Abstract
We previously demonstrated vast expansion of hypoxic areas in the leukemic microenvironment and provided a rationale for using hypoxia-activated prodrugs. PR104 is a phosphate ester that is rapidly hydrolyzed in vivo to the corresponding alcohol PR-104A and further reduced to the amine and hydroxyl-amine nitrogen mustards that induce DNA cross-linking in hypoxic cells under low oxygen concentrations. In this phase I/II study, patients with relapsed/refractory acute myeloid leukemia (n=40) after 1 or 2 prior treatments or acute lymphoblastic leukemia (n=10) after any number of prior treatments received PR104; dose ranged from 1.1 to 4 g/m2. The most common treatment-related grade 3/4 adverse events were myelosuppression (anemia 62%, neutropenia 50%, thrombocytopenia 46%), febrile neutropenia (40%), infection (24%), and enterocolitis (14%). Ten of 31 patients with acute myeloid leukemia (32%) and 2 of 10 patients with acute lymphoblastic leukemia (20%) who received 3 g/m2 or 4 g/m2 had a response (complete response, n=1; complete response without platelet recovery, n=5; morphological leukemia-free state, n=6). The extent of hypoxia was evaluated by the hypoxia tracer pimonidazole administered prior to a bone marrow biopsy and by immunohistochemical assessments of hypoxia-inducible factor alpha and carbonic anhydrase IX. A high fraction of leukemic cells expressed these markers, and PR104 administration resulted in measurable decrease of the proportions of hypoxic cells. These findings indicate that hypoxia is a prevalent feature of the leukemic microenvironment and that targeting hypoxia with hypoxia-activated prodrugs warrants further evaluation in acute leukemia. The trial is registered at clinicaltrials.gov identifier: 01037556.
Introduction
Therapy of acute myeloid leukemia (AML) results in high initial response rates, associated with the elimination of bulk leukemia cells, and, invariably, a high relapse rate.1 Recent studies indicate specific interactions between leukemia cells and the bone marrow (BM) microenvironment promote leukemic cell survival and confer drug resistance.2 Our pre-clinical data indicate an expanded hypoxic BM niche in AML compared to the discrete hypoxic niches observed in normal hematopoiesis.3 Similar findings have been reported in myeloma models,4 substantiating the concept of a prevalent hypoxic microenvironment in diverse hematologic malignancies. Recent gene expression profiling data confirmed highly distinct hypoxic and proinflammatory gene signatures in AML cells compared to normal hematopoietic stem cells.5 Hypoxia results in stabilization of the transcription factors hypoxia-inducible factor 1α (HIF-1α) and HIF-2α, which in turn induce a vast array of gene products controlling metabolism, angiogenesis, apoptosis, and cell cycle.6 In tumors, increased levels of HIF-1α activity are often associated with increased aggressiveness and therapeutic resistance.6,7 Importantly, HIF-1α has been shown to be over-expressed in clusters of leukemic cells in the BM of patients with acute lymphoblastic leukemia (ALL).8 A recent report suggests that quiescent HIF-expressing leukemic stem cells may contribute to minimal residual disease in AML9 and chronic myeloid leukemia (CML).10
Hypoxic tumors are resistant to chemotherapy and radiation and are associated with poor survival,11 suggesting hypoxia itself could be a therapeutic target. With the goal of exploiting hypoxia in tumors, several hypoxia-activated pro-drugs (HAPs) were designed to provide targeted release of toxins in tumors.11 A common mechanism by which a non-toxic prodrug can be activated is enzymatic addition of one electron, which initiates the formation of DNA reactive species, a process that can be inhibited by molecular oxygen. PR104 is a phosphate ester that is rapidly hydrolyzed in vivo to the corresponding alcohol PR104A, which acts as an HAP through its metabolic reduction to activated nitrogen mustards PR-104H and PR-104M.12 The second mechanism of PR104 activation is through hypoxia-independent two-electron reduction by enzyme aldo-keto reductase 1C3 (AKR1C3), which is highly expressed in AML blasts.13 In pre-clinical models of ALL, we showed PR104 prolonged survival and decreased the leukemia burden of immune-deficient mice injected with primary leukemia cells.3
In solid tumors, phase I clinical trials of single-agent PR104 given as a 1-h intravenous infusion every three weeks identified thrombocytopenia, neutropenia, infection, and fatigue as dose-limiting toxic effects and a maximum tolerated dose of 1.1 g/m2 (when given every 3 weeks)14 or 675 mg/m2 (when given on Days 1, 8, and 15 every 28 days).15 While no data on the clinical utility of HAPs in the setting of hematologic malignancies are available, this toxicity profile and pre-clinical data prompted us to hypothesize PR104 may demonstrate activity in patients with relapsed acute leukemia that harbors a hypoxic BM microenvironment.
To test this hypothesis, we conducted a phase I/II clinical trial of PR104 in relapsed or refractory AML or ALL. The primary objectives were to determine the toxic effects and recommended dose of PR104 in patients with relapsed/refractory leukemia. Secondary objectives were to evaluate the pharmacokinetics and anti-tumor effects of PR104, the expression of AKR1C3 in leukemic cells, and biomarkers of hypoxia.
