Among haematological malignancies, Hodgkin’s lymphoma (HL) remains a disease with a high cure rate and overall survival rate of >80% for patients under 60 years. Nevertheless, approximately 5–10% of HL patients are refractory to initial treatment and 10–30% relapse after achieving an initial complete remission. In this refractory setting, disease-free survival is very short,1–4 even when using an aggressive chemotherapy combination (platinum-based, ifosfamide/etoposide-based).3,5–7
Owing to these poor results, most experts recommend that eligible patients with relapsed or refractory disease undergo intensive therapy (auto- or allograft) after obtaining a good response. However, none of the existing options have been compared in a randomised trial, and there are many different combinations available. The literature reveals that 60–87% of refractory/relapsing patients are responders to second-line therapy3 and thus eligible for autologous stem cell transplantation, even if the length of the first remission (> or < 1 year) signifies that mortality is very predictable. The LYSA group reported an event-free survival (EFS) at 5 years of 46% and 73% when using double auto-SCT for poor risk patients and unique auto SCT for intermediate risk patients respectively.8 Furthermore, these salvage regimens were often associated with significant haematological toxicity (frequent patient hospitalisation for febrile neutropenia; high incidence of transfusion support).
In vitro studies have demonstrated the potential antitumor activity of anti-CD30 monoclonal antibodies (mAB) in HL and large B-cell anaplastic lymphoma, where CD30 is expressed selectively (84–91% of classic HL cells are detected as CD30 positive), creating an excellent target for immunotherapy.9,10,11 Moreover, the combination of cytostatic drugs such as gemcitabine with anti-CD30mAB can really enhance the antitumor activity of the immunotherapy.11
Brentuximab, an anti-CD30 antibody conjugated with the antimitotic agent monomethyl auristatin E (MMAE), has been successfully tested in heavily pre-treated CD30 HL patients in a large Phase II study, reporting 75% overall response rate (ORR) and 34% complete response (CR) rates, with a median remission duration of 20 months for complete responders. It also demonstrated a good safety profile, with low Grade ≥3 adverse events (20% neutropenia, 8% thrombocytopenia, 6% anaemia, and no treatment-related deaths).12 Recently, an update of 2 phase II studies reported the efficacy of brentuximab combined either with bendamustine13 (83% of RC) or followed by augmented ifosfamide carboplatin and etoposide (39% of PET negative before transplantation and a 2 year progression-free survival of 63%) for patients with relapsed or refractory HL.14
Based on this background, a sequential combination of GVD and brentuximab have been used in some French centres as a bridge to transplant after either a bleomycin or platinum based regimen.
This retrospective study sought to determine if a sequential combination of GVD and brentuximab would be an effective and safe regimen for relapsed or refractory HL patients to conduce them to transplantation.
From January 2013 to December 2014, we conducted a retrospective analysis of 11 patients recruited from the haematological departments of the following French centres: Centre Hospitalier Lyon sud; Hôpital Saint-Louis, Paris; Hôpital Cochin, Paris; Centre Hospitalier d’Annecy. All patients were analysed in accordance with the ethical standards of the institutional committee on human experimentation. The primary inclusion criterion was adults (aged >18 years) with HL who had relapsed or who had failed to achieve CR after at least 2 lines of treatment. These patients then subsequently received the combined GVD and brentuximab regimen, in accordance with the French marketing authorisation. Patients were evaluated at diagnosis and 3 weeks following the last course of chemotherapy, according to the Ann Arbor classification, consisting of a physical examination, bone marrow biopsy, computed tomography (CT) scan of the neck, chest, abdomen, and pelvis, as well as a positron emission tomography (PET) scan. For patients classified as Stage I or II, we have indicated the EORTC/LYSA prognosis staging, as being namely favourable or poor. Response to the induction treatment was evaluated according to the 2007 Cheson guidelines.
Treatment plan. On Day 1, gemcitabine was administrated at 1000mg/m2 intravenously (iv), vinorelbine at 20mg/m2 iv, and liposomal doxorubicin at 15mg/m2 iv, with brentuximab administered at 1.8mg/kg on Day 8 (with the exception of one centre, the dose was 100 mg for every patient), repeated every 21 days.
