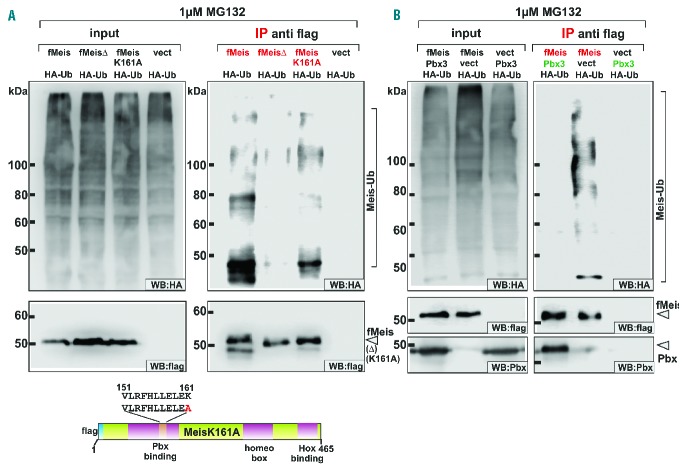
Figure 2.
Ubiquitination of Meis1 is blocked by interaction with Pbx3. (A) Mutations of the Pbx3-binding domain reduce ubiquitination of Meis1. Ubiquitination of Meis1, MeisΔ, and MeisK161A that carries an alanine substitution of the putative ubiquitin lysine acceptor within the Pbx3-binding domain, was investigated by co-expression with HA-modified ubiquitin (HA-Ub) in cells treated with MG132 to stabilize the transient ubiquitin-modified degradation intermediates. Meis-derivatives were specifically precipitated by anti-flag IP and analyzed for ubiquitin addition by an anti-HA immunoblot. The presence of Meis modified by addition of ubiquitin chains of varying length was indicated by the appearance of high molecular weight, HA-reactive material in western blotting (Meis-Ub). (B) Binding of Pbx3 blocks ubiquitination of Meis1. Meis1 alone or in combination with Pbx3 was co-expressed with HA-modified ubiquitin in cells treated with 1 μM proteasome inhibitor. Upon anti-flag precipitation ubiquitination was detected as described for (A) by anti-HA immunoblot.