Influenza infection can cause severe complications in patients with multiple myeloma. There are no systematic studies on the efficacy of influenza vaccines in patients with MM. In a retrospective, single-center study we assessed the immune response of MM patients to one and two doses of a trivalent influenza vaccine.
The secondary aim of the study was to correlate the immune responses with disease-specific parameters and anti-neoplastic treatment. While a single dose of influenza vaccine resulted in seroprotection in 14.6% of MM patients, the frequency of protective titers was more than doubled (31.3%) after a second dose of the vaccine.
There is increasing evidence that the immune response to influenza vaccine in MM patients is mostly insufficient.1–3 Protection against influenza is mediated by virus-specific antibodies and depends on the humoral immune response,4,5 which is impaired in patients with MM. Preliminary data support the assumption that the immune response can be improved by a second dose of vaccine.6,7
During the season 2012/13, MM patients were offered the seasonal influenza vaccination [(Optaflu®, Novartis, Basel, Switzerland; a trivalent cell-culture-based influenza vaccine, containing inactivated viral surface antigens of influenza A(H1N1)pdm09, influenza A(H3N2), and influenza B/Yamagata-lineage; hereafter referred to as A(H1N1), A(H3N2) and B/Yamagata)] in our myeloma outpatient clinic. Blood samples were taken before and 4 weeks after each vaccination. Patients were offered a second dose of vaccine if they responded insufficiently to the first vaccination but had not had any serious side effects. Antibody titers were analyzed using the hemagglutination inhibitory (HI) assay. HI titers <1:10, 1:10 to <1:40 and ≥1:40 were considered as negative, indicating immune memory and protection, respectively. Seroconversion was defined as having occurred in seronegative patients if the titer achieved after vaccination was ≥1:40, and in seropositive patients if the ratio achieved was ≥4-fold.
Apart from this intervention, diphtheria and tetanus (DT) immunization titers were determined as recall parameters using enzyme-linked immunoassays. DT titers between 0.1 and 1.0 IU/mL were considered as protective, titers >1 IU/mL as indicating long-term protection.
Forty-eight patients with smoldering myeloma and MM stage I or higher who had been vaccinated in our myeloma outpatient clinic during the season 2012/13 were included in this study, which was approved by the Institutional Review Board.
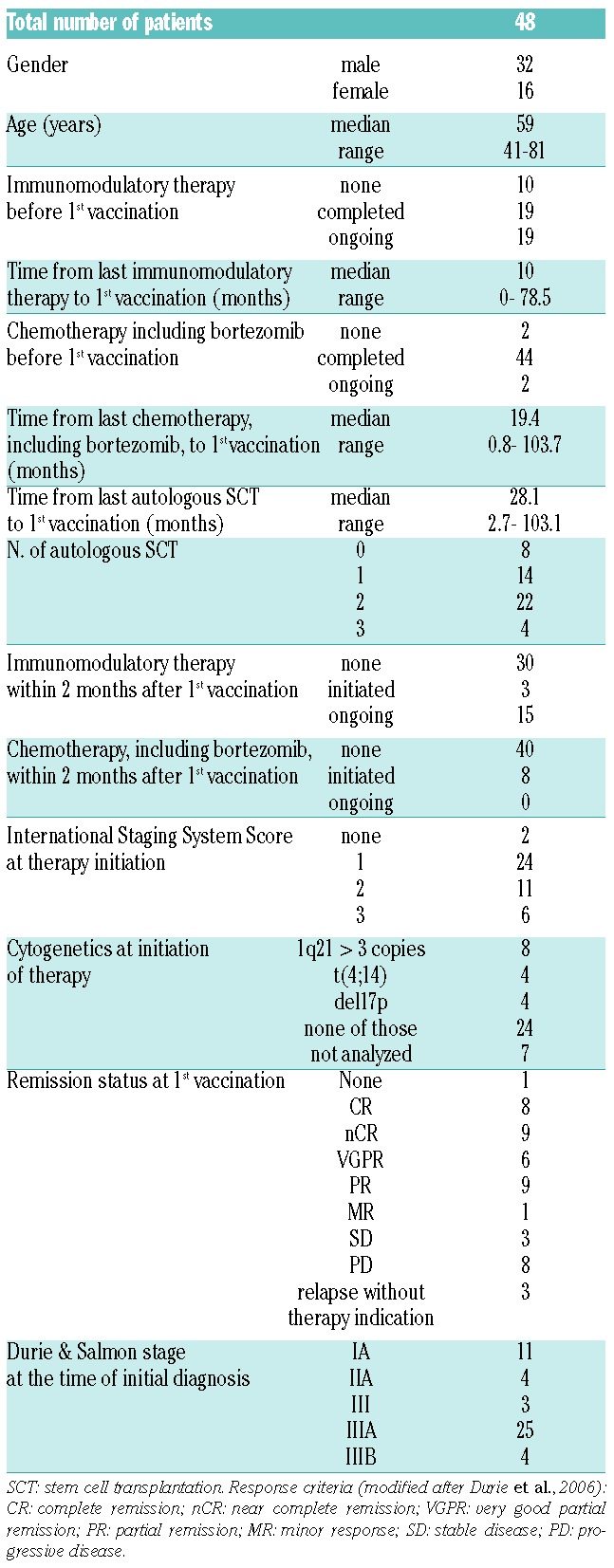
The patients’ characteristics are summarized in Table 1. Most of the patients had previously received intensive therapies; only two patients had not been given any chemotherapy before the first vaccination. Seventeen patients were in complete or near complete remission at the time of the first vaccination.
