Abstract
Study Design
An in vivo dosing study of vitamin D in a rat posterolateral spinal fusion model with autogenous bone grafting. Rats randomized to four levels of Vitamin D adjusted rat chow, longitudinal serum validation, surgeons/observers blinded to dietary conditions, and rats followed prospectively for fusion endpoint.
Objective
To assess the impact of dietary and serum levels of Vitamin D on fusion success, consolidation of fusion mass, and biomechanical stiffness after posterolateral spinal fusion procedure.
Summary of Background Data
Metabolic risk factors, including vitamin D insufficiency, are often overlooked by spine surgeons. Currently there are no published data on the causal effect of insufficient or deficient vitamin D levels on the success of establishing solid bony union after a spinal fusion procedure.
Methods
50 rats were randomized to four experimentally controlled rat chow diets: normal control, vitamin D-deficient, vitamin-D insufficient, and a non-toxic high dose of vitamin D, four weeks prior to surgery and maintained post-surgery until sacrifice. Serum levels of 25(OH)D were determined at surgery and sacrifice using radioimmunoassay. Posterolateral fusion surgery with tail autograft was performed. Rats were sacrificed 12 weeks post-operatively and fusion was evaluated via manual palpation, high resolution radiographs, μCT, and biomechanical testing.
Results
Serum 25(OH)D and calcium levels were significantly correlated with vitamin-D adjusted chow (p<0.001). There was a dose dependent relationship between vitamin D adjusted chow and manual palpation fusion with greatest differences found in measures of radiographic density between high and deficient vitamin D (p<0.05). Adequate levels of vitamin D (high and normal control) yielded stiffer fusion than inadequate levels (insufficient and deficient) (p<0.05).
Conclusions
Manual palpation fusion rates increased with supplementation of dietary vitamin D. Biomechanical stiffness, bone volume and density were also positively-related to vitamin D, and calcium.
Keywords: Vitamin D, lumbar spine, fusion, rat model, vitamin D deficiency, vitamin D insufficiency, microCT, biomechanics, 4-point bending, infection, calcium, Vitamin D3, posterolateral fusion, bone density, stiffness
INTRODUCTION
The formation of a solid spinal fusion is a complex process dependent upon many patient, metabolic, behavioral, biomechanical, and surgical variables.1-3 Surprisingly, there has been little to no experimental or clinical research on the effect of patients’ vitamin D status on the success of establishing a solid bony union after a spinal fusion procedure despite the large amount of scientific studies demonstrating its importance for overall skeletal health.4-15 This disregard for vitamin D is reflected clinically among physicians who often overlook blood levels of vitamin D prior to treatment. A 2009 study by Diapola et al. revealed only 12% of spine surgeons check metabolic tests, including serum levels of vitamin D, prior to fusion surgery and only 20% as part of a pseudoarthrosis workup.16
By underestimating the importance of vitamin D, surgeons may be neglecting a safe and cost effective means for improving fusion outcomes especially given the high prevalence of vitamin D insufficiency, particularly among orthopaedic patients. A retrospective review by Bogunovic et al. found 43% of orthopaedic patients had insufficient serum levels of vitamin D, of which 40% were classified as deficient.17 Stoker et al. followed this study with a retrospective review specifically looking at adults undergoing spinal fusion procedures and reported rates of vitamin D inadequacy and deficiency at 57% and 27%, respectively.18 Lastly, and perhaps most alarming, was a 2011 study by Parry et al. which revealed 90% of pediatric patients admitted for long bone or spinal fusion procedures were either vitamin D-deficient or insufficient.19
Only a few case studies highlighting the potential benefit of vitamin D are available to persuade the need for preoperative vitamin D screening among spine fusion patients.20-22 Most of the research on the importance of vitamin D during bone formation comes from studies on fracture healing.8,23,24 A 2009 study by Fu et al. found the administration of 1,25(OH)2D3 after a femoral osteotomy in ovarectomized rats increased the rate of healing, volume of callus, new bone volume, and trabecular number and density.24 In addition, they reported one fold higher load to failure during biomechanical testing and better bone remodeling with vitamin D supplementation. Similarly, a randomized placebo-controlled study of proximal humerus fracture healing demonstrated a significant increase in bone mineral density among patients who supplemented with vitamin D and calcium when compared to controls.8 While these studies provide important information on the role of vitamin D during healing, bone formation in the context of spinal fusion is a process with unique challenges and considerations.25
Therefore, the purpose of this study was to determine whether vitamin D can quantifiably improve the formation of a solid bony union after spinal fusion by performing an in vivo dosing study of vitamin D in a rat posterolateral fusion model with autogenous grafting. We hypothesized that a dose-dependent relationship exists between dietary vitamin D3 and fusion success as determined by the rate of fusion, stiffness, and density of the fusion mass formed.
MATERIAL AND METHODS
Subjects and Dietary Manipulation of Vitamin D
Approval for this study was obtained from the Institutional Animal Care and Use Committee (IACUC#003802) at Cedars-Sinai Medical Center, Los Angeles, CA. Fifty male Sprague-Dawley rats (220-250g) were randomized to four experimentally-manipulated vitamin D3(cholecalciferol) diets: 0 IU/g vitamin D3 rat chow (Deficient D, ‘DD’, Modified TestDiet 5A4Y, Newco Distributors, Inc.), 2.25 IU/g vitamin D3 rat chow (Insufficient D, ‘ID’, TestDietBV325), 5 IU/g vitamin D3 rat chow (Control D, ‘CD’, Diet 5001), and 40 IU/g vitamin D3 rat chow (Hyper-vitamin D, ‘HD’, TestDiet BV331), Table 1. Dietary environments were modified 4 weeks prior to surgery with maintenance from post-surgery through sacrifice. Rats were housed in a vivarium with no ultraviolet light exposure and allowed to drink and feed ad libitum.
TABLE 1.