Methods
Study population
Patients aged 18 years or older were eligible if they had persistent or relapsed AML (according to the 2008 World Health Organization classification16) requiring first or second salvage therapy. In the expansion phase of the study, patients with persistent or relapsed ALL were also eligible. Other entry criteria were standard for phase I studies. Cytogenetic risk group was defined according to the refined criteria of the National Cancer Research Institute/Medical Research Council.17
Treatment plan
In the phase I portion, patients with relapsed/refractory AML received PR104 as a 1-h intravenous infusion according to an escalating dose schedule. Induction therapy initially comprised administration of PR104 every 14–28 days for up to 3 cycles. Later, the induction treatment was limited to 1 cycle. Response and toxicity were assessed by day 42 (±2 days). A starting dose level of 1.1 g/m2 was based on the single-agent maximum tolerated dose for PR104 in patients with relapsed/refractory solid tumors.14 Patients who achieved complete remission (CR) or CR without platelet recovery (CRp) received consolidation therapy for up to 4 additional cycles, at 75% or 50% of the dose used for induction therapy. In the expansion phase of the study, patients received PR104 at a dose of 3 g/m2 or 4 g/m2, at the investigator’s discretion, toxic effects were monitored continuously, with a target of less than 30% grade 3 or 4 non-hematologic toxic effects. Response to treatment was assessed by International Working Group response criteria (Online Supplementary Appendix).18 The institutional review boards at MDACC and FHCRC approved this study, and patients gave consent in accordance with the Declaration of Helsinki.
Statistical analysis
The dose-finding portion of the study utilized the covariate-adjusted outcome-adaptive Bayesian method of Thall et al.19 to determine a recommended dose for specific subsets of patients as determined by 3 prognostic covariates. Patients had to achieve either CR or CRp by day 42 for PR104 administration to be considered efficacious. For the purpose of dose finding, toxicity was defined as treatment-related death or treatment-related grade 3 or 4 non-hematologic CTCAE toxicity within 42 days from the start of therapy. Once a potentially beneficial dose was determined, an expanded dose cohort of patients with AML or ALL received PR104 at uniform doses in the expansion phase of the study. Unadjusted overall survival (OS) time distributions were estimated by using the Kaplan-Meier method.20 Further details are provided in the Online Supplementary Appendix.
Pharmacokinetic assessment
Plasma pharmacokinetics was assessed by sampling 0.5, 1, 1.5, 2 and 3 h after the start of PR-104 infusions during cycle 1 only and included determination of PR104, PR104A and its O-glucuronide metabolite PR104G by LC-MS/MS, as described elsewhere.14
Biomarker analyses
Biomarkers analyzed included hypoxia markers pimonidazole (PIMO), HIF-1α and its target carbonic anhydrase IX (CAIX) evaluated in bone marrow biopsies, and AKR1C3 activity. (See the Online Supplementary Appendix for details of assays).
Results
Study population
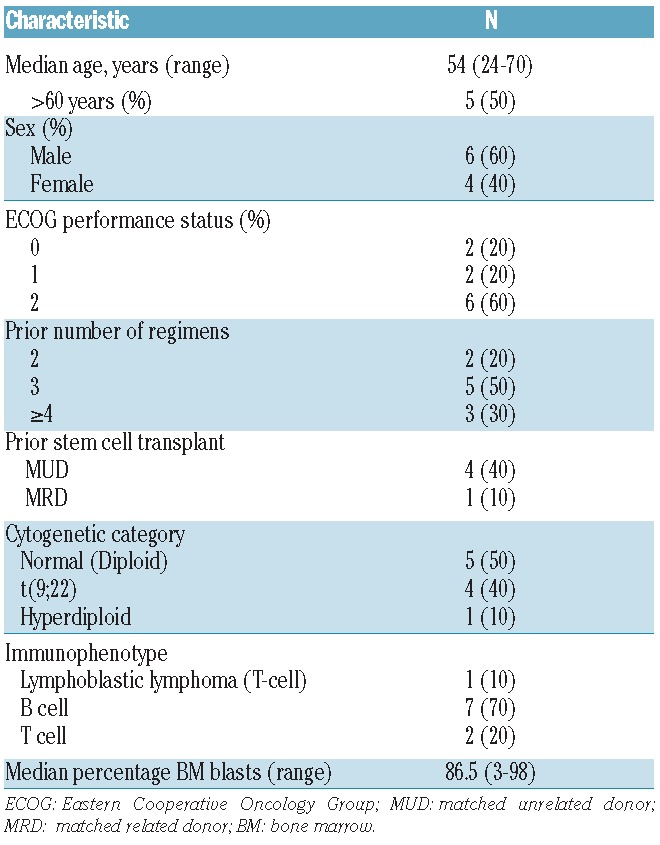
Fifty-three patients were enrolled and 50 patients [AML, n=40 (80%); ALL, n=10 (20%)] received PR104 at doses ranging from 1.1 g/m2 to 4 g/m2. Patients with ALL received PR104 doses of 3 or 4 g/m2 only. Patients’ characteristics are shown in Tables 1 and 2. Median age of the study population was 62 years (range 20–79 years). Among patients with AML, 29 (72.5%) had relapsed disease and 11 (27.5%) refractory disease; all except one patient had a first CR duration of 52 weeks or less. Eighteen (45%) patients with AML had adverse cytogenetics, and 4 patients with ALL had a translocation t[9;22]. Twenty-one (52.5%) patients with AML had undergone 2 prior therapies; and all 10 patients with ALL (100%) had received 2 or more prior treatments.
Table 1.
Baseline characteristics of acute myeloid leukemia patients (n=40).
