Heat shock protein 90 (HSP90) plays an important role in normal and malignant cells by stabilizing chaperone proteins. It is frequently overexpressed in lymphoma, is known to contribute to the stability of oncoproteins and is thus an attractive target of treatment.1 While first generation HSP90 inhibitors, namely geldanamycin derivatives, have shown some clinical activities in cancer, they apparently carried disadvantages in clinical use, including dependence on NAD(P)H: quinone oxidoreductase activity, difficult formulation with limited aqueous solubility, hepatotoxicity, and some unexpected deaths.2–6
AUY922 is a highly potent non-geldanamycin HSP90 inhibitor with improved bioavailability and aqueous solubility7 compared to the previously evaluated prototypes of HSP90 inhibitors.6 AUY922 has shown nanomolar efficacy against a wide range of human cancer cell models in vitro. Mice xenograft models showed that AUY922 was present in the tumor for >1 week following administration of AUY922, and significant tumor growth inhibition was observed when AUY922 was given on a weekly basis.8 A phase I study of AUY922 in patients with advanced solid tumors evaluated a weekly dosage of AUY922, and the recommended phase II dose was weekly intravenous infusions of 70mg/m2.9 We conducted a phase II trial of AUY922 in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and peripheral T-cell lymphoma (PTCL) to assess the activity and safety of this agent.
This was an open-label, single arm phase II study of AUY922 in 2 cohorts: patients with DLBCL and PTCL. This study was registered at clinicaltrails.gov (NCT01485536) and approved by the institutional review board. All patients enrolled in this study gave written informed consent. The primary objective of the study is to assess the overall response rate (complete [CR] plus partial response [PR]) to AUY922 treatment. The secondary objectives include an evaluation of the safety profile of AUY922. Eligible patients were required to have relapsed or refractory DLBCL or PTCL with radiographically measurable disease, with no limit on the number of prior treatment regimens. Patients were required to have adequate organ function, including a platelet count of 50,000/mm3 and a neutrophil count of 1,500/mm3. Patients were treated with weekly intravenous doses of AUY922 at 70 mg/m2 over 2 hours on days 1, 8, 15 and 22 of 28-day cycles, for up to 12 cycles until disease progression or toxicity.
Toxicity was graded based on Common Terminology Criteria for Adverse Events Version 4. If a patient experienced toxicity of Grade 3 or greater, the dose needed to be interrupted until the toxicity decreased to either Grade 1 or better or to baseline, and the patient was thereafter to resume treatment at the lower level of 55mg/m2 then 40mg/m2. Given the previous report of visual disturbance with AUY922, all patients were required to have a baseline ophthalmology evaluation. As diarrhea was an expected toxicity from this treatment, patients were recommended to take 4mg of loperamide orally following each infusion of AUY922.
Response assessments were planned every 2 cycles as per the revised response criteria for malignant lymphoma 2007. Simon’s two-stage minimax model was used to evaluate the response rate with alpha of 0.05 and a power of 0.8 in each cohort. We considered overall response rate ≥ 20% to be meaningful and ≤ 5% to be of no interest. If response was seen in ≥1 of the first 12 patients in each cohort, then accrual was to continue in order to include 21 patients in each cohort.
Between October 2012 and January 2014, 20 patients (14 in the DLBCL cohort and 6 in the PTCL cohort) were enrolled. The DLBCL cohort included 7 with germinal center phenotype, 5 with non-germinal center phenotype and 2 with inadequate phenotype information, based on the Hans algorithm. The PTCL cohort included 4 with PTCL not otherwise specified, 1 with angioimmunoblastic T-cell lymphoma and 1 with extranodal NK/T-cell lymphoma, nasal type. The median age of enrolled patients was 60 years (range 33–75 years), and the median number of prior treatment regimens was 4 (range 1–10). Fifteen patients (75%) were male and 5 (25%) were female. Although the study surpassed the first futility endpoint for DLBCL, we terminated the study early due to the limited responses and significant toxicities witnessed in the entire cohort of the study.