Diet composition (g) by Vitamin-D Dietary Groups
| Rat Chow Diet | 0 IU/g Vitamin D deficient D3, ‘DD’* | 2.25 IU/gVitamin D insufficient D3, ‘ID’* | 5 IU/g Vitamin-D sufficient D3, control diet, ‘CD’ | 40 IU/g Vitamin D hyper-vitamin D3, ‘HD’* |
|---|---|---|---|---|
| Percentage of fat (ether extract) | 4.5 | 4.5 | 5.0 | 4.5 |
| Percentage of fat (acid hydrolysis) | 5.5 | 5.2 | 5.7 | 5.2 |
| Kcal/g | 3.35 | 3.34 | 3.36 | 3.34 |
| Percentage of carbohydrates | 49.7 | 49.5 | 48.7 | 49.7 |
| Protein (%) | 23.9 | 23.9 | 23.9 | 23.8 |
| Vitamin A (IU/g) | 22 | 22 | 15 | 22 |
| Vitamins B-12 (mcg/kg) | 24 | 24 | 50 | 24 |
| Vitamins E (IU/kg) | 46 | 42 | 42 | 42 |
| Calcium (% weight) | 0.95 | 0.95 | 0.95 | 0.94 |
| Potassium (% weight) | 1.08 | 1.17 | 1.18 | 1.17 |
| Phosphorus (% weight) | 0.63 | 0.67 | 0.66 | 0.67 |
Diets were customized by LabDiet; 5 IU/g is 5001 Laboratory Rodent Diet from LabDiet. (www.LabDiet.com)
Blood Collection
Blood was collected under inhaled anesthesia immediately prior to spine surgery via tail cut to expose vein prior to tail amputation. A second collection by heart puncture was performed immediately after sacrifice. 200 mL samples were collected in heparin coated tubes, (Microvette 200ul Li Hep, Sardstedt, Newton NC) centrifuged for serum separation, and freeze-stored (−80°C). Serum levels of hydroxyvitamin D [25(OH)D, sVD] and calcium (Free, CaF; Bound, CaB; Total, CaT) were determined via radioimmunoassay (AniLyticsInc, Gaithersburg, MD).
Surgical Procedure
Rats were anesthetized (5% Isoflurane) and maintained under continuous anesthesia (2%Isoflurane). Posterolateral inter-laminar fusion with tail vertebral bone grafting was performed at L4/L5 by the same surgeon blinded to the experimental dietary condition of the rat. The lumbar spine and tail were sterilized and prepped for surgery. The tail was amputated via caudectomy and incision was closed by suture. Seven tail vertebrae were extracted and soft tissues removed, six were morselized using a Rongeur and one was ground using a dental bone mill, Figure 1A, B. The morselized and milled bone were mixed and inserted into a 1cc sterile syringe using a plunger to compact the bone.
Figure 1.

Instruments used to create tailbone graft: a) dental bone mill, b) enlargement of mill, and c) surgical image of placement of bone graft in fusion bed and across the laminar space (TP= transverse process, SP=spinous process).
While the bone graft was prepared, a surgical incision was made above the spinous processes of L4 with further dissection by blunt exposure followed by thorough cleaning of the transverse processes (TPs), facets, and laminar surfaces, and removal of the L4 spinous process. A high-speed drill was used to decorticate the TPs, facets and lamina just until punctate bleeding was observed. Prepared autograft (0.3cc per side, 0.6cc total) was implanted and tightly packed into the posterolateral gutter spanning the TPs and across the posterior laminar space, Figure 1C. The wound was sutured closed in two layers. Buprenorphine and lactated ringers were administered subcutaneously. No antibiotics were given.
Rats were individually caged after surgery. Health status was evaluated daily throughout the follow-up period. Rats were allowed to drink and feed ad libitum on specified vitamin D diets and sacrificed at 12 weeks post-operatively via CO2. Lumbar spine segments were harvested en bloc for fusion evaluation.
Fusion Assessment—Manual Palpation
Gross explanted L4-L5 segments were subjected to manual palpation performed by two researchers blinded to diet assignments. Manual palpation included bending in sagittal and coronal planes. No motion at the L4-L5 segment was determined as fusion success and any motion at the L4-L5 segment was deemed fusion failure.
Fusion Assessment—High Resolution Radiographs and μCT
Radiographic fusion was determined via high resolution radiographic images (LX-60, Faxitron X-Ray, LLC Lincolnshire, IL), taken at monthly intervals postoperatively under inhalation anesthesia. Radiographs were systematically reviewed by two researchers blinded to dietary conditions and characterized for radiographic fusion defined as density showing any continuous bridging of the TPs bilaterally ‘fused’ vs. ‘not fused’, fusion grade (Appendix A), percent remodeling, and presence of radiolucencies.
Ex vivo micro-computed tomography (μCT, vivaCT 40, Scanco USA Inc.) was performed on explanted spines to determine the structural properties of the fusion mass. The L4-L5 region of interest was contoured and segmented from soft tissue using a binary thresholding procedure. Direct three-dimensional morphometry was used to determine: (a) volume of mineralized bone tissue (BV, mm3), (b) bone volume fraction (BV/TV), (c) bone surface to volume ratio (BS/BV, mm−1), and (d) bone mineral density (BMD, HA/cm3) based on calibration with a commercially available μCT phantom containing hydroxyapatite (HA).
Fusion Assessment--Biomechanical Testing
Fused specimens were meticulously cleaned of nonstructural soft tissue and potted to isolate each L4-L5 motion segment. Potted specimens were mounted on to a servo-hydraulic actuator (MTS Bionix 370.02, MTS Corp., Eden Prairie, MN) equipped with a mini load cell (MINI45, API Corp., Apex, NC) and four-point bending apparatus (MTS 642.001A-02, MTS Corp.). The actuator was lowered at a rate of 3 mm/min imposing a flexural pure-moment to the L4-L5 motion segment. Vertical load vs. deflection curves were produced for each specimen and converted to moment-deflection curves where the slope of the first linear region was taken as a measure of stiffness.
Statistical Analysis
Fishers Exact test and Logistic Regression were applied to frequency data of manual palpation fusion (fused =1, not fused =0) to test for differences among dietary groups and to model the serum Vitamin-D dose-response relationship. Analysis of Variance (ANOVA) with a dietary group factor was applied to stiffness and continuous measures of density to test for overall group effect. Tukey test was applied for intergroup and pairwise comparisons. Interrelationships among serum Vitamin D, calcium, and rat weight were evaluated with Person Correlation. ANOVA (GLM, SAS) or Logistic Regression (SAS, LOGISTIC) was applied for multiple variable analyses to evaluate the influence of covariates.