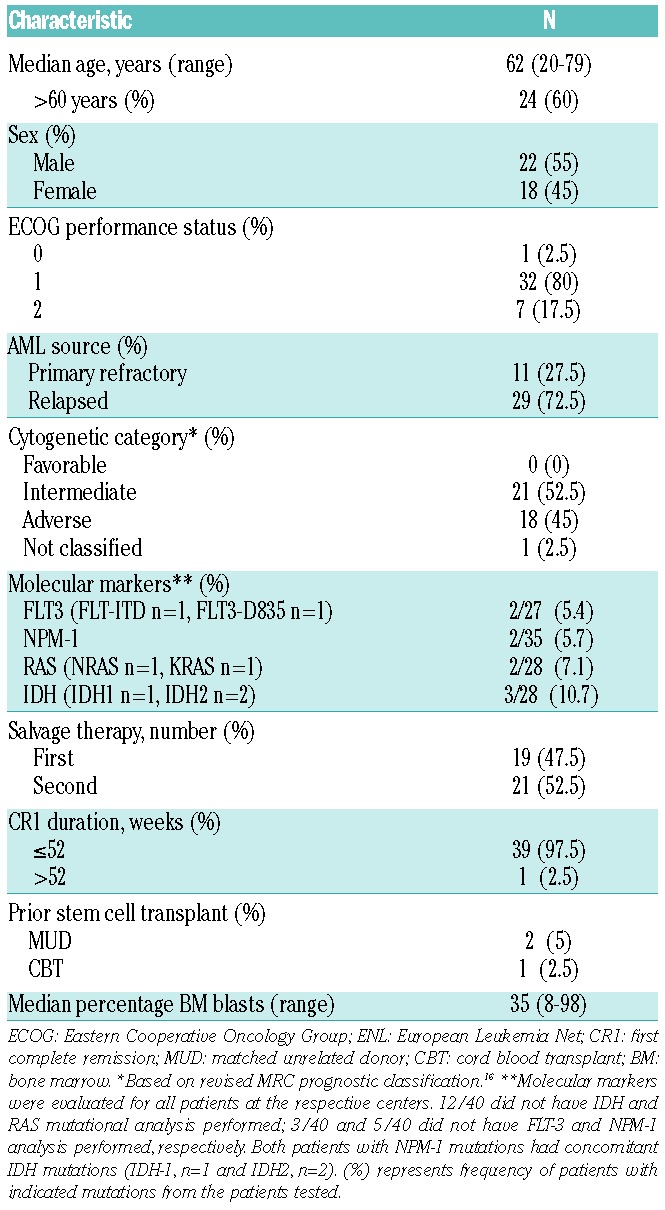
Table 2.
Baseline characteristics of acute lymphoblastic leukemia patients (n=10).
Phase I/II dose-finding
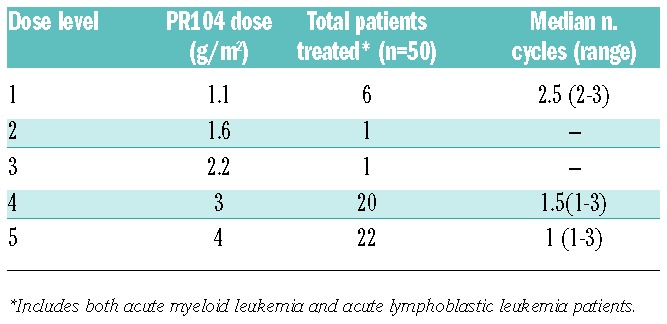
In the dose-finding phase, 25 patients with AML received PR104 at doses varying from 1.1 g/m2 to 4 g/m2 [1.1 g/m2 (n=6), 1.6 g/m2 (n=1), 2.2 g/m2 (n=1), 3 g/m2 (n=9), or 4 g/m2 (n=8)] for a median of 1 cycle (range 1–3 cycles) (Table 3). Of these, 17 patients were treated using adaptive dose selection, and 8 additional patients were treated at 3 g/m2 or 4 g/m2 to further evaluate safety and efficacy and establish a recommended dose for the expansion phase. During this portion of the study, PR104 was associated with acceptable toxicity at the 3 g/m2 and 4 g/m2 dose levels. Non-hematologic grade 3/4 treatment-related adverse events at 3 g/m2 and 4 g/m2 included febrile neutropenia (8 patients, 33%), infections (5 patients, 21%) and anorexia, nausea, vomiting, fatigue, colitis, pruritus, and rash (1 subject each, 4%). PR104 caused suppression of hematologic parameters, with grade 3–4 leukopenia and lymphopenia seen in 68%, neutropenia or anemia in 56%, and thrombocytopenia in 52%. In 2 patients treated at 3 g/m2, prolonged BM suppression (<5% marrow cellularity beyond day 42 without evidence of leukemia) was observed. Febrile neutropenia was more common at 4 g/m2 (5 of 8 patients vs. 3 of 16 at other doses combined).
Table 3.
PR104 dose levels.
Phase II dose expansion
In the expansion phase of the study, a total of 25 patients (AML, n=15; ALL, n=10) were treated at doses of 3 g/m2 (n=11) or 4 g/m2 (n=14) for a median of 1 cycle (range 1–3 cycles). Of the 17 patients initially enrolled in the phase II, 4 developed grade 3 enteritis [24%; standard error (SE) 17% to 43%], and one patient developed grade 5 liver failure. The patient who developed liver failure was a 53-year old female with history of AML with complex cytogenetics, refractory to decitabine who relapsed two months after salvage with idarubicin/cytarabine; she developed conjugated bilirubinemia and hepatomegaly after the second course of PR104, with liver biopsy demonstrating hepatocellular and canalicular cholestasis without portal inflammation or evidence of bile duct damage, most consistent with drug injury. Two of these adverse events resulted in treatment-related deaths; both were patients with AML treated at 4 g/m2 after receiving a second cycle of induction therapy at the full PR104 dose. This prompted a trial amendment restricting induction to only 1 cycle in the last 8 patients treated on study. Of these 8 patients, one died of pneumonia in the setting of drug-induced myelosuppression, and another developed grade 3 esophagitis and grade 4 small bowel obstruction.
