Abstract
The JAK2 V617F mutation, the thrombopoietin receptor MPL W515K/L mutation and calreticulin (CALR) mutations are mutually exclusive in essential thrombocythemia and support a novel molecular categorization of essential thrombocythemia. CALR mutations account for approximately 30% of cases of essential thrombocythemia. In a retrospective study, we examined the frequency of MPL and CALR mutations in JAK2 V617F-negative cases of essential thrombocythemia (n=103). In addition, we compared the clinical phenotype and outcome of CALR mutant cases of essential thrombocythemia with a cohort of JAK2 V617F-positive essential thrombocythemia (n=57). CALR-positive cases represented 63.7% of double-negative cases of essential thrombocythemia, and most carried CALR type 1 or type 2 indels. However, we also identified one patient who was positive for both the JAK2 V617F and the CALR mutations. This study revealed that CALR mutant essential thrombocythemia is associated with younger age, higher platelet counts, lower erythrocyte counts, leukocyte counts, hemoglobin, and hematocrit, and increased risk of progression to myelofibrosis in comparison with JAK2 V617F-positive essential thrombocythemia. Analysis of the CALR mutant group according to indel type showed that CALR type 1 deletion is strongly associated with male gender. CALR mutant patients had a better overall survival than JAK2 V617F-positive patients, in particular patients of age 60 years or younger. In conclusion, this study in a Belgian cohort of patients supports and extends the growing body of evidence that CALR mutant cases of essential thrombocythemia are phenotypically distinct from JAK2 V617F-positive cases, with regards to clinical and hematologic presentation as well as overall survival.
Introduction
The JAK2 V617F mutation is found in about 50–60% of cases of essential thrombocythemia (ET) while 5–10% of JAK2 V617F-negative ET patients carry a W515K/L gain-of-function mutation in exon 10 of MPL, the thrombopoietin receptor gene.1–5 Recently, indels in CALR have been described in about 70% of cases of JAK2/MPL wild-type (double-negative) ET and primary myelofibrosis (PMF), in a mutually exclusive pattern with JAK2 and MPL mutations. CALR mutations were not found in other myeloid malignancies, with the exception of the myelodysplastic syndrome, refractory anemia with ring sideroblasts and thrombocytosis.6,7 CALR indels are variable but all cause a +1 frameshift leading to a novel C-terminal lacking the KDEL endoplasmic reticulum targeting sequence. CALR mutations have also been identified in familial cases of ET (11%) and PMF (30%).8
Several recent studies have analyzed the clinical and hematologic features of ET or PMF patients according to their mutational genotype.6,7,9–13 These studies have shown that CALR-mutated cases are associated with younger age, higher platelet counts, lower erythrocyte counts, lower leukocyte counts, and lower hemoglobin levels compared with JAK2-mutated cases. In addition, in most of these studies CALR mutations were linked to male gender. However, the impact of the mutations on overall survival seems to differ between cases of ET and PMF.
We retrospectively analyzed a Belgian cohort of 103 JAK2 V617F-negative ET patients for the presence of MPL and CALR mutations. In addition, we compared the clinical and hematologic phenotypes of CALR mutant cases with those of a control cohort of 57 JAK2 V617F-positive ET patients, as well as with a group of triple-negative (JAK2V617F-negative, MPL W515K/L-negative, and CALR mutant-negative) patients with ET.