The toxicity assessment was conducted based on the brentuximab “Autorisation Temporaire d’Utilisation” (ATU), an early-access program available in France, which enables the use of severe disease-targeting drugs which have not yet been granted marketing authorisation for patients with no other therapeutic options and who cannot be included in a clinical trial.
In our study, 11 patients were treated as described above. The patient characteristics have been listed in Table 1. The median age at diagnosis was 25 years (range: 18–38). Nine (82%) were male, while only 2 (18%) were female. The majority presented with poor prognosis at diagnosis, with 7 exhibiting extensive disease (63.5% Stage III/IV) and 8 (72.5%) B symptoms (fever, night sweats, or weight loss). The median delay between diagnosis of the disease and treatment with brentuximab was 10.7 months (7.1–35.3). After first-line therapy, 5 patients (45.5%) were primary refractory and another 5 (45.5%) had suffered early relapse, whereas only 1 (9%) had had a late relapse. Seven patients (63.5%) were initially treated with BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, and procarbazine, prednisolone) and 4 (36.5%) with ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine). Only 1 received radiotherapy as first-line treatment. For second-line therapy, 5 patients (45.5%) were given a combination of IVOx, five (45.5%) a DHAOx regimen, and only 1 (9%) a BEACOPP combination.
Table 1.
Patients’ characteristics of the entire cohort at initial diagnosis.
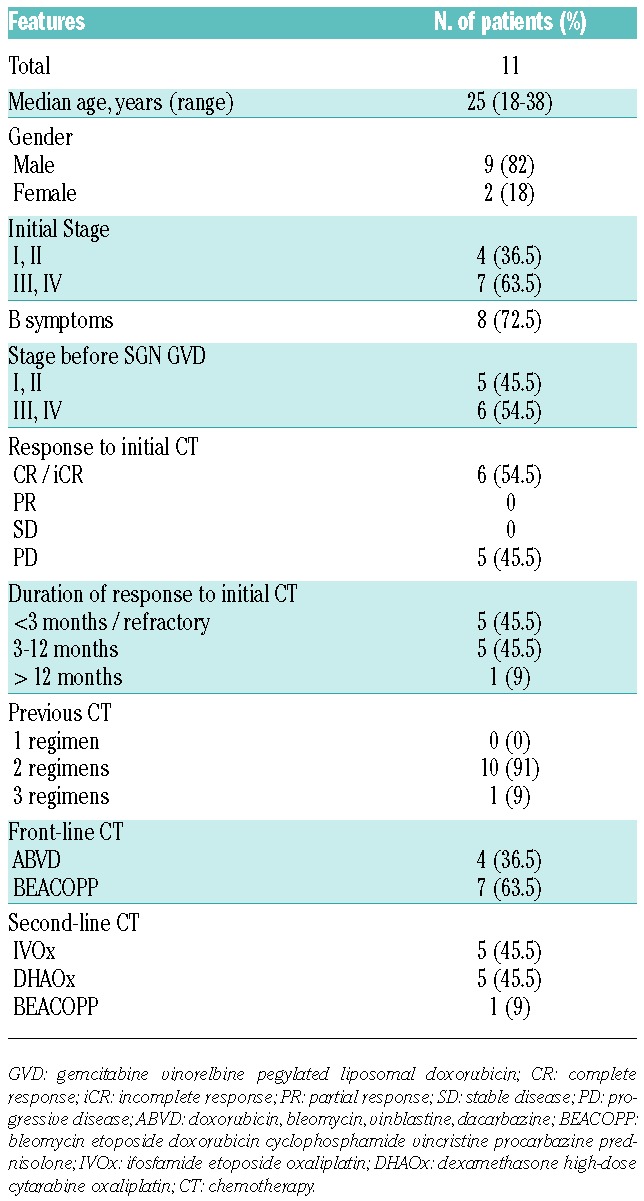
Although our patients were young and had not undergone heavy prior treatments, the greater number having previously received only 2 lines, this cohort exhibited poor prognosis with extensive and primary refractory disease for the majority.