RESULTS
Overall mortality rate was 4% (2/50); both deaths occurred in the deficient-D group, Table 2. Non-terminal infections were classified as either Type I or Type II based on severity. Minor type I infections were only observed in the CD and HD groups, while more severe type II infections occurred in the ID and DD groups (Table 2, Not Significant, NS). Average weight gain did not vary significantly among the four vitamin D-adjusted dietary groups (NS). During the month of pre-surgical dietary conditioning, there was an average weight gain of 159.98 g overall. Similarly, there was an average weight gain of 126.59 g from surgery to sacrifice, Table 3.
TABLE 2.
Fusion outcomes
| Rat Chow Diet | 0 IU/g Vitamin D deficient D3, ‘DD’ Ca = 0.95% | 2.25 IU/gVitamin D insufficient D3, ‘ID’ Ca = 0.95% | 5 IU/g Vitamin-D sufficient D3, normal control diet ‘CD’ Ca = 0.95% | 40 IU/g Vitamin D hyper-vitamin D3, ‘HD’, Ca= 0.94% | total | P values |
|---|---|---|---|---|---|---|
| Manual Palpation (inclusive, # fused/n) | 45.5% (5/11) | 58.3% (7/12 ) | 61.5% (8/13) | 83.3% (10/12) | ns | |
| Manual Palpation (# fused/n without infection/wound) | 33.3% (3/9) | 50.0% (5/10 ) | 63.6% (7/11) | 81.8% (9/11) | p< 0.05* | |
| Rate of Infection (no terminus intervention, resolution without antibiotic) | 18% (2/11) | 16.7% (2/12) | 15.4%(2/13) | 8% (1/12) | ns | |
| Type I (minor local abnormality observed only on gross dissection) | 0 | 0 | 2 | 1 | ||
| Type 2 (seroma, superficial wound developed) | 2 | 2 | 0 | 0 | ||
| Death < 1 weekpost surgical, suspected due to perioperative sepsis (blood or other infection, no use of antibiotics postoperatively in any rats) | 15.4% (2/13) | -- | --- | -- | 4% (2/50) | ns |
Mantel-Haenszel Chi-Square (1) = 3.7663, p= 0.0523
TABLE 3.
Description of Rat Characteristics and serum parameters by Vitamin-D Dietary Groups
| Rat Chow Diet | 0 IU/g Vitamin D deficient D3, ‘DD’ Ca = 0.95% | 2.25 IU/gVitamin D insufficient D3, ‘ID’ Ca = 0.95% | 5 IU/g Vitamin-D sufficient D3, control diet ‘CD’ Ca = 0.95% | 40 IU/g Vitamin D hyper-vitamin D3, ‘HD’, Ca = 0.94% |
|---|---|---|---|---|
| Weights (I_wt g, at intake) | 271.00 ± 4.80 | 272.50 ± 4.17 | 270.00 ± 7.04 | 270.50 ± 7.69 |
| Weights (Srg_wt g, at surgery) | 421.64 ± 83.27 | 446.42 ± 34.54 | 424.15 ± 70.61 | 431.42 ± 70.99 |
| Weights (Sac_wt g, at sacrifice) | 589.71 ± 19.18 | 576.11 ± 44.30 | 578.56± 34.09 | 601.00 ±10.90 |
| Serum Vitamin D (sVD, at surgery)* | 9.25 ± 1.61 | 20.64 ± 3.27 | 28.30± 4.57 | 97.37± 13.59 |
| Serum Vitamin D (sVD at sacrifice)* | 6.25 ± 1.02 | 15.58 ± 5.04 | 19.59 ± 5.64 | 88.44 ± 9.90 |
| Serum Calcium-Total (CaT,, at sacrifice) | 11.69 ± 1.20 | 11.78 ± 0.49 | 11.48 ± 0.89 | 12.86 ± 0.76 |
| Serum Calcium-Free (CaF, at sacrifice) | 6.59 ± 0.85 | 6.83 ± 0.81 | 6.84 ± 1.07 | 7.11 ± 1.09 |
| Serum Calcium-Bound (CaB, at sacrifice) | 5.10 ± 1.74 | 4.95 ± 0.72 | 4.65 ± 1.29 | 5.75 ± 1.15 |
p<0.0001 overall
Intracorrelations among dietary and serum status variables:
Vitamin D adjusted diet and serum Total Ca: dVD3 vs. sCaT, r=0.52, p<0.0002;
Serum Vitamin D and serum Total Ca: sVD vs. sCaT, r=0.51, p<0.0002;
Marginal correlation between Serum Vitamin D and serum Bound Ca: sVD vs. sCaB, r=0.25, p=0.08;
Correlation between serum Total Ca and serum Bound Ca: sCaT vs. sCaB, r=0.68, p<0.0001;
Correlation between sCaF and sCaB (r= −0.64, p<0.0001)
Serum levels of Vitamin D and Calcium
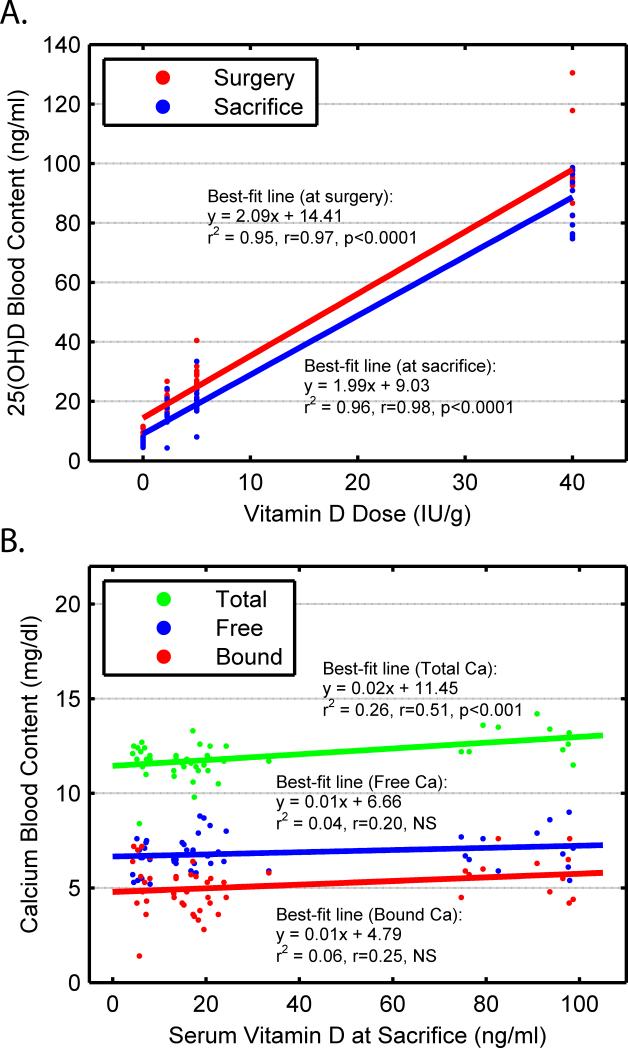
Circulating levels of serum 25(OH)D (sVD) were highly correlated between surgical and sacrificial time points with a shared variability of 93% (r= 96, p<0.0001). Thus, after one month of dietary conditioning, sVD was relatively stable over time and was significantly associated with the level of vitamin D3 adjusted in the rat chow (p<0.0001), Figure 2a.