Methods
Patients and clinical information
This study was approved by the local ethical committee of UZ Leuven (study S53745). All patients selected for this study were seen at hematology units of academic or large regional Belgian hospitals between 1980 and 2013. Inclusion criteria were a diagnosis of ET [based on World Health Organization (WHO) 2008 criteria14], and the availability of stored bone marrow or peripheral blood for molecular analysis. Given that an initial bone marrow biopsy was not available for all cases, the presence of any two minor criteria for PMF (WHO 2008) was an exclusion criterion for this study, if no bone marrow biopsy was available, thus eliminating all cases with potential PMF. First, a cohort of 103 JAK2 V617F-negative ET cases diagnosed between 1980 and 2013 at several Belgian hospitals was collected and analyzed for CALR and MPL mutations. We also collected a control cohort of 57 JAK2 V617F-positive ET patients diagnosed between 1987 and 2009 in the University Hospitals Leuven. The median follow-up of the whole cohort of 160 patients was 8 years (range, 1–34 years). Hematologic parameters (platelet counts, erythrocyte counts, leukocyte counts, hemoglobin, and hematocrit) at diagnosis were retrieved as was information on cardiovascular events and complications (arterial thrombosis, and venous events). During follow-up, progression to myelofibrosis, progression to acute myeloid leukemia, need for cytoreductive treatment, and presence of splenomegaly were recorded.
Sequencing
Genomic DNA was extracted from stored bone marrow or blood samples, and analyzed for the JAK2 V617F mutation by allele-specific polymerase chain reaction testing.1 The mutational load of JAK2 V617F was determined with the Ipsogen JAK2 MutaQuant Kit (Qiagen, the Netherlands). JAK2 V617F-negative patients were sequenced for MPL W515K/L mutations. For the detection of MPL exon 10 or CALR exon 9 mutations, we applied polymerase chain reaction amplification and Sanger sequencing, using previously described primers.6 JAK2 V617F-positive and double-negative ET patients were sequenced for CALR indels. Sequencing was done on an ABI PRISM® 3100 Genetic Analyzer and the sequencing traces obtained were analyzed on CLC Main Workbench 6 (CLC bio, Denmark). Indels in CALR were labeled in accordance with the study by Klampfl et al.6
Statistics
The non-parametric Mann-Whitney test was used for numerical comparisons while a chi-square test or Fisher exact test was used for nominal comparisons. The analyses were two-tailed, and P values <0.05 were considered statistically significant. Overall survival, leukemia-free survival, and myelofibrosis-free survival were estimated by Kaplan-Meier analysis and compared using the logrank test. Statistical analyses were performed with SPSS software (version 20).
Results and discussion
First, the JAK2 V617F-negative group of patients (n=103) diagnosed since 1980 was sequenced for MPL W515K/L mutations and CALR indels. Sequencing revealed 58 CALR mutations, 12 MPL W515K/L mutations and no MPL or CALR mutations in 33 patients (Figure 1). Therefore, 63.7% of JAK2 V617F- and MPL W515K/L-negative patients were carrying an indel in CALR, which is in line with previously reported percentages indicating that the percentage of CALR mutations in this group of patients is ≥50% making CALR the second most recurrently mutated gene in ET.6,7,9,12 For the comparison of the hematologic and clinical phenotypes, we selected 57 JAK2 V617F-positive patients from our internal database of patients diagnosed since 1987. In contrast with the first published results stating that JAK2 V617F and CALR mutations are mutually exclusive,6,7 we identified one JAK2 V617F ET patient with a type 2 CALR indel who was excluded from further statistical analysis. This is, to the best of our knowledge, the fourth ET patient to be reported with both a CALR mutation and a JAK2 V617F mutation. This combination has so far been reported in several cases of myeloproliferative neoplasms.11,15–18
Figure 1.
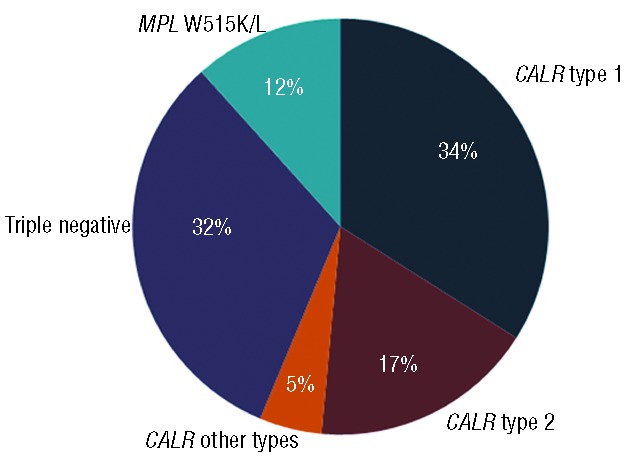
Distribution of JAK2 V617F-negative ET patients (n=103) according to their mutational status.