The ORR for all patients was 100%, with eight (72.5%) achieving CR and three PR prior to transplantation, as shown in Table 2A. All patients received an intensification treatment, with either autologous stem cell transplant (SCT) (82%) or allogeneic SCT (18%). Six patients (54.5%) were led to a tandem therapy (five of them (83%) an autologous SCT, and only one an allogeneic SCT).
Table 2A.
Efficacy of the sequential combination of gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD) with brentuximab.
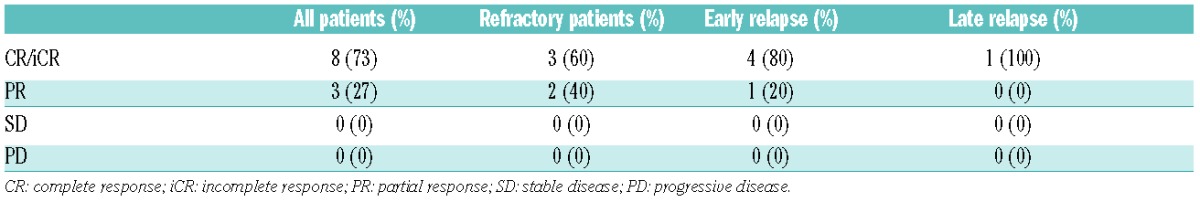
At the last follow-up, eight patients (72.5%) had achieved CR and one (9%) exhibited disease progression. With a median follow-up of 8.4 months (5.7–19.9), median progression-free survival (PFS) was 11.2 months, and overall survival at 12 months was estimated at 60%. Two patients (18%) had died before the time of writing, one from hepatic graft versus host disease, and one from other causes.
A total of 33 courses were administered, with a median of three cycles. Few Grade 3–4 toxicities were reported and these were mostly haematological, but with no related severe infection or death, as shown in Table 2B. Only one patient (9%) presented an anaphylactic shock reaction with the utilization of liposomal doxorubicin, from which he fully recovered. This very promising toxicity profile could be partially explained by the young age of our cohorts and the limited amount of treatment they had previously received, namely two or three lines. These positive results were, likewise, in agreement with the recent reports of very few Grade 3/4 toxicities under GVD, again mostly haematological (63% neutropenia and only 7% febrile neutropenia). One Phase III trial from CALGB using the combination of GVD with SGN30 was prematurely discontinued due to very severe pneumonitis reported in one case. This complication was not reported with brentuximab administered solely,12,15 nor was it observed in our study.
Table 2B.
Toxicities (Grade 3 or 4 adverse events) of the combined strategy.
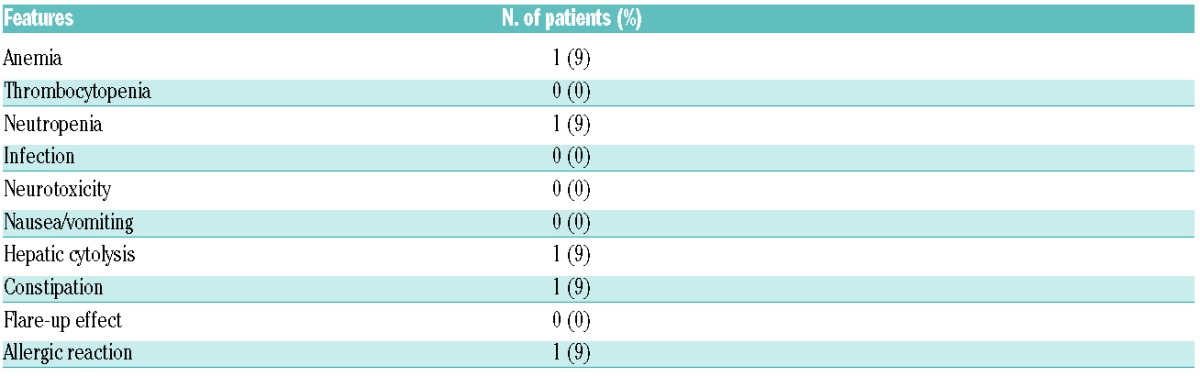
In conclusion, the sequential combination as described herein, has been revealed by our study to be effective and safe as a bridge to transplant, whereas most second-line therapies induced significant toxicities. These results support the recommendation of using this combined strategy for patients with early relapse or refractory disease.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97(3):616–623. [DOI] [PubMed] [Google Scholar]
- 2.Josting A, Rudolph C, Reiser M, et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin’s disease. Ann Oncol. 2002;13(2):1628–1635. [DOI] [PubMed] [Google Scholar]
- 3.Kuruvilla J, Keating A, Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. 2011;117(16):4208–4217. [DOI] [PubMed] [Google Scholar]