Safety and tolerability
Online Supplementary Table S1 provides a summary of adverse events occurring in 15% or more of patients regardless of attribution; all drug-related adverse events are summarized in Online Supplementary Table S3. All laboratory abnormalities that changed by at least 1 grade (based on CTCAE v.4.0) from baseline (or cycle 1 day 1) were considered drug-related adverse events and are listed in the Online Supplementary Table S1. The most common treatment-related grade 3/4 adverse events were myelosuppression (anemia 62%, leukopenia 64%, neutropenia 50%, thrombocytopenia 46%); febrile neutropenia (40%); infections, including lung infection (16%); sepsis (8%); and gastrointestinal toxic effects. Grade 3–4 effects in the lower gastrointestinal tract (enteritis, enterocolitis, colitis, and small bowel obstruction) were seen in 7 patients (14%), all treated at the 3 g/m2 or 4 g/m2 dose. Nausea, vomiting, and diarrhea were common but were predominantly grade 1/2. The most common treatment-related laboratory abnormalities other than myelosuppression were hypoalbuminemia (76%), hyperglycemia (68%), hyperbilirubinemia (52%), and creatinine elevation (36%). There was no statistically significant difference in adverse events between different dose levels except for anemia (Fisher’s exact test, P=0.023). Analysis of associations between toxic effects and covariates showed that neutropenia was significantly associated with the number of prior induction regimens (Online Supplementary Table S3). Seven deaths occurred during treatment (14%), one at the dose of 1.1 g/m2, 3 at 3 g/m2, and 3 at 4 g/m2. All 3 treatment-related deaths occurred at 4 g/m2, and included: hepatic failure (n=1), sepsis and unresolved enteritis (n=1), and pneumonia in the setting of drug-induced myelosuppression (n=1).
Responses and outcomes
Anti-leukemic activity was observed at PR104 doses of 3 g/m2 and 4 g/m2. Nine patients (18%) had reduction of BM blasts by more than 50% from baseline on day 14. In addition, 9 patients (18%) had BM aspirates on day 14 that yielded insufficient sample and therefore the percentage of blasts could not be assessed. None of the 8 patients who received less than 3 g/m2 had clearance of BM blasts.
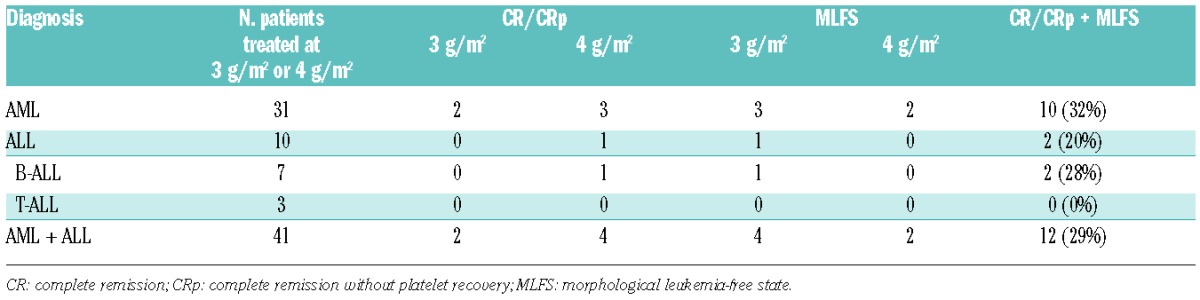
Forty-nine of the 50 patients were evaluable for response. One patient achieved CRp but was considered not evaluable due to recent participation in a different clinical trial. Among the 49 evaluable patients, 12 (24%) exhibited anti-leukemic activity (AML, n=10; B-cell ALL, n=2). All responses occurred at PR104 doses of 3 g/m2 or 4 g/m2 (Table 4). Ten of the 31 evaluable patients with AML treated at one of these doses [32%, confidence interval (CI): 0.19, 0.5] and 2 of 10 patients with ALL patients (20%, CI: 0.06, 0.51) had responses (CR, n=1; CRp, n=5; MLFS, n=6). Of the 21 patients with AML who had received one prior treatment, responses were seen in 7 (CR, n=1; CRp, n=3; MLFS, n=3). One patient with AML, an 81-year old man with normal AML karyotype (NPM1 and IDH2 mutations) in whom induction chemotherapy with omacetaxine and low-dose cytarabine had failed, achieved CR after 1 induction cycle (3 g/m2) followed by 2 consolidation cycles (1.5 g/m2) of PR104, the CR lasting for 13 months. Four patients underwent a stem cell transplant following response to PR104. The characteristics of responding patients are shown in Online Supplementary Table S4.
Table 4.
Responses to PR104 at 3 g/m2 or 4g/m2 in acute myeloid leukemia and acute lymphoblastic leukemia.
Median remission duration in the responders was 57 days (95%CI: 21, 366). The median OS of patients treated at 3 g/m2 or 4 g/m2 was 86 days (95%CI: 64,152). In patients who achieved CR/CRp/MLFS, the PFS and OS were 99 (95%CI: 69, 408) and 175 (95%CI: 85, NA) days, respectively (Figure 1A and B). None of the co-variates (age, PR104 dose, CR1 duration, prior number of inductions) was significantly associated with response to PR104 (Online Supplementary Tables S5 and S6) or significantly predicted either OS or PFS (data not shown).
Figure 1.
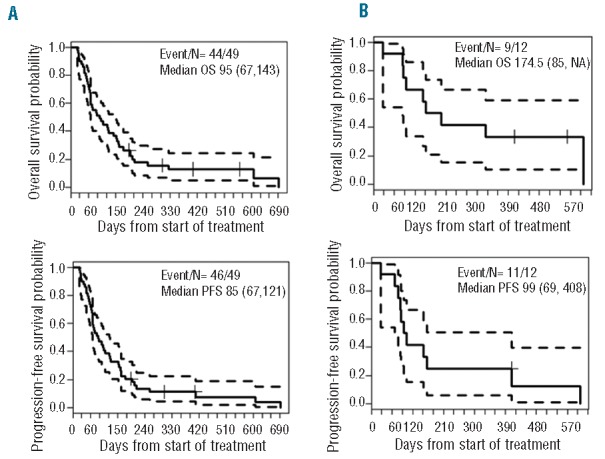
(A). Kaplan-Meier curves of overall survival (OS) and progression-free survival (PFS) for all patients (n=49) from start of treatment. (B). Kaplan-Meier curves of OS and PFS for responders (n=12).