Type 1 (c.1092_1143del) and type 2 (c.1154_1155insTTGTC) indels occurred in 35 cases (60.3%) and 18 cases (31%), respectively. The remaining indels included five other types of which three were novel; they were labeled type 41, 42, and 43 in accordance with Klampfl et al. (Online Supplementary Table S1). Although novel, these types also lead to typical loss of the acidic bases and the gain of a novel basic C-terminal, as with the previously described types.6 Similar frequencies of type 1 deletion and type 2 insertion in ET have been reported in several other studies. In the first study by Klampfl et al. in a cohort of patients with myeloproliferative neoplasms, 53% and 31.7% carried type 1 or type 2, respectively.6 In later studies, the incidences of type 1 and type 2 in the cohorts studied by Tefferi et al., Rumi et al. and Andrikovics et al. were 48% and 35%, 46% and 38%, and 50% and 32%, respectively.
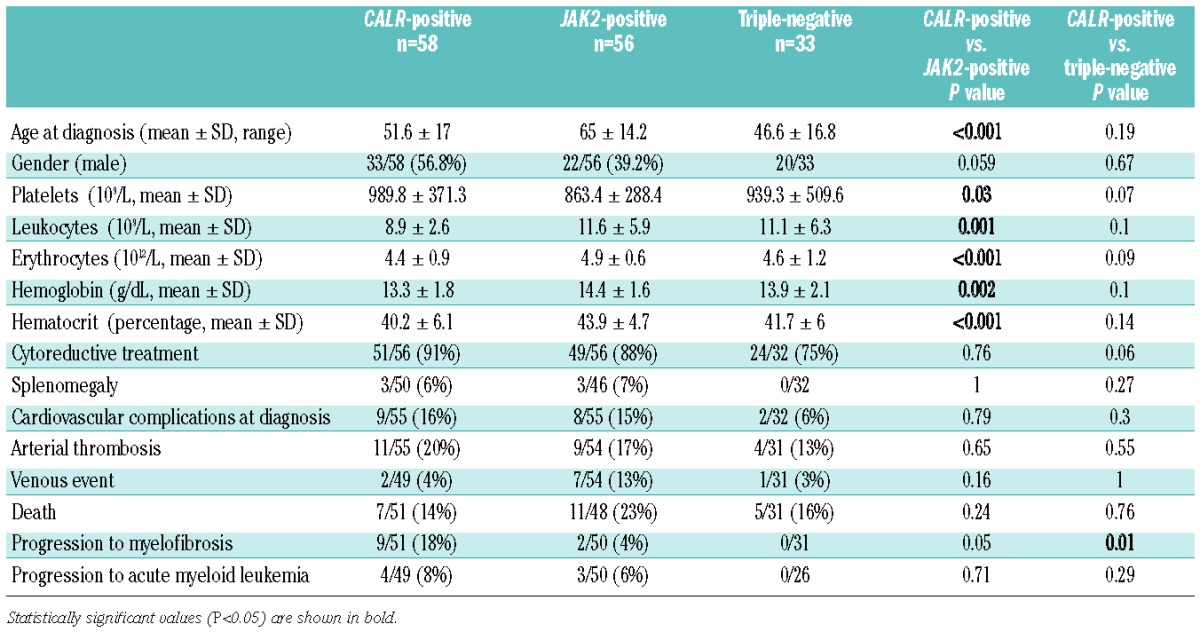
The mean age of patients with CALR mutations was 51.6±17 years (range, 23 and 84), whereas the mean age of triple-negative patients and JAK2 V617F-positive patients was 46.6±16.8 years (range, 17–78) and 65±14 years (range, 36–94), respectively. CALR mutant patients were younger than JAK2 V617F-positive ones (P<0.001), consistent with previous studies on JAK2 V617F-positive versus JAK2 V617F-negative myeloproliferative neoplasms.19,20 Also in agreement with previously published data, the CALR mutation-positive group had more males (56.8 %) compared to the JAK2 V617F-positive group (39.2%).9,12,13 Patients with a CALR mutation also had higher platelet counts (mean ± SD: 989.8±371.3×109/L versus 863.4±288.4 ×109/L; P=0.03), lower erythrocyte counts (4.4±0.9×1012/L versus 4.9±0.6×1012/L; P<0.001), lower hemoglobin concentration (13.3±1.8 g/dL versus 14.4±1.6 g/dL; P=0.002), lower hematocrit (40.2±6.1% versus 43.9±4.7%; P<0.001) and lower leukocyte counts (8.9±2.6×109/L versus 11.6±5.9 ×109/L; P=0.001) (Table 1). Several patients progressed to myelofibrosis or acute myeloid leukemia during the course of the disease. The percentage of ET patients who progressed to post-ET myelofibrosis was higher in the CALR-positive group than in the triple-negative or JAK2 V617F-positive groups (18%, 0%, and 4%, respectively; P=0.01 and P=0.05, respectively). Overall these comparisons indicate that CALR mutation-positive patients appear to have a higher risk of evolution to myelofibrosis compared to the JAK2 V617F-positive patients. However, the median follow-up of the CALR mutation-positive group (12 years) was longer than that of the JAK2 V617F-positive group (7 years). The natural history of early/prefibrotic PMF is known to be different from that of true ET, in particular due to higher rates of evolution to overt myelofibrosis and blast transformation.21 The possibility of early/prefibrotic PMF