- 4.Corazzelli G, Angrilli F, D’Arco A, et al. Efficacy and safety of bendamustine for the treatment of patients with recurring Hodgkin lymphoma. Br J Haematol. 2013;160(2):207–215. [DOI] [PubMed] [Google Scholar]
- 5.Longo DL, Duffey PL, Young RC, et al. Conventional-dose salvage combination chemotherapy in patients relapsing with Hodgkin’s disease after combination chemotherapy: the low probability for cure. J Clin Oncol. 1992;10(8):210–218. [DOI] [PubMed] [Google Scholar]
- 6.Bonfante V, Santoro A, Viviani S, et al. Outcome of patients with Hodgkin’s disease failing after primary MOPP-ABVD. J Clin Oncol. 1997;15(2):528–534. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002; 359(9323):2065–2071. [DOI] [PubMed] [Google Scholar]
- 8.Morschhauser F, Brice P, Fermé C, et al. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin’s lymphomas: results of the prospective multicenter H96 trial by the GELA/SFGM study group. J Clin Oncol. 2008;26(36):5980–5987. [DOI] [PubMed] [Google Scholar]
- 9.Tian ZG, Longo DL, Funakoshi S, et al. In vivo antitumors effects of unconjugated CD30 monoclonal antibodies on human anaplastic large -cell lymphoma xenografts. Cancer Res. 1995;55(22):5325–5341. [PubMed] [Google Scholar]
- 10.Heuck F, Ellermann J, Borchmann P, et al. Combination of the human anti-CD30 antibody 5F11 with cytostatic drugs enhances its antitumor activity against Hodgkin and anaplastic large cell lymphoma cell lines. J Immunother. 2004; 27(5):347–353. [DOI] [PubMed] [Google Scholar]
- 11.Cerveny CG, Law CL, McCormick RS, et al. Signaling via the anti-CD30 mAb SGN-30 sensitizes Hodgkin’s disease cells to conventional chemotherapeutics. Leukemia. 2005;19(9):1648–1655. [DOI] [PubMed] [Google Scholar]
- 12.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. Journal of clinical oncology. J Clin Oncol. 2012;30(18):2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedberg JW, Cohen P, Chen L, et al. Bendamustine in patients with rituximab-refractory indolent and transformed non-hodgkin’s lymphoma: results from a phase II multicenter, single agent study. J Clin Oncol. 2008;26(2):204–210. [DOI] [PubMed] [Google Scholar]
- 14.Moskowitz AJ, Schöder H, Yahalom J, et al. PET-adapted sequential salbage therapy with brentuximab vedotin followed by augmented ifosamide, carbopaltin, and etoposide for patients with relapsed and refractory Hodgkin’s lymphoma: a non randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16(3):284–292. [DOI] [PubMed] [Google Scholar]
- 15.Monjanel H, Deville L, Ram-Wolff C, et al. Brentuximab vedotin in heavily treated Hodgkin and anaplastic large-cell lymphoma, a single centre study on 45 patients. Br J. Haematol. 2014;166(2):306–308. [DOI] [PubMed] [Google Scholar]