One patient with DLBCL with germinal center phenotype achieved a complete response after 2 cycles (Figure 1A). This patient discontinued therapy after 4 cycles due to continued toxicity (Grade 3 diarrhea and fatigue) despite dose reduction to 40mg/m2, but the response was durable, lasting longer than 24 months. One patient with angioimmunoblastic T-cell lymphoma achieved a partial response after 2 cycles, which was short lived (Figure 1B). No other patient experienced a response to AUY922. Therefore, overall response rate in the entire cohort was 10%, including CR seen in 7% of DLBCL and PR seen in 17% of PTCL. In fact, 13 patients (65%) received only 1 cycle of AUY922 or less, due to apparent disease progression during that time, prior to the planned evaluation after 2 cycles. The median progression free survival was 1 month.
Figure 1.
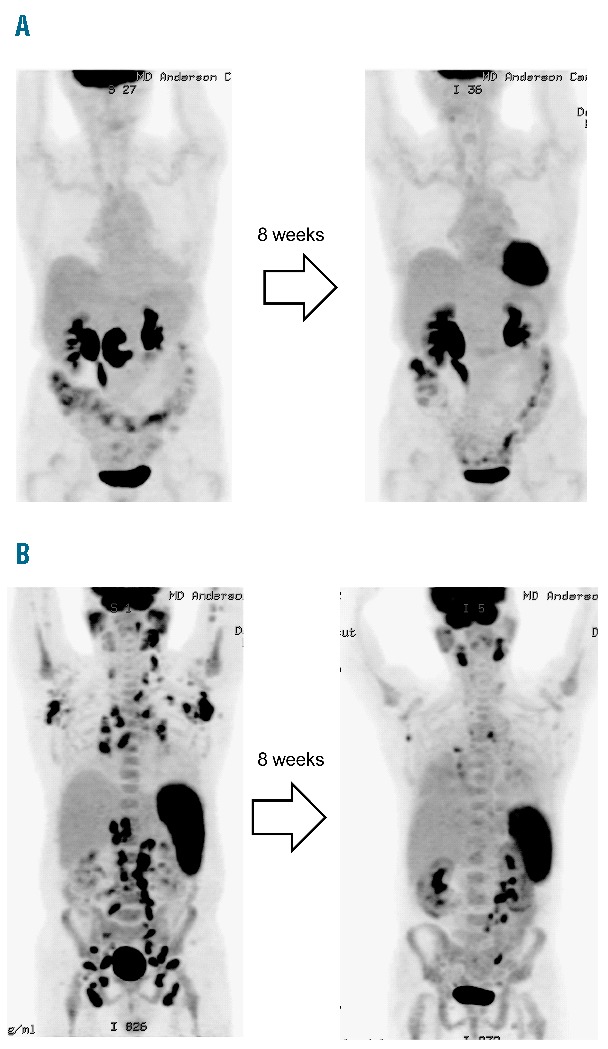
PET scans after 8 weeks of treatment. A. A 74-year-old female with diffuse large B-cell lymphoma with 7 prior treatment regimens. The patient achieved complete response after 2 cycles, and discontinued therapy after 4 cycles due to toxicity. The response is ongoing and the patient has remained in complete remission for more than 24 months B. A 60-year-old male with angioimmunoblastic lymphoma with 10 prior treatment regimens. The patient achieved partial response but the response was short lived < 1 month.