at diagnosis was specifically ruled out in those evolving to myelofibrosis, based on review of the initial bone marrow biopsy and histopathological criteria for PMF.14 The bone marrow biopsies showed a proliferation mainly of the megakaryocytic lineage with increased numbers of enlarged, mature megakaryocytes, rather than bizarre highly atypical megakaryocytes. There was no significant increase or left-shift of neutrophil granulopoiesis or erythropoiesis. Previous studies by Nangalia et al. and Andrikovics et al. also found higher risks of progression among CALR mutation-positive versus JAK2 V617F-positive ET (15% versus 6%, P=0.034 and 19% versus 2%; P=0.03).7,22 Other studies found comparable progression rates to myelofibrosis in CALR mutation-positive and JAK2 V617F-positive ET.12,13,23
Table 1.
Hematologic and clinical features of CALR-positive versus JAK2 V617F-positive and triple-negative patients.
Eighty-six percent of the patients in our study group started on cytoreductive therapy, most commonly hydroxyurea, upon diagnosis. The triple-negative group contained significantly fewer treated patients compared to CALR mutation-positive and JAK2 V617F-positive patients (P=0.04, not shown).
ET is associated with cardiovascular complications including stroke, acute coronary syndrome, transient ischemic attack, pulmonary embolism, abdominal thrombosis, deep-vein thrombosis, and peripheral arterial thrombosis. We assessed whether the mutational status was associated with differences in the incidence of cardiovascular complications at diagnosis and arterial thrombosis or venous events. In our cohort of patients the risks of developing any of these complications did not differ significantly between the different molecular subtypes.
We also compared the clinical and hematologic data of the CALR type 1 and type 2 subgroups and compared them to those of the JAK2 V617F-positive group (Online Supplementary Table S2). Remarkably, the male predilection was confined to the group with CALR type 1 mutation, and this group also had a tendency to the highest platelet counts and was associated with younger age compared to the JAK2 V617F-positive group. Tefferi et al. also reported that CALR type 1 is associated with male gender. However, they also reported that type 2 is associated with younger age and higher platelet counts,24 which was not confirmed in our study or by another study published by Andrikovics et al.22
We divided our JAK2 V617F-positive group into a group with low mutational load (≤10%; n=11) and another with high mutational load (>10%; n=46), as determined by quantitative polymerase chain reaction. However, no significant differences between the two groups were revealed (Online Supplementary Table S3). Given the low number of patients with a low load of JAK2, this comparison needs to be conducted again in larger groups.