Correlative studies
Pharmacokinetics
Plasma pharmacokinetic data for PR-104 and its two major metabolites (PR-104A and glucuronide PR-104G) were available for 25 patients with AML. Non-compartmental pharmacokinetic parameters for each patient are listed in Online Supplementary Table S7. AUC values were highly variable between patients, but showed dose dependence broadly consistent with linear pharmacokinetics for each analyte (Figure 2).13,14,21 The mean PR-104 clearance was 127 L/h-m2 (SD 99 L/h-m2) for these patients, similar to that for solid tumor oncology patients.13,14 The mean AUC for PR104A, the pharmacokinetic parameter most relevant to cytotoxicity in vitro,22,23 was significantly higher at 3 g/m2 and 4 g/m2 than at 1.1 g/m2, the maximum tolerated doses achieved in solid tumor oncology patients13 (Online Supplementary Table S8). Although highly variable between patients, PR-104A AUC was not associated with response or toxicity in this small group of patients (Online Supplementary Table S9).
Figure 2.
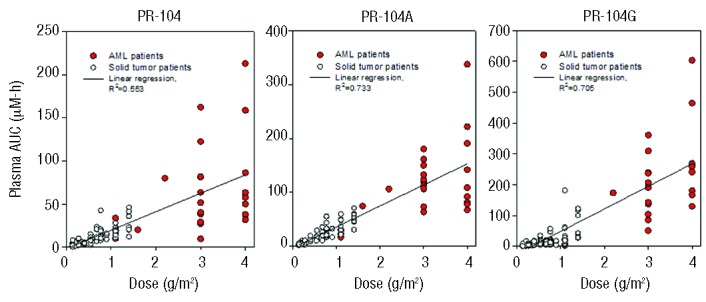
Plasma pharmacokinetics of PR104 and its major metabolites PR104A and PR104G, comparing acute myeloid leukemia (AML) patients (colored symbols) and solid tumor patients (data from refs. 13,14,32). Values are AUC (area under the concentration-time curve) for each patient, and lines are the linear regressions through all data.
Hypoxia-related biomarkers
To characterize the extent of pre-treatment and/or post treatment BM hypoxia, we utilized hypoxia marker PIMO and immunostaining for HIF-1α and CAIX in patients who consented to these studies. Five patients received PIMO injection prior to PR104 therapy. All 5 patients showed positive BM signal (Figure 3A). Quantification of PIMO signal in the BM showed levels of positive cells ranging between 50% and 88%. In 4 of the 5 patients, PIMO was used to detect BM hypoxia on day 14 after PR104 treatment; the percentage of PIMO-positive cells in the BM was reduced from an average of 74.7±4.4% to 23.3±3.5% (P=0.002) after treatment (Figure 3B and C). In an additional 10 patients who consented to the study after the baseline BM aspirations had already been performed for clinical purposes, the percentage of PIMO positivity on days 14–42 ranged from 10% to 89% (Figure 3D and Online Supplementary Figure S1). No correlation between PIMO positivity and percentage of BM blasts was observed (Online Supplementary Figure S2E and F).
Figure 3.
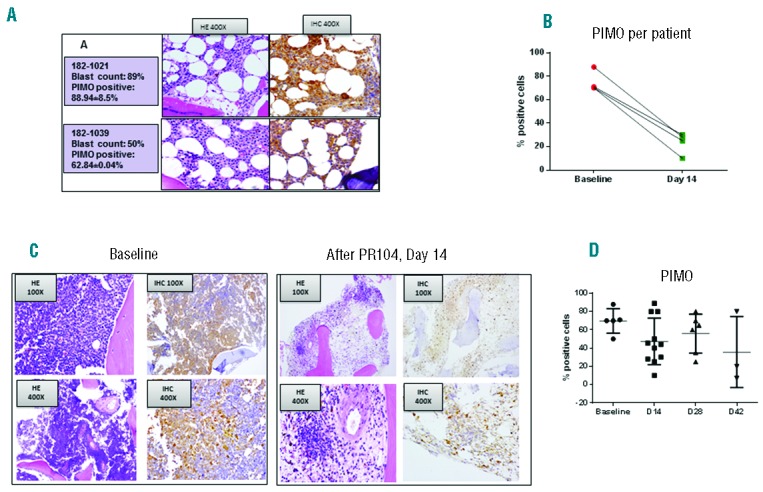
(A). Examples of hematoxylin and eosin (HE) staining (left) and PIMO immunohistochemical (IHC) staining (right) in bone marrow (BM) biopsy specimens from 2 representative patients. Original magnification, ×400. (B). Quantification of PIMO-positive cells in BM before and after PR104 (day 14) treatment by CRi spectral imaging and Inform software analysis (=4). None of these patients achieved an objective response. (C). Example of paired BM biopsy specimens (before and after PR104) in patient 182-1017 (ALL). Baseline BM blasts, 98%; day 14, 43%. Fraction of PIMO-positive cells: baseline, 82%; day 14, 28%. Original magnification is shown in the boxed areas. (D). Fraction of PIMO-positive cells in all BM specimens tested.