Table 1.
Patients’ characteristics.
Differences of HI titers between two time points (before/after first vaccination, after first/after second vaccination and before first/after second vaccination) were analyzed using the signed-rank Wilcoxon test. Seroconversion and seroprotection rates were analyzed comparing the times before, after first and after second vaccination by pairwise McNemar test. The association between medical history and response to vaccine was examined by means of a Fisher exact test for nominal variables and the Wilcoxon test for continuous variables.
The absolute increment in titer due to the first vaccina tion was statistically significant for all three strains (Figure 1A).
Figure 1.
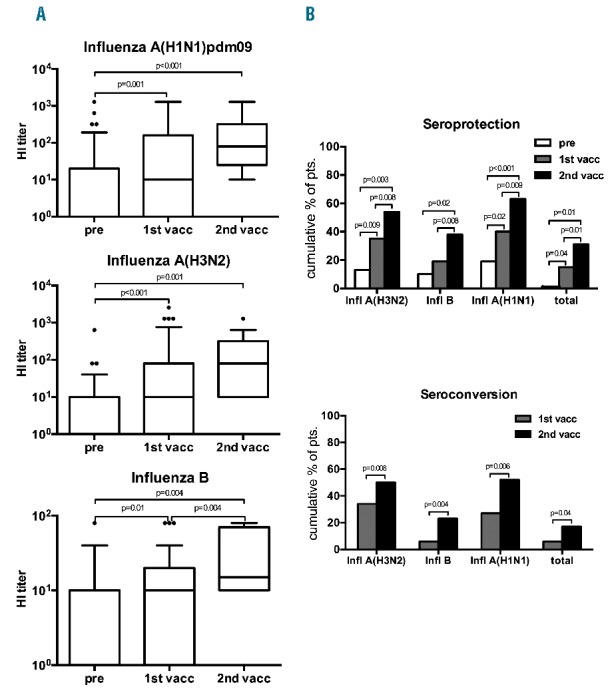
(A) Antibody titers against different influenza virus strains after sequential vaccination in myeloma patients. Box plots depict the increase of antibody titers against the different influenza strains. Differences of HI titers between two time points were analyzed using the signed-rank Wilcoxon test. Statistically significant P-values are shown in the plots. (B) Immune response to double vaccination against seasonal influenza. Bar charts depict seroprotection (upper panel) and seroconversion (lower panel) rates before, after the first and second vaccinations. Differences were analyzed by pairwise McNemar test. P-values are shown.
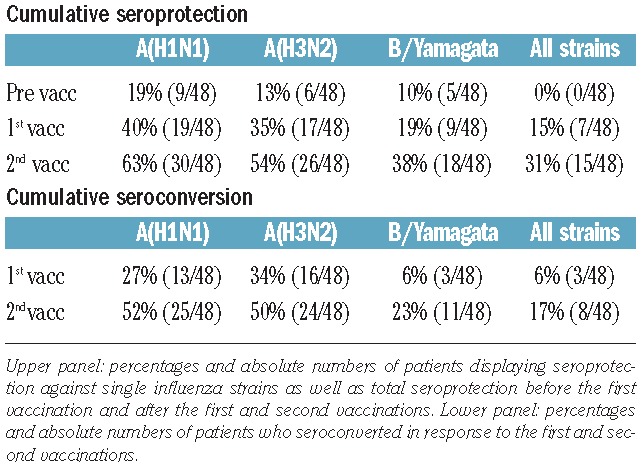
The seroprotection rates against A(H1N1), A(H3N2), B/Yamagata and against all three viruses (total influenza seroprotection) before/after the first vaccination were 19% (9/48)/40% (19/48), 13% (6/48)/35% (17/48), 10% (5/48)/19% (9/48) and 0% (0/48)/15% (7/48), respectively.