The mean follow-up of the CALR mutation-positive, the JAK2 V617F-positive and the triple-negative groups was 14, 7, and 7 years, respectively. The median overall survivals, as evaluated by Kaplan-Meier analysis, were 30 years (95% confidence interval, 25.4 to 34.5), 21 years (95% confidence interval, not reached), and not reached, respectively (P=0.015) (Figure 2A). Myelofibrosis-free survival (Figure 2B) and leukemia-free survival (Figure 2C) were not significantly different between the CALR mutation-positive group and the JAK2 V617F-positive group (P=0.65 and P=0.09, respectively). In our study, none of the triple-negative patients progressed to develop myelofibrosis or acute myeloid leukemia.
Figure 2.
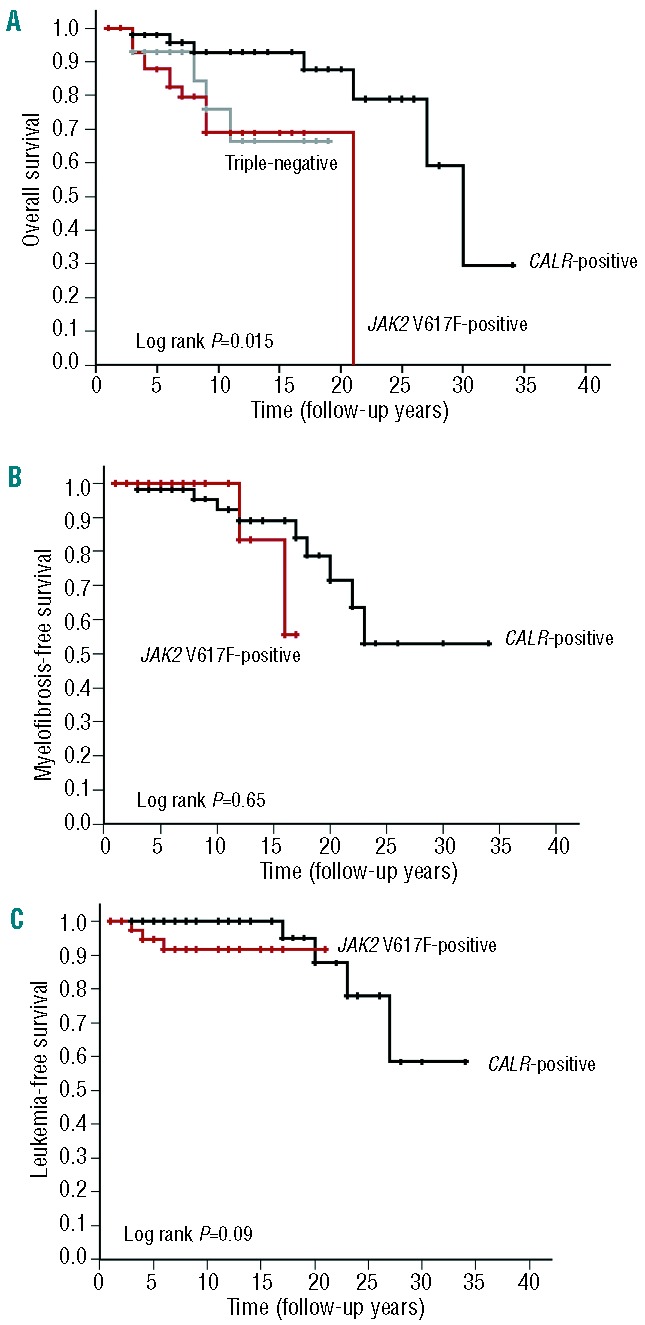
Overall survival, myelofibrosis-free survival, and leukemia-free survival. Kaplan-Meier estimate of (A) overall survival of CALR-positive, JAK2 V617F-positive, and triple-negative groups, (B) myelofibrosis-free survival and (C) leukemia-free survival of the CALR-positive group and JAK2 V617F-positive group.
