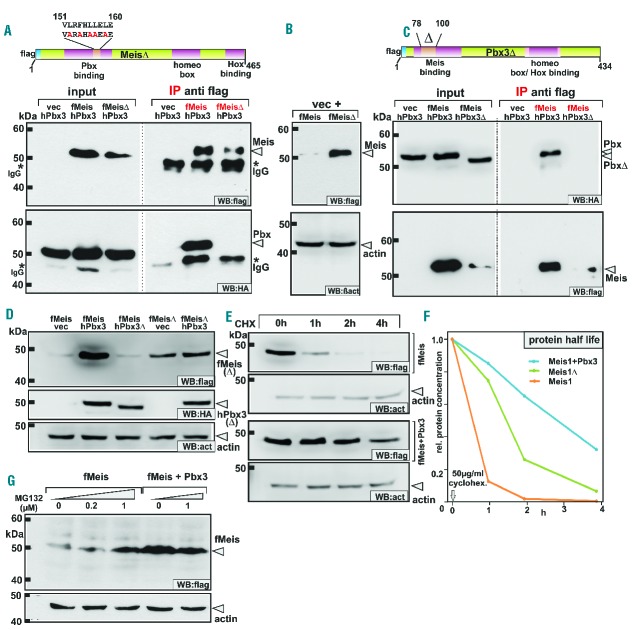
Figure 1.
Binding of Pbx3 or deletion of the Pbx-binding domain stabilizes Meis1 by prolonging protein half-life and blocking proteasomal degradation. (A) Generation of a Pbx-binding defective Meis1 mutant. Five alanine substitutions were introduced into the Pbx-binding domain of Meis1 to create a MeisΔ construct as schematically indicated. The lower panels demonstrate the results of immunoprecipitation experiments confirming that MeisΔ lost detectable affinity for Pbx3. f = flag tag, h = HA tag. (B) Deletion of the Pbx3-binding domain in MeisΔ leads to protein stabilization. Extracts from cells transfected with equal amounts of MeisΔ or wt Meis1 constructs were tested for expression of flag-tagged MeisΔ and wt Meis1 in immunoblots (upper panel). β-actin served as a loading control (lower panel). (C) Construction of a Meis1-interaction defective Pbx3. A Pbx3Δ mutant was cloned introducing a small deletion within the N-terminal Meis-interaction domain. In immunoprecipitation experiments this mutant lost its ability to bind to Meis1 (upper right panel). Concomitant with the loss of Meis1 binding capacity Pbx3Δ also did not stabilize Meis1 protein (lower left panel). (D) Comparison of the Meis1 stabilization effect achieved by co-expression of Pbx3 or by deletion of the Pbx-binding domain. Wt Meis1 or the Pbx-binding defective MeisΔ mutant were expressed alone or in combination with Pbx3 or together with Pbx3Δ that has lost Meis-binding capability. Accumulated Meis protein was detected by immunoblotting. (E) Instability of Meis1 is due to short half-life. Protein decay rates were determined for Meis1 and a combination of Meis1 + Pbx3 in a time course after shut-off of synthesis by addition of cycloheximide (CHX). Samples were drawn at the indicated time points and analyzed by western blot for the remaining quantity of Meis1. Please note that protein amounts and exposure times were adjusted to start with an equal signal intensity for Meis1 and Meis1 + Pbx3 samples. (F) Half-life determination of Meis1. Quantification of detectable Meis1 protein after shutting down protein synthesis was done in a parallel experiment as described in (B) by densitometric analysis. Representative values for one out of two experiments are given. (G) Small molecule inhibitor of the proteasome system phenocopies the presence of Pbx3. Meis1 alone or in combination with Pbx3 was expressed in cells incubated for 24 h after transfection with increasing amounts of the proteasome inhibitor MG132 as indicated. Flag-tagged protein was detected by immunoblot with β-actin as a loading control.