The improvement of seroprotection provided by the first vaccination was statistically significant for A(H1N1), A(H3N2) and for all strains taken together (Figure 1B).
The corresponding seroconversion rates for A(H1N1), A(H3N2), B/Yamagata and for all three antigens were 27% (13/48), 34% (16/48), 6% (3/48), and 6% (3/48), respectively (Figure 1B). Except for shivering after the first vaccination, reported by one patient, no adverse events were observed in any patient.
Out of the remaining 40 patients who did not display seroprotection after the first vaccination, one dropped out for loss of follow-up, two underwent autologous stem cell transplantation (SCT), five rejected a second vaccination and eight did not receive a second dose for various logistic reasons. The remaining 24 patients were given a second, booster dose of vaccine. Subsequently, the absolute HI titers increased significantly for all three antigens, compared to pre-vaccination titers (Figure 1A).
The cumulative seroprotection rates for A(H1N1), A(H3N2), B/Yamagata and for all three antigens after the second vaccination were 63% (30/48), 54% (26/48) 38% (18/48), and 31% (15/48), respectively. Compared to the frequency of seroprotection after the first vaccination, there were significant improvements in seroprotection against all strains and also against each single strain after the second vaccination (Figure 1B).
Cumulative seroconversion rates after the second vaccination for A(H1N1), A(H3N2), B/Yamagata and for all three antigens were 52% (25/48), 50% (24/48), 23% (11/48) and 17% (8/48), respectively. Seroconversion improved significantly after the second vaccination (Figure 1B).
The seroprotection and seroconversion rates are summarized in Table 2.
Table 2.
Response to vaccination. Cumulative seroprotection
The patients’ characteristics (Table 1) were analyzed for their impact on immunization results. Most of the tested variables, including chemotherapy, immunomodulatory therapy and number of autologous stem cell transplants, did not show a significant association with seroprotection or seroconversion rates after the first and second vaccinations. Neither was the outcome after the first vaccination different according to chemotherapy or immunomodulatory therapy within the last 6 months or according to autologous SCT status. Differentiation according to the latter mentioned variables was not feasible after the second vaccination because of small sample sizes.
The period of time from last autologous SCT to first vaccine was significantly longer in patients mounting A(H3N2) seroprotection (median 18.9 versus 56.4 months; P=0.006) as well as seroconversion of A(H3N2) titers (19.0 versus 56.2 months; P=0.01) and A(H1N1) titers (20.9 versus 37.6 months; P=0.04) after the first vaccination.
Only two of the 24 patients receiving a second vaccination had not undergone autologous SCT. Of those two, one developed total seroprotection after the second vaccination. All 24 patients had received chemotherapy before the first vaccination, and 20 had received or were being treated with immunomodulatory therapy. Of four patients who had not been treated with preceding immunomodulatory therapy, two developed total protection after the second vaccination.
Regarding DT titers, seroprotection against diphtheria (P<0.001) and long-term protection against tetanus (P=0.007) were found at significantly higher frequencies in patients who also had total seroprotection against influenza after the second vaccination. Accordingly, anti-diphtheria titers (P=0.0007) and anti-tetanus titers (P=0.02) were significantly higher in patients with seroprotection against all strains after the second vaccination. Remarkably, after the first vaccination, no significant correlation between seroprotection and recall antigen titers was detected.
Similar results were found for seroconversion: in patients who had seroconverted for all three influenza antigens after the second vaccination (P=0.007), seroprotection against diphtheria was significantly more frequent, and anti-diphtheria titers were significantly higher in those patients (P=0.02).
This pilot study suggests that a second vaccine dose may boost the immune response against influenza in MM patients. The frequency of total seroprotection was increased from 15% after the first vaccination to 31% after the second, booster dose. Likewise, the rate of overall seroconversion increased from 6% to 17%. The highest success rates were achieved for A(H1N1), with seroprotection rates of 40% and 63% and seroconversion rates of 27% and 52% after the first and second vaccinations, respectively.