Expression of hypoxia markers HIF-1α and CAIX was studied in BM samples at baseline and after the first cycle of PR104 in a subset of patients (n=27 and 25, respectively) treated at the 3 g/m2 or 4 g/m2 dose for whom BM biopsy specimens were available for research studies. HIF-1α was positive in all but one of the 27 samples at baseline, with the percentage of positive cells ranging between 20% and 100%; CAIX was positive in all 25 samples at baseline, and the percentage of positive cells was between 40% and 100%. There was a significant correlation between proportions of HIF-1α– and CAIX-positive cells (Spearman r = 0.491, P<0.0001) (Figure 4A). In 8 of the 16 paired specimens, fewer HIF-1α–positive cells were detectable after PR104 administration (mean±SEM, 76.8±3.8% vs. 53.1±7.4%; P=0.004) (Figure 4B and C), and 3 cases became negative after treatment, including a responding patient whose percentage of positive cells at baseline was 40%. Likewise, in 11 of the 16 paired specimens, fewer CAIX-positive cells were detectable after PR104 administration (75±4.7% vs. 60.6±6.8%; P=0.03) (Figure 4D). Lower fraction of HIF-1α– and CAIX-positive cells after therapy may reflect preferential killing of hypoxic cells or merely decrease of the tumor burden after PR104 therapy. Expression of both markers was significantly correlated with percentage of BM blasts in pre-treatment and post-treatment samples (Online Supplementary Figure S2A-D). No correlation was found between HIF-1α and PIMO or CAIX and PIMO, at baseline or after therapy (Online Supplementary Figure S3A-D).
Figure 4.
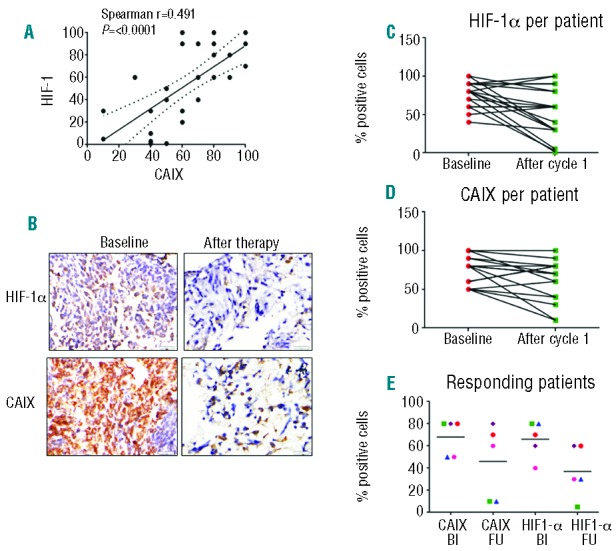
(A). Correlation between proportions of HIF-1α– and CAIX-positive cells (all samples included, before or after therapy). (B). HIF-1α and CAIX were detected by immunohistochemical staining in BM biopsy specimens of ALL Patient 182-1018 (baseline BM blasts, 67%; day 14, 40%). HIF-1α– and CAIX-positive cells, before and after PR104: 80% vs. 19% and 80% vs. 5%. (C and D). Proportions of HIF-1α– and CAIX-positive cells before and after PR104, in all samples tested. (E). Proportions of HIF-1α– and CAIX-positive cells before and after PR104, in 5 responding patients; Bl: baseline; FU: follow up. Symbols represent patients as follows: green square: Patient 1018 (best response, MLFS); blue triangle: Patient 1023 (best response, CRp); red circle.
Of the 8 responding patients who had biomarker studies performed, 3 were not evaluable because of “empty” marrow on day 14. In 3 of the 5 patients who achieved CRp or MLFS, proportions of both HIF-1α– and CAIX-positive cells decreased. In one patient, the fraction of PIMO-positive cells tested decreased from 50% at baseline to 7% on day 42 (Online Supplementary Figure S4). No significant change in HIF-1α/CAIX was seen in BM samples from 2 of the 5 patients, whose best response was MLFS; one of these had no change in percentage of blasts after the first cycle when the biomarker assessment was performed. No significant associations between biomarkers and dose or toxicity were found (data not shown).
AKR1C3
Given the enzyme AKR1C3 can lead to PR104 activation in the absence of hypoxia, we investigated its activity in baseline samples [peripheral blood (PB) or BM] from 16 patients. The activity in samples from trial patients was measured by a fluorometric assay13 in which AKR1C3 specific activity results in the conversion of coumberone to coumberol and it is inhibited by SN34037. In 16 patients the amount of SN34037-sensitive coumberol formation was on average 1.24±0.3 μM (range 0.06–3.83). This mean was 1.6- or 2.8-fold higher, respectively than the value for BM or PB from normal donors.13 No correlation was found between percentage of blasts and AKR1C3 activity (P=0.19). Only 2 responding patients had the AKR1C3 assay performed, and no statistical association was found between response or toxicity and AKR1C3 activity.
Discussion
In this study, we demonstrated that the BM in patients with advanced leukemia is markedly hypoxic, and the HAP PR104 had measurable clinical activity in patients with relapsed/refractory AML or ALL. This agent had significant toxicity, primarily myelosuppression and gastrointestinal effects.
Objective responses (CR/CRp) were seen in 6 of 31 (19%) patients with AML treated at 3 g/m2 or 4 g/m2. An additional 6 patients cleared BM blasts (MLFS), and a significant fraction had temporary blast reductions at day 14 assessment. These findings constitute signs of clinical activity in these cases of relapsed/refractory AML with poor-risk features (short CR duration all except 1, 45% adverse cytogenetics, 52% having undergone ≥2 prior chemotherapy regimens). Two responses (one CRp, one MLFS) were seen in heavily pre-treated patients with ALL (median 3 prior regimens) Notably, 4 patients who showed a response to PR104 later underwent stem cell transplantation, suggesting the potential utility of this therapy as a myeloablative “bridge to transplant”.