Figure 2.
(a) Serum level of circulating vitamin D via radioimmunoassay at surgery (p<0.0001) and sacrifice (p<0.0001) as a function of dietary levels of vitamin D. (b) Calcium determined by radioimmunoassay as Free (NS), Bound (NS), and Total Calcium (p<0.01) levels as a function of serum vitamin D.
Dietary calcium was held constant across the VD3 adjusted diets, although significant inter-correlations were seen between serum levels of calcium and VD3 diets [VD3 vs. CaT, (r=0.52, p<0.0002) and VD vs. CaB (r=0.28, p=0.057)]. Likewise, a significant positive relationship was found between sVD and CaT (r=0.51, p<0.001) and a marginal positive relationship was found between sVD and bound calcium (sVD vs. sCaB, r=0.25, p=0.08), Figure 2b. There was no relationship observed between VD3 and CaF. Serum CaT was positively related to CaB (r=0.68, p<0.0001) and CaF was negatively related to sCaB (r=-0.64, p<0.0001), as expected.
Manual Palpation Fusion Rates
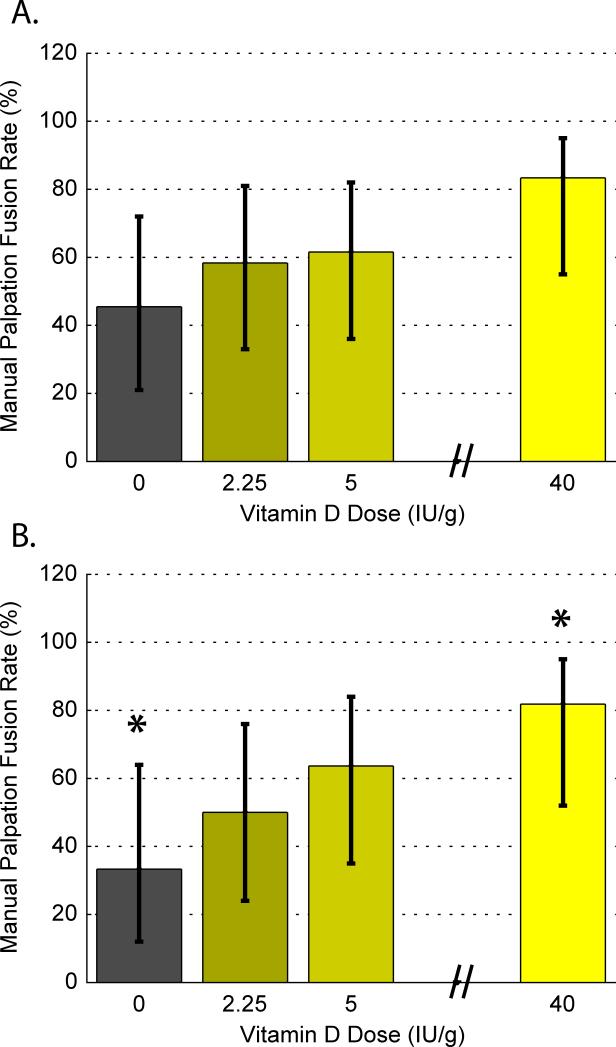
Manual palpation fusion rates were 83.3% for HD fed rats, 61.5% fused for CD, 58.3% fused for ID, and 45.5 % fused for DD and were marginally dose-dependent on the VD3 adjusted in the chow (p≤0.07) and were marginally positively related to serum Vitamin D levels taken at sacrifice (p≤0.07), Figure 3a. When data from infected rats were removed, manual palpation fusion rates were significantly related to serum Vitamin D levels with HD vs. DD accounting for the greatest difference (p<0.05), Figure 3b.
Figure 3.
(a) Percent fused as determined by manual palpation with (+/−) confidence intervals for each of the 4 vitamin-D adjusted diet groups: No vitamin D (ND, 0 IU/g), Insufficient D (ID, 2.25 IU/g), Control D (CD, 5 IU/g), and High D (HD, 40 IU/g), and (b) excluding data from infected rats (*p<0.05).
Biomechanical Testing
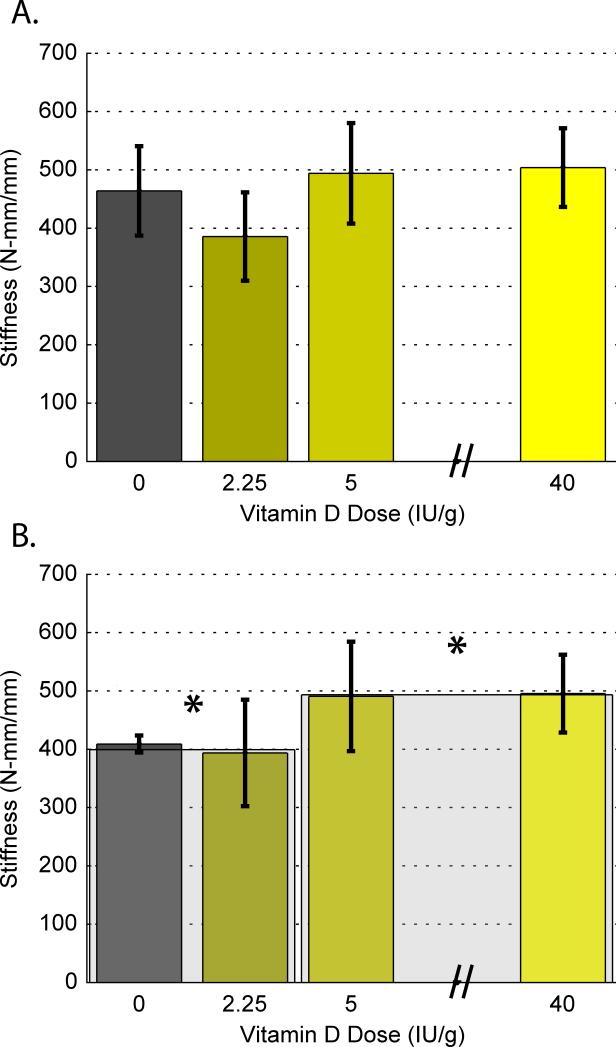
Four-point bending of fused specimens indicated an overall difference among mean stiffness values (p<0.05) across dietary groups, Table 4 and Figure 4A. Excluding data from infected rats in a separate analysis, the CD and HD groups combined were significantly stiffer than the ID and DD groups combined (p<0.05), Figure 4B.