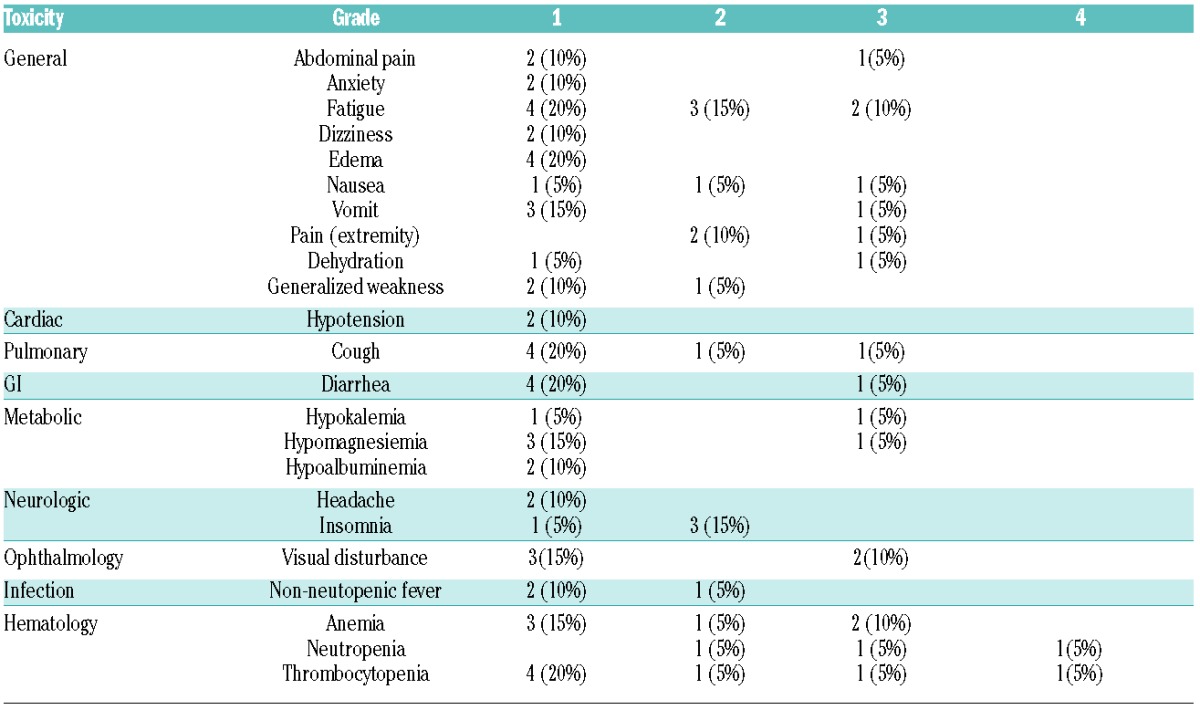
A detailed toxicity profile is summarized in Table 1. Fatigue seems to be a significant toxicity, with Grade 3 fatigue observed in 10%. Other Grade 3/4 toxicities included Grade 3 visual disturbance (10%) and Grade 3 anemia (10%). Grade 3 visual disturbance led to treatment discontinuation in 2 patients. At the time when patients reported visual impairment, an ophthalmology evaluation was performed. Both patients showed impairment in visual acuity, visual field and color vision. However, evaluation with a slit-lamp examination and a dilated fundus examination of the optic nerve and the retina were essentially normal. Before electro-retinogram was scheduled, their vision improved relatively quickly and completely normalized in both patients within 2 months after drug discontinuation. This reversible ocular toxicity is consistent with previous experience with AUY922 in other tumors. Grade 3/4 cytopenia was observed in 10% (for anemia, neutropenia and thrombocytopenia), but the study included a heavily treated population and these patients had intermittent cytopenias prior to study enrollment. Therefore, actual association with cytopenias and AUY922 is unclear.
Table 1.
Summary of toxicity (n=20).
AUY922 is a second-generation, non–geldanamycin isoxazole HSP90 inhibitor, and this is the first phase II report of this drug in hematologic malignancy. Our data demonstrate the relative safety of single agent AUY922 in patients with relapsed lymphoma, though with limited clinical activity, we observed one remarkably durable complete response in relapsed/refractory DLBCL. Correlative analysis to investigate the association between the characteristics of the disease and the response would be of interest, but the limited number of responses observed in the study and the finite amount of tissues available precluded such exploratory analysis.