Doses of PR104 administered in this trial (3 g/m2 and 4 g/m2) were 3–4 times higher than those used in solid tumor studies, resulting in approximately 3-fold higher AUCs for PR104 and PR104A. This could explain the gastrointestinal toxicity observed with repeated dosing. Severe gastrointestinal toxicity (enteritis, enterocolitis, small bowel obstruction) was seen in 14% of patients (17% of those treated at the highest dose) and caused sepsis and death in one patient. Review of the literature indicates the occurrence of neutropenic enterocolitis in 5.6%–15% of patients with hematologic malignancies treated with chemotherapy24–27 and the higher incidence in the setting of drug-induced neutropenia. It is possible PR104 has a direct cytotoxic effect on the gastrointestinal tract, via biliary excretion of its O-glucuronide metabolite (PR-104G) which is the major PR-104 metabolite in solid tumor patients.28 as it is in the present study (Figure 2); β-glucuronidase activity in the gut flora can regenerate PR-104A, which in turn is reduced to the active cytotoxin PR-104H by bacterial nitroreductases.28
Overall, the most common severe adverse effects (CTC grades 3–5) were myelosuppression, febrile neutropenia, and infection. Neutropenia and thrombocytopenia constituted dose-limiting toxic effects in solid tumor studies of single-agent PR104.13,14 These observations point to possible toxicity against normal hematopoietic stem/progenitor cells, residing within hypoxic BM niches.29,30 This toxicity against normal hematopoiesis makes it difficult to conclude whether there is a therapeutic window in the setting of leukemia with profound underlying myelosuppression. A different hypoxia-activated cytotoxin could possibly be less toxic against normal BM. In fact, the initial results of the phase I clinical trial with TH-302,31 an HAP of the cytotoxin bromoisophosphoramide mustard, did not show myelosuppression as a prevalent toxic effect, although dose escalation was interrupted due to DLT of mucositis. Alternatively, combining these agents at less toxic doses with low-dose chemotherapy could conceivably decrease chemoresistance and improve treatment outcomes.
In this study, we have demonstrated that hypoxia is highly prevalent in human leukemic BM, confirming our pre-clinical findings in murine leukemia models. Although stabilization of HIF-1α in leukemic marrows has been shown previously,9,32 this is the first utilization of PIMO for measuring hypoxia levels in leukemia. These data support recently published findings of a distinct hypoxia gene expression signature in patients with AML.6 PR104 significantly decreased leukemia cells residing in the hypoxic BM. We were able to utilize PIMO prior to PR104 treatment in only a handful of patients, since the majority of patients underwent routine BM testing prior to enrollment in the PR104 study. Although this limited our ability to conclusively compare PIMO and HIF-1/CAIX measurements, we did not find a correlation between these 2 separate assessments, suggesting that mechanisms of HIF-1/CAIX stabilization besides intracellular hypoxia may play a role in leukemic cells. This is not unexpected given the multitude of upstream regulators of HIF-1 expression.7 On the other hand, expression of HIF-1 and CAIX showed correlation in both pre-treatment and post-treatment BM samples, supporting the validity of these markers. Importantly, expression of HIF and CAIX correlated with the percentage of BM blasts, indicating that stabilization of HIF-1α and CAIX tightly correlates with the extent of leukemia infiltration and most likely represents activation of HIF signaling within leukemia blasts. These findings implicate HIF-1α as one of the molecules to be targeted in adjuvant anti-leukemia therapy. Of interest, IDH mutations common in AML were shown to cause HIF-1α stabilization in one study21 but were associated with the lower HIF-1α levels in another.33 Although therapeutic targeting of transcription factors has proven challenging, several agents commonly used in AML (doxorubicin; topoisomerase I inhibitor topotecan) directly affect HIF protein levels,34,35 which could contribute to their anti-leukemia efficacy.
Despite the fact our biomarker analysis supported the hypoxic nature of the leukemic microenvironment, the expression levels of the biomarkers did not directly correlate with the anti-leukemia efficacy of PR104. Although this could be explained by the recently discovered hypoxia-independent activation of PR104 through 1-electron reductase AKR1C3,13 we found no correlation between functional AKR1C3 activity and drug efficacy; the caveat low AKR1C3 levels could reflect low leukemia burden in the blood samples used interchangeably with BM for these measurements. An alternative explanation is that despite adequate activation of the cytotoxin in the hypoxic leukemic marrows, it was unable to induce substantial killing in refractory leukemia cells possessing multiple mechanisms of resistance to alkylating agents, even upon achieving high local concentrations. This would imply that for successful application of the HAP concept, targeted agents such as selective kinase inhibitors in a genetically defined context may have a higher potential for success. This strategy would likewise limit toxicity to normal hematopoietic stem cells that do not express the oncogenic target.
In summary, this study demonstrated BM hypoxia to be a possible target for HAPs such as PR104. However, this strategy is unlikely to be successful for long-term leukemia control because of on-target toxicity of PR-104 to the BM. Further studies may be warranted to determine whether lower doses of PR104 combined with agents that target non-hypoxic cells yield better efficacy and tolerability. Alternatively, the use of HAPs to target residual hypoxic cells residing within protective BM niches is another attractive strategy. Finally, the appreciation of the hypoxic nature of leukemic cells gained through this study warrants further investigations to fully understand the mechanisms of this hypoxia and the biological consequences of the metabolically altered leukemia microenvironment, which have clear implications for chemoresistance and relapse.
Acknowledgments
We would like to thank Kathryn Hale, Scientific Editor, MD Anderson Cancer Center, for editorial assistance.