TABLE 4.
Quantitative Measures of Bone Quality by Vitamin-D Dietary Groups
| Rat Chow Diet | 0 IU/g Vitamin D deficient D, ‘DD’ | 2.25 IU/g Vitamin D insufficient D, ‘ID’ | 5 IU/g Vitamin-D sufficient D, ‘CD’ | 40 IU/g Vitamin-D hyper-vitamin D, ‘HD’ | p-value |
|---|---|---|---|---|---|
| Biomechanical Stiffness (Nmm/mm) , average ± sd | 463.99±76.67 (n=5) | 385.67 ± 75.78 (n=7) | 494.04±86.17 (n=8) | 503.91 ± 67.44 (n=10) | p<0.01 |
| U.CT Bone Mineral Density (BMD), average ± sd | 409.98±58.40 | 422.36±37.11 | 441.00±40.53 | 483.83±56.50 | p<0.01 |
| U.CT Mineralized Bone Volume (BV) average ± sd | 575.58 ± 81.00 | 488.27 ± 87.27 | 512.97 ± 110.14 | 651.61 ± 162.71 | P<0.01 |
| U.CT Bone Volume Measures (BS/BV), average ± sd | 4.91±0.86 | 4.81±0.51 | 4.44±0.48 | 3.83 ± 0.65 | p<0.01 |
| U.CT Bone Volume Fraction (BV/TV), average ± sd | 0.71±0.09 | 0.74±0.051 | 0.76±0.05 | 0.81 ± 0.05 | p<0.01 |
For pairwise comparison: **p<0.05, Tukey stiffness: HD vs.ID, CD vs.ID; ** p<0.05, Tukey: BMD: HD vs. ID, HD vs. DD; ** p<0.05, Tukey: μ.CT BV: HD vs.CD, HD vs. ID; **p<0.05, Tukey: μCT BS/BV: HD vs. ID, HD vs. DD; ** p<0.05 Tukey: μ.CT BV/TV: HD vs. ID, HD vs. DD.
Figure 4.
(a) Biomechanical testing results showing the average stiffness (Nmm/mm) as determined by four-point bending in flexion for all specimens, and (b) excluding data from infected rats. Adequate (CD and HD, mean = 493.30 +/−76.06) vs. inadequate (DD and ID, mean = 399.31 +/−69.89) dietary vitamin D groups are presented separately in the shadow bars (*p<0.05).
Micro CT Analysis
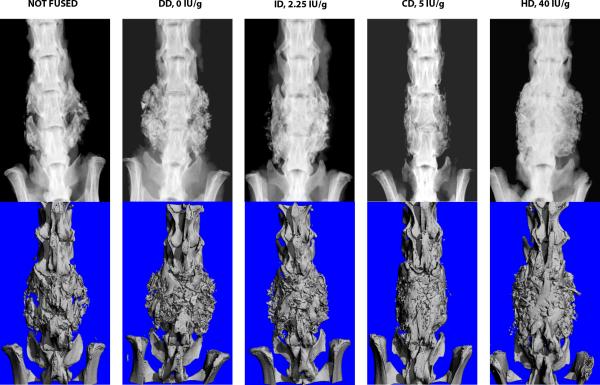
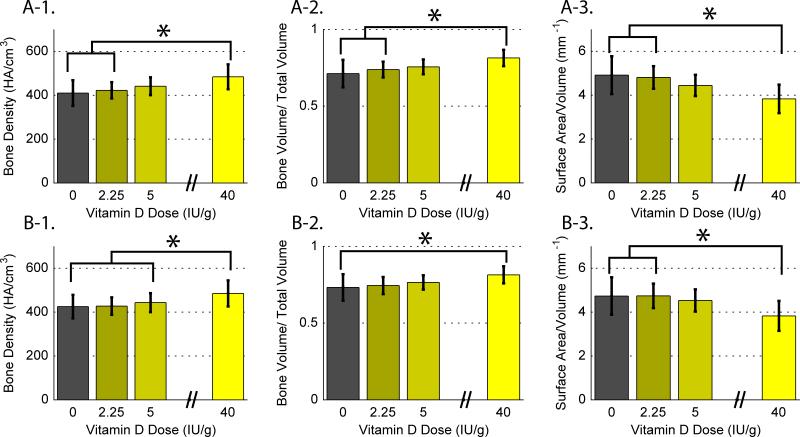
Micro CT images confirmed bone volume in specimens that were fused, Figure 5. The hyper-vitamin D group had significantly higher average Bone Mineral Density (BMD, HA/cm3) and Bone volume fraction (BV/TV) than the ID and DD groups, and significantly lower bone surface to volume ratio (BS/BV, mm−1) than the ID and DD groups, Figure 6A. Similar results were found when data from infected rats were removed, Figure 6B.
Figure 5.
Example high resolution radiographs (top) with μCT (below) of ex vivo specimens from each of the vitamin D-adjusted chow groups. Robust radiographic fusion is observed in the High Vitamin D (40 IU/g) specimen. A radiographic example of ‘not fused’ is included for comparison (left most radiograph).
Figure 6.
MicroCT data for each vitamin D diet group, presented as the average +/−sd. (6-1) bone mineral density (BMD, HA/cm3) (a) for all specimens, and (b) excluding infected rat data, (6-2) bone volume fraction (BV/TV) (a) for all specimens, and (b) excluding infected rat data, and (6-3) bone surface to volume ratio (BS/BV, mm-1) (a) for all specimens, and (b) excluding infected rat data.