In the first paper by Klampfl et al., the CALR mutant group had a better overall survival compared to JAK2 V617F-positive cases or MPL mutant cases in 311 ET cases and 203 PMF cases.6 However, subsequent studies by Tefferi et al., Andrikovics et al., and Rumi et al. showed better overall survival for CALR mutant cases than for JAK2 V617F-positive cases in PMF,11,22,25 but not in ET.8,11,13,22 Tefferi et al. addressed this divergence by studying survival in 299 ET cases with long follow-up, but did not find a survival advantage for CALR-mutated cases even after adjusting for age (≤ or > 65 years).12 Since JAK2 V617F-positive patients are generally older at diagnosis than CALR mutation-positive patients, we also divided our patients according to age (> 60 or ≤60 years). In the group of patients ≤60 years old, CALR mutation-positive patients had a better overall survival than the JAK2 V617F-positive group (P=0.04). On the other hand, in the group of patients older than 60 years there was no difference in the overall survival between the CALR mutation-positive group and the JAK2 V617F-positive group (P=0.19) (Figure 3). This indicates that the prognosis of CALR/JAK2 mutated ET is different only in younger age groups.
Figure 3.
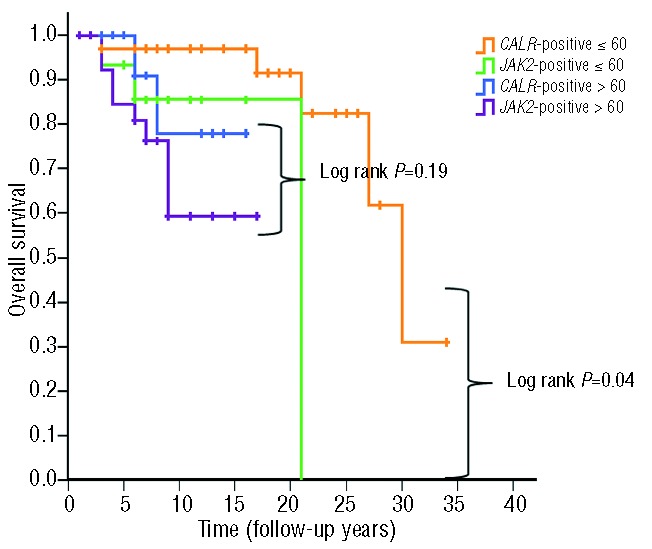
Overall survival of the CALR-positive and the JAK2 V617F-positive groups stratified into age groups. Kaplan-Meier estimate according to age groups: greater than 60 and younger or equal to 60 years old.
We also describe, what is to the best of our knowledge, the fourth ET patient with both a JAK2 V617F and a CALR mutation. In this patient the JAK2 V617F mutational load assessed by allele-specific polymerase chain reaction analysis was around 0.2%, while the mutational load of CALR type 2 estimated by Sanger sequencing was around 40%. Based on the differential mutational loads two scenarios are possible; either the JAK2 V617F is a subclonal mutation in the major CALR mutated clone, or the two mutations arose in two different clones with the CALR clone being the larger, with the JAK2 V617F as a minor clone. Given that no viable frozen material is available, this issue unfortunately cannot be further investigated in vitro.
In summary, this study of 160 ET patients confirms that CALR mutation-positive ET is phenotypically and biologically distinct from JAK2 V617F-positive ET, in terms of clinical presentation and disease evolution. We also observed that CALR mutant ET was associated with better overall survival in younger ET patients. Our study also suggests the possibility of phenotypic differences within the CALR mutant group according to the mutation type, in line with a few other studies.22,24 Although the analysis of CALR mutations is rapidly being implemented in the diagnostic work-up of ET, the molecular subtypes of ET are, at the time of writing, not taken into account in treatment decisions in ET.