Improved immunization results against A(H1N1) as a result of double vaccination have also been reported for other immunocompromised hosts, e.g. patients positive for human immunodeficiency virus (seroconversion rates of 68.2% and 91.9% after the first and second doses, respectively)8 and hemodialysis patients (seroprotection rates of 64.1% and 88.6% after the first and second doses, respectively).7
Double vaccination against A(H1N1) in patients after allogeneic or autologous SCT9 improved the seroprotection rate from 17.9% at baseline to 44.2% after the first vaccination and to 48.8% after the second. Gueller et al. showed seroconversion rates of A(H1N1) titers in SCT patients of 41.2% and 81.8% after the first and second vaccinations, respectively.6
In our study, the interval from last SCT to first vaccine was found to be associated with protective HI titers and with seroconversion. This is in line with data from Engelhard et al., who found a correlation between the time from SCT to immunization and seroconversion in a population of 48 SCT recipients (of whom only 13 were autologous graft recipients).10
In our analysis, no association with chemotherapy or immunomodulatory treatment (thalidomide, lenalidomide, interferon) was found. This was unexpected, as previous studies suggested an adverse impact of chemotherapy,3,11 but a beneficial effect of immunomodulatory therapy12 on vaccine effectiveness. Our finding may be due to the overall low number of subjects in the study and the heterogeneity of the population.
Patients in our study who responded to prior DT vaccination were more likely to respond adequately to the influenza vaccination. Seroprotection against diphtheria and long-term protection against tetanus were found significantly more frequently in patients who also had seroprotection against all three influenza antigens after the second vaccination.
Girndt et al. reported a strong relation of vaccination responses to hepatitis B and tetanus toxoid in dialysis patients.13 The response to tetanus and diphtheria vaccines might serve as a convenient and informative marker for assessing an impaired immune response to other vaccinations.
Double vaccination against influenza in MM patients seems to enhance protection and should be systematically studied. A larger and stratified cohort of patients would be needed for systematic assessment of associations between immunization results and clinical parameters. Furthermore, clinical effectiveness should also be studied, particularly with regards to the impact on influenza incidence, morbidity and mortality.
Acknowledgments
We would like to thank Cathrin Hollenbach, Ines Fischer and Beate Kopp for their excellent secretarial and administrative support of this study.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9(8):493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant. 2008;42(10):637–641. [DOI] [PubMed] [Google Scholar]
- 3.Robertson JD, Nagesh K, Jowitt SN, et al. Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br J Cancer. 2000;82(7):1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–549. [DOI] [PubMed] [Google Scholar]
- 5.Ada GL, Jones PD. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. [DOI] [PubMed] [Google Scholar]
- 6.Gueller S, Allwinn R, Mousset S, et al. Enhanced immune response after a second dose of an AS03-adjuvanted H1N1 influenza A vaccine in patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(10):1546–1550. [DOI] [PubMed] [Google Scholar]
- 7.Dikow R, Eckerle I, Ksoll-Rudek D, et al. Immunogenicity and efficacy in hemodialysis patients of an AS03(A)-adjuvanted vaccine for 2009 pandemic influenza A(H1N1): a nonrandomized trial. Am J Kidney Dis. 2011;57(5):716–723. [DOI] [PubMed] [Google Scholar]
- 8.Bickel M, von Hentig N, Wieters I, et al. Immune response after two doses of the novel split virion, adjuvanted pandemic H1N1 influenza A vaccine in HIV-1-infected patients. Clin Infect Dis. 2011;52(1):122–127. [DOI] [PubMed] [Google Scholar]
- 9.Engelhard D, Zakay-Rones Z, Shapira MY, et al. The humoral immune response of hematopoietic stem cell transplantation recipients to AS03-adjuvanted A/California/7/2009 (H1N1)v-like virus vaccine during the 2009 pandemic. Vaccine. 2011;29(9):1777–1782. [DOI] [PubMed] [Google Scholar]
- 10.Engelhard D, Nagler A, Hardan I, et al. Antibody response to a two-dose regimen of influenza vaccine in allogeneic T cell-depleted and autologous BMT recipients. Bone Marrow Transplant. 1993;11(1):1–5. [PubMed] [Google Scholar]
- 11.Ortbals DW, Liebhaber H, Presant CA, Van Amburg AL, 3rd, Lee JY. Influenza immunization of adult patients with malignant diseases. Ann Intern Med. 1977;87(5):552–557. [DOI] [PubMed] [Google Scholar]
- 12.Noonan K, Rudraraju L, Ferguson A, et al. Lenalidomide-induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res. 2012;18(5):1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girndt M, Pietsch M, Kohler H. Tetanus immunization and its association to hepatitis B vaccination in patients with chronic renal failure. Am J Kidney Dis. 1995;26(3):454–460. [DOI] [PubMed] [Google Scholar]