Bone mineral density was related to all measures of serum vitamin D, p<0.001. BMD was also related to bound (CaB) and total (CaT) calcium, p<0.01. Similar relationships were observed for BV/TV (p<0.001) and BS/BV (p<0.001). Further, bone volume (BV, mm3) was positively correlated to biomechanical stiffness (r=0.42, p<0.05).
No relationship was observed between BMD and manual palpation fusion or stiffness. Likewise, there was no relationship between BV/TV and BS/BV with either manual palpation fusion or stiffness.
Qualitative Radiographic Fusion
Cohen’ Kappa calculated between the two reviewers blinded to treatment for radiographic fusion (fused vs. not fused), was 0.31(DD) indicating fair agreement, 0.43 (ID) and 0.53 (CD) moderate agreement, to 1 perfect agreement for the HD rats. Radiographic fusion grade (1- 4, see Appendix A), percent remodeling, and disc space density were significantly related to manual palpation fusion results (all p< 0.0001, Table 5). Vitamin D adjusted diets were related to radiographic grade (p<0.01), radiographic percent remodeled bone (p<0.02), and radiographic fusion (0 vs.1, p=0.066).
TABLE 5.
Descriptive radiographic measures of bone quality by Vitamin-D Dietary Groups
| Rat Chow Diet | 0 IU/g Vitamin D deficient D, ‘DD’ | 2.25 IU/g Vitamin D insufficient D, ‘ID’ | 5 IU/g Vitamin-D sufficient D, ‘CD’ | 40 IU/g Vitamin-D hyper-vitamin D, ‘HD’, | p-value |
|---|---|---|---|---|---|
| Radiographic Fusion rate (1 vs. 0), average of 2RB [Radiographic fused of manually fused] | 45.5% (5/11) 100% (5/5) | 75.0% (9/12) 100% (7/7) | 46.% (6/13) 75% (6/8) | 83.3% (10/12) 100% (10/10) | NS |
| Kappa= 43% (CI 0.17 to 0.69, overall 77.1% (37/48) agree | Kappa = 0.31 (95%CI = 0.08-0.7) | Kappa = 0.43 (95%CI = 0.16-1.0) | Kappa = 0.53 (95%CI = 0.07-0.98) | Kappa~, 10/12 agree SAS, not calculated (***note1, 83%,) | |
| *Radiographic Score (4 point scale), average of 2RB | p<0.001 | ||||
| 1. None or limited density, evidence of graft/bone resorption, bilaterally | -- | 8.3% (1/12) | 7.7% (1/13) | -- | |
| 2. Some density (bone formation) not solid, no side has connectivity of the grafting material/bone mass to TPs | 54.5% (6/11) | 16.7% (2/12) | 38.5% (5/13) | -- | |
| 3. Some solid appearing 'fusion' dense mass connecting TPs one side, some contralateral bone formation or smaller mass, less consistent | 18.1% (2/11) | 33.3 % (4/12) | 23.1% (3/13) | 33.3 % (4/12) | |
| 4. Consistent large dense, mass connecting TPs, bilaterally | 27.3% (3/11) | 41.7 % (5/12) | 30.8% (4/13) | 66.7% (8/12) | |
| % Remodeled on Radiographic, average of 2RB** | 53.18±27.8 | 61.66±28.29 | 46.3±36.40 | 77.5±18.7 | p<0.05 |
2RB = averaged over 2 reviewers that were blinded to Vitamin D dietary assignments; Table numbers presented with '1.5' taken as '1' the lower of the nearest category
TPs = transverse processes;
Mantel-Haenszel Chi-Square = 6.5, p<0.01
p<0.05 Tukey: %Remodeled: 40 vs. 5.
Note1: Kappa will be specific for a given population so presented as a function of vitamin D dietary group as probability of manual palpation fusion may differ. Radiographic fusion 2RB agreement in for the 40 IU/g was 10 (fused) of 12 (2 deemed not fused by one reviewer) yielding a low Kappa value, although this was almost perfect agreement. Kappa is maximum when the probability of a true 'fused' is 0.5 and as the probability gets closer to 0 or 1, the expected Kappa gets smaller. Unless agreement is perfect, Kappa will be small or 0 (SAS), no matter how good the agreement is so Kappa of 0 is misleading.
**** Radiographic Score: 66.7% same grade 2RB (32/48), yields 43% Agreement, Overall Kappa=0.43, CI=0.17 to 0.69;
DISCUSSION
This study demonstrates that vitamin D modulates the consolidation of bone after grafting for spinal posterolateral fusion in a rat model. Specifically, our results indicate that increased levels of dietary Vitamin D are directly related to the density of the fusion mass, and marginally related to the rate of manual palpation fusion and stiffness of the fusion formed.
While this is the first study suggesting vitamin D benefits bone formation after spinal fusion surgery, numerous studies examining the effect of vitamin D on bone strength and fracture healing support these findings.24,26-37 A study by Erben et al. demonstrated increased trabecular width and cancellous bone mass with increased vitamin D supplementation in an ovariectomized rat model.26 While their study only used two vitamin D doses [low (0.025 μg/kg) vs. high (0.1 μg/kg)], their results are consistent with ours and suggest supplementation with vitamin D dose-dependently increases bone mass.
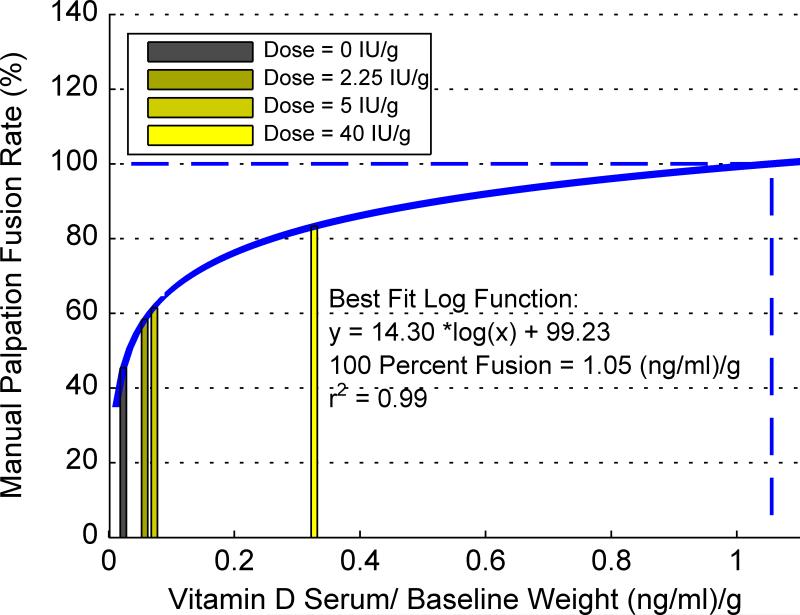
Rate of manual palpation fusion was also dose-dependent on vitamin D and can be modeled as a log function of serum vitamin D adjusted by weight (r2=0.99, Figure 7). Extrapolation to 100% rate of rat fusion equates to a serum level of 284 ng/ml (ED100%), which is considered toxic in humans.38 Thus, caution should be used when projecting animal data to clinical applications. Further clinically-based research is needed to determine optimal blood levels for patients undergoing fusion surgery.