Acknowledgments
PV is a senior clinical investigator of FWO-Vlaanderen. This study was supported by grants from FWO (G.0509.10N), Stichting tegen Kanker (2010-204), GOA/11/010, and FP7 (NGS-PTL-306242). The authors would like to thank Sanne Smits for excellent technical assistance.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. [DOI] [PubMed] [Google Scholar]
- 5.Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–2390. [DOI] [PubMed] [Google Scholar]
- 7.Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rumi E, Harutyunyan AS, Pietra D, et al. CALR exon 9 mutations are somatically acquired events in familial cases of essential thrombocythemia or primary myelofibrosis. Blood. 2014;123(15):2416–2419. [DOI] [PubMed] [Google Scholar]
- 9.Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123(10):1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tefferi A, Guglielmelli P, Lasho TL, et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014;28(7):1494–1500. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Lasho TL, Finke CM, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28(7): 1472–1477. [DOI] [PubMed] [Google Scholar]
- 12.Tefferi A, Wassie EA, Lasho TL, et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia. 2014;28(12):2300–2303. [DOI] [PubMed] [Google Scholar]
- 13.Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123(10):1552–1555. [DOI] [PubMed] [Google Scholar]
- 14.Thiele J, Kvasnicka HM, Tefferi A, Barosi G, Orazi A, Vardiman JW. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2008:44–50. [Google Scholar]
- 15.Xu N, Ding L, Yin C, et al. A report on the co-occurrence of JAK2V617F and CALR mutations in myeloproliferative neoplasm patients. Ann Hematol. 2015;94(5):865–867. [DOI] [PubMed] [Google Scholar]
- 16.McGaffin G, Harper K, Stirling D, McLintock L. JAK2 V617F and CALR mutations are not mutually exclusive; findings from retrospective analysis of a small patient cohort. Br J Haematol. 2014;167(2): 276–278. [DOI] [PubMed] [Google Scholar]
- 17.Lundberg P, Karow A, Nienhold R, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123(14):2220–2228. [DOI] [PubMed] [Google Scholar]
- 18.Broseus J, Lippert E, Harutyunyan AS, et al. Low rate of calreticulin mutations in refractory anaemia with ring sideroblasts and marked thrombocytosis. Leukemia. 2014; 28(6):1374–1376. [DOI] [PubMed] [Google Scholar]
- 19.Andrikovics H, Meggyesi N, Szilvasi A, et al. HFE C282Y mutation as a genetic modifier influencing disease susceptibility for chronic myeloproliferative disease. Cancer Epidemiol Biomarkers Prev. 2009;18(3):929–934. [DOI] [PubMed] [Google Scholar]
- 20.Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol. 2005;131(2):208–213. [DOI] [PubMed] [Google Scholar]
- 21.Barosi G. Essential thrombocythemia vs. early/prefibrotic myelofibrosis: why does it matter. Best Pract Res Clin Haematol. 2014;27(2):129–140. [DOI] [PubMed] [Google Scholar]
- 22.Andrikovics H, Krahling T, Balassa K, et al. Distinct clinical characteristics of myeloproliferative neoplasms with calreticulin mutations. Haematologica. 2014;99(7):1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CC, Gau JP, Chou HJ, et al. Frequencies, clinical characteristics, and outcome of somatic CALR mutations in JAK2-unmutated essential thrombocythemia. Ann Hematol. 2014;93(12):2029–2036. [DOI] [PubMed] [Google Scholar]
- 24.Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol. 2014;89(8):E121–124. [DOI] [PubMed] [Google Scholar]
- 25.Rumi E, Pietra D, Pascutto C, et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood. 2014;124(7):1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]