The toxicity profile seems to be acceptable in most patients, but the management of fatigue and diarrhea can be critical. In addition, although completely reversible after drug discontinuation, visual disturbance was a significant toxicity which affected the daily activity of 2 patients. Visual disturbance is reported to be dose dependent in the phase I study,9 and is a common toxicity associated with other HSP inhibitors including both geldanamycin and non-geldanamycin derivatives. Mice models suggested that such toxicity is considered attributable to the drug-induced photoreceptor degeneration.10
In conclusion, single AUY922 has limited activity against lymphoma. A rare but durable response, however, suggests that the HSP90 inhibitor is a valid target of therapy in some cases. Several in vitro studies have demonstrated synergistic antitumor activity of the HSP90 inhibitor against hematologic malignancies when combined with cytarabine,11 fludarabine,12 melphalan,13 doxorubicin13 or histone deacetylase inhibitors.13 A possible explanation for such synergistic effect with histone deacetylase inhibitors is that hyperacetylation of HSP90 by histone deacetylase inhibitors increase the effect of HSP90 inhibitors to induce apoptosis.14 Synergistic effects with chemotherapy seem to depend on the sequence of administration, as HSP90 inhibitors can induce cell cycle arrest, resulting in a subsequent decrease in sensitivity to chemotherapy whose cytotoxicity depends on cell cycle.15 Mechanisms of combination therapies with chemotherapy or other molecular target agents may be investigated.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerji U, O’Donnell A, Scurr M, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygel-danamycin in patients with advanced malignancies. J Clin Oncol. 2005;23(18):4152–4161. [DOI] [PubMed] [Google Scholar]
- 3.Goetz MP, Toft D, Reid J, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol. 2005;23(6):1078–1087. [DOI] [PubMed] [Google Scholar]
- 4.Grem JL, Morrison G, Guo XD, et al. Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J Clin Oncol. 2005;23(9):1885–1893. [DOI] [PubMed] [Google Scholar]
- 5.Kelland LR, Sharp SY, Rogers PM, Myers TG, Workman P. DT-Diaphorase expression and tumor cell sensitivity to 17-allylamino, 17-demethoxygeldanamycin, an inhibitor of heat shock protein 90. J Natl Cancer Inst. 1999;91(22):1940–1949. [DOI] [PubMed] [Google Scholar]
- 6.Oki Y, Copeland A, Romaguera J, et al. Clinical experience with the heat shock protein-90 inhibitor, tanespimycin, in patients with relapsed lymphoma. Leuk Lymphoma. 2012;53(5):990–992. [DOI] [PubMed] [Google Scholar]
- 7.Eccles SA, Massey A, Raynaud FI, et al. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;15;68(8):2850–2860. [DOI] [PubMed] [Google Scholar]
- 8.Jensen MR, Schoepfer J, Radimerski T, et al. NVP-AUY922: a small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models. Breast Cancer Res. 2008;10(2):R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sessa C, Shapiro GI, Bhalla KN, et al. First-in-human phase I dose-escalation study of the HSP90 inhibitor AUY922 in patients with advanced solid tumors. Clin Cancer Res. 2013;19(13):3671–3680. [DOI] [PubMed] [Google Scholar]
- 10.Zhou D, Liu Y, Ye J, et al. A critical role for the tissue distribution profile in heat shock protein (Hsp) 90 inhibitor-induced ocular toxicity in rats. Mol Cancer Ther. 2011;10(11 Suppl):Abstr C212. [Google Scholar]
- 11.Walsby EJ, Lazenby M, Pepper CJ, Knapper S, Burnett AK. The HSP90 inhibitor NVP-AUY922-AG inhibits the PI3K and IKK signalling pathways and synergizes with cytarabine in acute myeloid leukaemia cells. Br J Haematol. 2013;161(1):57–67. [DOI] [PubMed] [Google Scholar]
- 12.Best OG, Mulligan SP. Heat shock protein-90 inhibitor, NVP-AUY922, is effective in combination with fludarabine against chronic lymphocytic leukemia cells cultured on CD40L-stromal layer and inhibits their activated/proliferative phenotype. Leuk Lymphoma. 2012;53(11):2314–2320. [DOI] [PubMed] [Google Scholar]
- 13.Kaiser M, Lamottke B, Mieth M, et al. Synergistic action of the novel HSP90 inhibitor NVP-AUY922 with histone deacetylase inhibitors, melphalan, or doxorubicin in multiple myeloma. Eur J Haematol. 2010;84(4):337–344. [DOI] [PubMed] [Google Scholar]
- 14.Rao R, Fiskus W, Yang Y, et al. HDAC6 inhibition enhances 17-AAG--mediated abrogation of hsp90 chaperone function in human leukemia cells. Blood. 2008;112(5):1886–1893. [DOI] [PubMed] [Google Scholar]
- 15.Munster PN, Basso A, Solit D, Norton L, Rosen N. Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule-dependent manner. See: E. A. Sausville, Combining cytotoxics and 17-ally-lamino, 17-demethoxygeldanamycin: sequence and tumor biology matters, Clin. Cancer Res., 7: 2155–2158, 2001 Clin Cancer Res. 2001;7(8):2228–2236. [PubMed] [Google Scholar]