Figure 7.
Manual palpation fusion rate as a function of serum vitamin-D/baseline rat weight (g), presented per diet group, in a log-based curve with extrapolation to estimated minimum-dose needed for 100% manual palpation fusion.
An incidental finding of this study was rates of mortality and infection were lower in rats with adequate vitamin D. Of the 50 rats included, two died within the first week after surgery and both were given deficient-D chow. Deaths were most likely a result of post-surgical infection/sepsis as no rats were treated with antibiotics. Greater severity of infection was observed among inadequate-D (ID, DD) rats, whereas only minor infections were observed among adequate-D (CD, HD) rats, Table 3. Neither our mortality rate nor infection rate were statistically significant but are consistent with research emphasizing the importance of vitamin D for immune function.39 Recent clinical studies investigating pre-surgical serum 25(OH)D found deficient levels are associated with increased rates of peri-operative infection and mortality.40-42 Thus, decreased risk of infection may be another potential benefit of optimal serum levels of vitamin D prior to spinal fusion surgery.
Interestingly, rats with either type of infection formed a robust fusion mass. Research on this phenomenon is limited, although Lutton et al. demonstrated granulation tissues does indeed support neoangiogenesis through the release of growth factors and cytokines, and significantly increased bone formation in their sheep femoral defect model.43 Due to this potential infection-granulation phenomenon, fusion outcome measures were presented with and without data from infected rats. When data from infected rats was excluded from the analysis, two findings were significant: 1) manual palpation fusion rate was a significant function of vitamin D, and 2) fusion stiffness was significantly reduced in rats fed deficient chow compared to those fed sufficient chow.
The present study used a challenging model of bone formation through the use of autogenous tail bone grafting, as opposed to high-dose growth factor-induced fusion, with no use of antibiotics. This allowed for greater sensitivity in observing specific biologic responses to fusion in relation to vitamin D with multiple dependent measures. A balanced cohort design allowed any variation unrelated to diet to be evenly distributed across diet groups, with serum validation of the dietary intervention of vitamin D and covariates of calcium.
Limitations of this study include small sample size, limited number of vitamin D groups particularly at the higher range, and exclusive use of male rats in this fusion challenge model. No gender differences in dietary or target level of serum vitamin D has been described in the literature, and hormonal interactions are unknown. Other limitations include the inability to control for serum levels of calcium and other potential nutritional modulators of bone formation that may have indirectly affected some of our outcome measures.
In summary, this study suggests Vitamin D dose-dependently increases the quality of bone after a spinal fusion procedure. Adequate serum 25(OH)D levels have been associated with many health benefits and may be an important modifiable risk factor prior to fusion surgery. While prospective clinical studies are needed, vitamin D supplementation is a readily available, safe, and cost-effective pre-surgical intervention for patients undergoing spinal fusion that can potentially reduce the rate of pseudarthrosis and post-operative infection.
Supplementary Material
Acknowledgments
The manuscript submitted does not contain information about medical device(s)/drug(s). The Scoliosis Research Society, The National Center for Advancing Translational Sciences (NCATS), Grant UL1TR000124, and the department of Surgery at Cedars-Sinai Medical Center internal research funds were received in support of this work. Relevant financial activities outside the submitted work: grants, consultancy, royalties.
References
- 1.Kim YJ, Bridwell KH, Lenke LG, et al. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine (Phila Pa 1976) 2006;31:2329–36. doi: 10.1097/01.brs.0000238968.82799.d9. [DOI] [PubMed] [Google Scholar]
- 2.Kim YJ, Bridwell KH, Lenke LG, et al. Pseudarthrosis in primary fusions for adult idiopathic scoliosis: incidence, risk factors, and outcome analysis. Spine (Phila Pa 1976) 2005;30:468–74. doi: 10.1097/01.brs.0000153392.74639.ea. [DOI] [PubMed] [Google Scholar]
- 3.Steinmann JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop Relat Res. 1992:80–90. [PubMed] [Google Scholar]
- 4.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:551–61. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 5.Boonen S, Bischoff-Ferrari HA, Cooper C, et al. Addressing the musculoskeletal components of fracture risk with calcium and vitamin D: a review of the evidence. Calcif Tissue Int. 2006;78:257–70. doi: 10.1007/s00223-005-0009-8. [DOI] [PubMed] [Google Scholar]
- 6.Brinker MR, O'Connor DP, Monla YT, et al. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007;21:557–70. doi: 10.1097/BOT.0b013e31814d4dc6. [DOI] [PubMed] [Google Scholar]
- 7.Cranney A, Weiler HA, O'Donnell S, et al. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr. 2008;88:513S–9S. doi: 10.1093/ajcn/88.2.513S. [DOI] [PubMed] [Google Scholar]
- 8.Doetsch AM, Faber J, Lynnerup N, et al. The effect of calcium and vitamin D3 supplementation on the healing of the proximal humerus fracture: a randomized placebo-controlled study. Calcif Tissue Int. 2004;75:183–8. doi: 10.1007/s00223-004-0167-0. [DOI] [PubMed] [Google Scholar]
- 9.Harwood RH, Sahota O, Gaynor K, et al. A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: The Nottingham Neck of Femur (NONOF) Study. Age Ageing. 2004;33:45–51. doi: 10.1093/ageing/afh002. [DOI] [PubMed] [Google Scholar]