Footnotes
Funding
This research was supported by Proacta Inc.; the National Institutes of Health Leukemia 5 R21 CA153019-02, Leukemia and Lymphoma Society Scholar 2189-12 and Leukemia Spore 5 P50 CA100632-08 DRP award (to MK); the Leukemia Spore Pathology Core (CBR); and the Cancer Center Support Grant P30CA016672.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Estey EH. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88(4):318–327. [DOI] [PubMed] [Google Scholar]
- 2.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. 2011;29(5):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benito J, Shi Y, Szymanska B, et al. Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. PLoS One. 2011;6(8):e23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azab AK, Hu J, Quang P, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119(24):5782–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapin N, Bagger FO, Jendholm J, et al. Comparing cancer vs normal gene expression profiles identifies new disease entities and common transcriptional programs in AML patients. Blood. 2014;123(6):894–904. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. [DOI] [PubMed] [Google Scholar]
- 7.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437–443. [DOI] [PubMed] [Google Scholar]
- 8.Wellmann S, Guschmann M, Griethe W, et al. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia. 2004;18(5):926–933. [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga T, Imataki O, Torii E, et al. Elevated HIF-1alpha expression of acute myelogenous leukemia stem cells in the endosteal hypoxic zone may be a cause of minimal residual disease in bone marrow after chemotherapy. Leuk Res. 2012; 36(6):e122–124. [DOI] [PubMed] [Google Scholar]
- 10.Ng KP, Manjeri A, Lee KL, et al. Physiologic hypoxia promotes maintenance of CML stem cells despite effective BCR-ABL1 inhibition. Blood. 2014;123(21):3316–3326. [DOI] [PubMed] [Google Scholar]
- 11.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. [DOI] [PubMed] [Google Scholar]
- 12.Patterson AV, Ferry DM, Edmunds SJ, et al. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clin Cancer Res. 2007;13(13):3922–3932. [DOI] [PubMed] [Google Scholar]
- 13.Jamieson SM, Gu Y, Manesh DM, et al. A novel fluorometric assay for aldo-keto reductase 1C3 predicts metabolic activation of the nitrogen mustard prodrug PR-104A in human leukaemia cells. Biochem Pharmacol. 2014;88(1):36–45. [DOI] [PubMed] [Google Scholar]
- 14.Jameson MB, Rischin D, Pegram M, et al. A phase I trial of PR-104, a nitrogen mustard prodrug activated by both hypoxia and aldoketo reductase 1C3, in patients with solid tumors. Cancer Chemother Pharmacol. 2010;65(4):791–801. [DOI] [PubMed] [Google Scholar]
- 15.McKeage MJ, Gu Y, Wilson WR, et al. A phase I trial of PR-104, a pre-prodrug of the bioreductive prodrug PR-104A, given weekly to solid tumour patients. BMC Cancer. 2011;11:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. [DOI] [PubMed] [Google Scholar]
- 17.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. [DOI] [PubMed] [Google Scholar]
- 19.Thall PF, Nguyen HQ, Estey EH. Patient-specific dose finding based on bivariate outcomes and covariates. Biometrics. 2008;64(4):1126–1136. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324(5924):261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel K, Choy SS, Hicks KO, et al. A combined pharmacokinetic model for the hypoxia-targeted prodrug PR-104A in humans, dogs, rats and mice predicts species differences in clearance and toxicity. Cancer Chemother Pharmacol. 2011;67(5):1145–1155. [DOI] [PubMed] [Google Scholar]
- 23.Foehrenbacher A, Patel K, Abbattista MR, et al. The Role of Bystander Effects in the Antitumor Activity of the Hypoxia-Activated Prodrug PR-104. Front Oncol. 2013;3:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davila ML. Neutropenic enterocolitis: current issues in diagnosis and management. Current infectious disease reports. 2007;9(2):116–120. [DOI] [PubMed] [Google Scholar]
- 25.Ebert EC, Hagspiel KD. Gastrointestinal manifestations of leukemia. Journal of gastroenterology and hepatology. 2012;27(3):458–463. [DOI] [PubMed] [Google Scholar]
- 26.Gorschluter M, Mey U, Strehl J, et al. Neutropenic enterocolitis in adults: systematic analysis of evidence quality. Eur J Haematol. 2005;75(1):1–13. [DOI] [PubMed] [Google Scholar]
- 27.Hogan WJ, Letendre L, Litzow MR, et al. Neutropenic colitis after treatment of acute myelogenous leukemia with idarubicin and cytosine arabinoside. Mayo Clin Proc. 2002;77(8):760–762. [DOI] [PubMed] [Google Scholar]
- 28.Gu Y, Tingle MD, Wilson WR. Glucuronidation of anticancer prodrug PR-104A: species differences, identification of human UDP-glucuronosyltransferases, and implications for therapy. J Pharmacol Exp Ther. 2011;337(3):692–702. [DOI] [PubMed] [Google Scholar]
- 29.Eliasson P, Jonsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222(1):17–22. [DOI] [PubMed] [Google Scholar]
- 30.Winkler IG, Barbier V, Wadley R, et al. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116(3):375–385. [DOI] [PubMed] [Google Scholar]
- 31.Konopleva M, Handisides D, Lorente GA, et al. Phase I study of TH-302, a hypoxia-activated cytotoxic prodrug, in subjects with advanced leukemias. J Clin Oncol. 2012;30(Abstract 6585). [Google Scholar]
- 32.Deeb G, Vaughan MM, McInnis I, et al. Hypoxia-inducible factor-1alpha protein expression is associated with poor survival in normal karyotype adult acute myeloid leukemia. Leuk Res. 2011;35(5):579–584. [DOI] [PubMed] [Google Scholar]
- 33.Koivunen P, Lee S, Duncan CG, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483(7390):484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K, Qian DZ, Rey S, et al. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci USA. 2009;106(7):2353–2358. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Rapisarda A, Uranchimeg B, Sordet O, et al. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 2004; 64(4):1475–1482. [DOI] [PubMed] [Google Scholar]


