.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28(23):3730–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kebriaei P, Kline J, Stock W, et al. Impact of disease burden at time of allogeneic stem cell transplantation in adults with acute myeloid leukemia and myelodysplastic syndromes. Bone Marrow Transplant. 2005;35(10):965–970. [DOI] [PubMed] [Google Scholar]
- 41.Ustun C, Wiseman AC, Defor TE, et al. Achieving stringent CR is essential before reduced-intensity conditioning allogeneic hematopoietic cell transplantation in AML. Bone Marrow Transplant. 2013; 48(11):1415–1420. [DOI] [PubMed] [Google Scholar]
- 42.Schoch C, Kern W, Schnittger S, Buchner T, Hiddemann W, Haferlach T. The influence of age on prognosis of de novo acute myeloid leukemia differs according to cytogenetic subgroups. Haematologica. 2004;89(9):1082–1090. [PubMed] [Google Scholar]
- 43.Forman SJ, Rowe JM. The myth of the second remission of acute leukemia in the adult. Blood. 2013;121(7):1077–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tallman MS, Rowlings PA, Milone G, et al. Effect of postremission chemotherapy before human leukocyte antigen-identical sibling transplantation for acute myelogenous leukemia in first complete remission. Blood. 2000;96(4):1254–1258. [PubMed] [Google Scholar]
- 45.Cahn JY, Labopin M, Sierra J, et al. No impact of high-dose cytarabine on the outcome of patients transplanted for acute myeloblastic leukaemia in first remission. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Br J Haematol. 2000;110(2):308–314. [DOI] [PubMed] [Google Scholar]
- 46.Warlick ED, Paulson K, Brazauskas R, et al. Effect of postremission therapy before reduced-intensity conditioning allogeneic transplantation for acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2014;20(2):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeshurun M, Labopin M, Blaise D, et al. Impact of postremission consolidation chemotherapy on outcome after reduced-intensity conditioning allogeneic stem cell transplantation for patients with acute myeloid leukemia in first complete remission: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer. 2014;120(6):855–863. [DOI] [PubMed] [Google Scholar]
- 48.Yanada M, Kurosawa S, Yamaguchi T, et al. Effect of related donor availability on outcome of AML in the context of related and unrelated hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48(3):390–395. [DOI] [PubMed] [Google Scholar]
- 49.Ho VT, Kim HT, Aldridge J, et al. Use of matched unrelated donors compared with matched related donors is associated with lower relapse and superior progression-free survival after reduced-intensity conditioning hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(8):1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saber W, Opie S, Rizzo JD, Zhang MJ, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012;119(17):3908–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devillier R, Harbi S, Furst S, et al. Poor outcome with nonmyeloablative conditioning regimen before cord blood transplantation for patients with high-risk acute myeloid leukemia compared with matched related or unrelated donor transplantation. Biol Blood Marrow Transplant. 2014; 20(10):1560–1565. [DOI] [PubMed] [Google Scholar]
- 52.Oran B, Wagner JE, DeFor TE, Weisdorf DJ, Brunstein CG. Effect of conditioning regimen intensity on acute myeloid leukemia outcomes after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2011;17(9):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weisdorf D, Eapen M, Ruggeri A, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a Center for International Blood and Marrow Transplant Research-Eurocord analysis. Biol Blood Marrow Transplant. 2014; 20(6):816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Majhail NS, Brunstein CG, Tomblyn M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008;14(3):282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013; 31(10):1310–1316. [DOI] [PubMed] [Google Scholar]
- 56.Di Stasi A, Milton DR, Poon LM, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014; 20(12) 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morton J, Hutchins C, Durrant S. Granulocyte-colony-stimulating factor (G-CSF)-primed allogeneic bone marrow: significantly less graft-versus-host disease and comparable engraftment to G-CSF-mobilized peripheral blood stem cells. Blood. 2001;98(12):3186–3191. [DOI] [PubMed] [Google Scholar]
- 58.Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100(5):1525–1531. [DOI] [PubMed] [Google Scholar]
- 59.Anasetti C, Logan BR, Confer DL. Peripheral-blood versus bone marrow stem cells. N Engl J Med. 2013;368(3):288. [DOI] [PubMed] [Google Scholar]
- 60.Nagler A, Labopin M, Shimoni A, et al. Mobilized peripheral blood stem cells compared with bone marrow from HLA-identical siblings for reduced-intensity conditioning transplantation in acute myeloid leukemia in complete remission: a retrospective analysis from the Acute Leukemia Working Party of EBMT. Eur J Haematol. 2012;89(3):206–213. [DOI] [PubMed] [Google Scholar]
- 61.Nagler A, Labopin M, Shimoni A, et al. Mobilized peripheral blood stem cells compared with bone marrow as the stem cell source for unrelated donor allogeneic transplantation with reduced-intensity conditioning in patients with acute myeloid leukemia in complete remission: an analysis from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(9):1422–1429. [DOI] [PubMed] [Google Scholar]
- 62.Sorror ML. Comorbidities and hematopoietic cell transplantation outcomes. Hematology Am Soc Hematol Educ Program. 2010;2010:237–247. [DOI] [PubMed] [Google Scholar]
- 63.Sorror ML, Storer B, Storb RF. Validation of the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) in single and multiple institutions: limitations and inferences. Biol Blood Marrow Transplant. 2009;15(6):757–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25(27):4246–4254. [DOI] [PubMed] [Google Scholar]
- 65.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32(29):3249–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Versluis J, Labopin M, Niederwieser D, et al. Prediction of non-relapse mortality in recipients of reduced intensity conditioning allogeneic stem cell transplantation with AML in first complete remission. Leukemia. 2015;29(1):51–57. [DOI] [PubMed] [Google Scholar]
- 67.Giebel S, Labopin M, Mohty M, et al. The impact of center experience on results of reduced intensity: allogeneic hematopoietic SCT for AML. An analysis from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2013;48(2):238–242. [DOI] [PubMed] [Google Scholar]
- 68.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baron F, Labopin M, Blaise D, et al. Impact of in vivo T-cell depletion on outcome of AML patients in first CR given peripheral blood stem cells and reduced-intensity conditioning allo-SCT from a HLA-identical sibling donor: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49(3):389–396. [DOI] [PubMed] [Google Scholar]
- 70.Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8(9):468–476. [DOI] [PubMed] [Google Scholar]
- 71.Alatrash G, de Lima M, Hamerschlak N, et al. Myeloablative reduced-toxicity i.v. busulfan-fludarabine and allogeneic hematopoietic stem cell transplant for patients with acute myeloid leukemia or myelodysplastic syndrome in the sixth through eighth decades of life. Biol Blood Marrow Transplant. 2011;17(10):1490–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohty M, Malard F, Blaise D, et al. Reduced-toxicity conditioning with fludarabine, once-daily intravenous busulfan, and antithymocyte globulins prior to allogeneic stem cell transplantation: results of a multicenter prospective phase 2 trial. Cancer. 2015;121(4)562–569. [DOI] [PubMed] [Google Scholar]
- 73.Oudin C, Chevallier P, Furst S, et al. Reduced-toxicity conditioning prior to allogeneic stem cell transplantation improves outcome in patients with myeloid malignancies. Haematologica. 2014;99(11):1762–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23(24):5675–5687. [DOI] [PubMed] [Google Scholar]
- 75.Schmid C, Schleuning M, Schwerdtfeger R, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006; 108(3):1092–1099. [DOI] [PubMed] [Google Scholar]
- 76.Schmid C, Schleuning M, Hentrich M, et al. High antileukemic efficacy of an intermediate intensity conditioning regimen for allogeneic stem cell transplantation in patients with high-risk acute myeloid leukemia in first complete remission. Bone Marrow Transplant. 2008;41(8):721–727. [DOI] [PubMed] [Google Scholar]
- 77.Shimoni A, Shem-Tov N, Chetrit A, et al. Secondary malignancies after allogeneic stem-cell transplantation in the era of reduced-intensity conditioning; the incidence is not reduced. Leukemia. 2013;27(4):829–835. [DOI] [PubMed] [Google Scholar]
- 78.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Majhail NS, Brazauskas R, Rizzo JD, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117(1):316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Atsuta Y, Suzuki R, Yamashita T, et al. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol. 2014; 25(2):435–441. [DOI] [PubMed] [Google Scholar]
- 81.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimoni A. Second malignancies after allogeneic stem cell transplantation with reduced-intensity conditioning: is the incidence reduced? Biol Blood Marrow Transplant. 2014;20(11):1669–1670. [DOI] [PubMed] [Google Scholar]
- 83.Ringden O, Brazauskas R, Wang Z, et al. Second solid cancers after allogeneic hematopoietic cell transplantation using reduced-intensity conditioning. Biol Blood Marrow Transplant. 2014;20(11):1777–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rovo A, Tichelli A, Passweg JR, et al. Spermatogenesis in long-term survivors after allogeneic hematopoietic stem cell transplantation is associated with age, time interval since transplantation, and apparently absence of chronic GvHD. Blood. 2006;108(3):1100–1105. [DOI] [PubMed] [Google Scholar]
- 85.Savani BN, Kozanas E, Shenoy A, Barrett AJ. Recovery of spermatogenesis after total-body irradiation. Blood. 2006; 108(13):4292–4293; author reply 4293–4294. [DOI] [PubMed] [Google Scholar]
- 86.Savani BN, Griffith ML, Jagasia S, Lee SJ. How I treat late effects in adults after allogeneic stem cell transplantation. Blood. 2011;117(11):3002–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blaise D, Vey N, Faucher C, Mohty M. Current status of reduced-intensity-conditioning allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2007;92(4):533–541. [DOI] [PubMed] [Google Scholar]
- 88.Mawad R, Gooley TA, Rajendran JG, et al. Radiolabeled anti-CD45 antibody with reduced-intensity conditioning and allogeneic transplantation for younger patients with advanced acute myeloid leukemia or myelodysplastic syndrome. Biol Blood Marrow Transplant. 2014;20(9):1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rio B, Chevret S, Vigouroux S, et al. Decreased nonrelapse mortality after unrelated cord blood transplantation for acute myeloid leukemia using reduced-intensity conditioning: a prospective phase II multicenter trial. Biol Blood Marrow Transplant. 2015;21(3):445–453. [DOI] [PubMed] [Google Scholar]
- 90.Gotoh M, Yoshizawa S, Katagiri S, et al. A novel reduced-intensity umbilical cord blood transplantation using a recombinant G-CSF combined with high-dose Ara-C for active myeloid malignancies. Bone Marrow Transplant. 2014;49(7):955–960. [DOI] [PubMed] [Google Scholar]
- 91.Peffault de Latour R, Brunstein CG, Porcher R, et al. Similar overall survival using sibling, unrelated donor, and cord blood grafts after reduced-intensity conditioning for older patients with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2013;19(9): 1355–1360. [DOI] [PubMed] [Google Scholar]
- 92.Majhail NS, Brunstein CG, Shanley R, et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone Marrow Transplant. 2012;47(4):494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ponce DM, Sauter C, Devlin S, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(5): 799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yahng SA, Kim JH, Jeon YW, et al. A well-tolerated regimen of 800 cGy TBI-fludarabine-busulfan-ATG for reliable engraftment after unmanipulated haploidentical peripheral blood stem cell transplantation in adult patients with acute myeloid leukemia. Biol Blood Marrow Transplant. 2015;21(1):119–29. [DOI] [PubMed] [Google Scholar]
- 95.Di Stasi A, Milton DR, Poon LM, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014;20(12): 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao L, Wen Q, Chen X, et al. Effects of priming with recombinant human granulocyte colony-stimulating factor on conditioning regimen for high-risk acute myeloid leukemia patients undergoing human leukocyte antigen-haploidentical hematopoietic stem cell transplantation: a multicenter randomized controlled study in southwest China. Biol Blood Marrow Transplant. 2014;20(12):1932–1939. [DOI] [PubMed] [Google Scholar]
- 97.Solomon SR, Sizemore CA, Sanacore M, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012; 18(12):1859–1866. [DOI] [PubMed] [Google Scholar]
- 98.Huang XJ, Zhu HH, Chang YJ, et al. The superiority of haploidentical related stem cell transplantation over chemotherapy alone as postremission treatment for patients with intermediate- or high-risk acute myeloid leukemia in first complete remission. Blood. 2012;119(23):5584–5590. [DOI] [PubMed] [Google Scholar]