- 10.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 11.LeBoff MS, Hawkes WG, Glowacki J, et al. Vitamin D-deficiency and post-fracture changes in lower extremity function and falls in women with hip fractures. Osteoporos Int. 2008;19:1283–90. doi: 10.1007/s00198-008-0582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lips P, Bouillon R, van Schoor NM, et al. Reducing fracture risk with calcium and vitamin D. Clin Endocrinol (Oxf) 2010;73:277–85. doi: 10.1111/j.1365-2265.2009.03701.x. [DOI] [PubMed] [Google Scholar]
- 13.Mezquita-Raya P, Munoz-Torres M, Luna JD, et al. Relation between vitamin D insufficiency, bone density, and bone metabolism in healthy postmenopausal women. J Bone Miner Res. 2001;16:1408–15. doi: 10.1359/jbmr.2001.16.8.1408. [DOI] [PubMed] [Google Scholar]
- 14.Need AG. Bone resorption markers in vitamin D insufficiency. Clin Chim Acta. 2006;368:48–52. doi: 10.1016/j.cca.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Priemel M, von Domarus C, Klatte TO, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305–12. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 16.Dipaola CP, Bible JE, Biswas D, et al. Survey of spine surgeons on attitudes regarding osteoporosis and osteomalacia screening and treatment for fractures, fusion surgery, and pseudoarthrosis. Spine J. 2009;9:537–44. doi: 10.1016/j.spinee.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Bogunovic L, Kim AD, Beamer BS, et al. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92:2300–4. doi: 10.2106/JBJS.I.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoker GE, Buchowski JM, Bridwell KH, et al. Preoperative vitamin D status of adults undergoing surgical spinal fusion. Spine (Phila Pa 1976) 2013;38:507–15. doi: 10.1097/BRS.0b013e3182739ad1. [DOI] [PubMed] [Google Scholar]
- 19.Parry J, Sullivan E, Scott AC. Vitamin d sufficiency screening in preoperative pediatric orthopaedic patients. J Pediatr Orthop. 2011;31:331–3. doi: 10.1097/BPO.0b013e3182104a94. [DOI] [PubMed] [Google Scholar]
- 20.Plehwe WE, Carey RP. Spinal surgery and severe vitamin D deficiency. The Medical journal of Australia. 2002;176:438–9. doi: 10.5694/j.1326-5377.2002.tb04487.x. [DOI] [PubMed] [Google Scholar]
- 21.Schwalfenberg G. Improvement of chronic back pain or failed back surgery with vitamin D repletion: a case series. J Am Board Fam Med. 2009;22:69–74. doi: 10.3122/jabfm.2009.01.080026. [DOI] [PubMed] [Google Scholar]
- 22.Waikakul S. Serum 25-hydroxy-calciferol level and failed back surgery syndrome. Journal of orthopaedic surgery. 2012;20:18–22. doi: 10.1177/230949901202000104. [DOI] [PubMed] [Google Scholar]
- 23.Delgado-Martinez AD, Martinez ME, Carrascal MT, et al. Effect of 25-OH-vitamin D on fracture healing in elderly rats. J Orthop Res. 1998;16:650–3. doi: 10.1002/jor.1100160604. [DOI] [PubMed] [Google Scholar]
- 24.Fu L, Tang T, Miao Y, et al. Effect of 1,25-dihydroxy vitamin D3 on fracture healing and bone remodeling in ovariectomized rat femora. Bone. 2009;44:893–8. doi: 10.1016/j.bone.2009.01.378. [DOI] [PubMed] [Google Scholar]
- 25.Reid JJ, Johnson JS, Wang JC. Challenges to bone formation in spinal fusion. J Biomech. 2011;44:213–20. doi: 10.1016/j.jbiomech.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Erben RG, Bromm S, Stangassinger M. Therapeutic efficacy of 1alpha,25-dihydroxyvitamin D3 and calcium in osteopenic ovariectomized rats: evidence for a direct anabolic effect of 1alpha,25-dihydroxyvitamin D3 on bone. Endocrinology. 1998;139:4319–28. doi: 10.1210/endo.139.10.6249. [DOI] [PubMed] [Google Scholar]
- 27.Andreen O, Larsson SE. Effects of parathyroidectomy and vitamin D on fracture healing. Fracture biomechanics in rats after parathyroidectomy and treatment with 1,25-dihydroxycholecalciferol. Acta Orthop Scand. 1983;54:805–9. doi: 10.3109/17453678308992913. [DOI] [PubMed] [Google Scholar]
- 28.Andreen O, Larsson SE. Effects of 1,25-dihydroxycholecalciferol on fracture healing. Calcium, phosphate, and zinc in callus and serum. Arch Orthop Trauma Surg. 1984;103:257–62. doi: 10.1007/BF00387331. [DOI] [PubMed] [Google Scholar]
- 29.Aerssens J, Van Audekercke R, Talalaj M, et al. Effect of 1 alpha-vitamin D3 on bone strength and composition in growing rats with and without corticosteroid treatment. Calcif Tissue Int. 1994;55:443–50. doi: 10.1007/BF00298558. [DOI] [PubMed] [Google Scholar]
- 30.Aerssens J, van Audekercke R, Talalaj M, et al. Effect of 1alpha-vitamin D3 and estrogen therapy on cortical bone mechanical properties in the ovariectomized rat model. Endocrinology. 1996;137:1358–64. doi: 10.1210/endo.137.4.8625911. [DOI] [PubMed] [Google Scholar]
- 31.Atkin I, Dean DD, Muniz OE, et al. Enhancement of osteoinduction by vitamin D metabolites in rachitic host rats. J Bone Miner Res. 1992;7:863–75. doi: 10.1002/jbmr.5650070803. [DOI] [PubMed] [Google Scholar]
- 32.Erben RG, Scutt AM, Miao D, et al. Short-term treatment of rats with high dose 1,25-dihydroxyvitamin D3 stimulates bone formation and increases the number of osteoblast precursor cells in bone marrow. Endocrinology. 1997;138:4629–35. doi: 10.1210/endo.138.11.5511. [DOI] [PubMed] [Google Scholar]
- 33.Finkelman RD, Linkhart TA, Mohan S, et al. Vitamin D deficiency causes a selective reduction in deposition of transforming growth factor beta in rat bone: possible mechanism for impaired osteoinduction. Proc Natl Acad Sci U S A. 1991;88:3657–60. doi: 10.1073/pnas.88.9.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly J, Lin A, Wang CJ, et al. Vitamin D and bone physiology: demonstration of vitamin D deficiency in an implant osseointegration rat model. J Prosthodont. 2009;18:473–8. doi: 10.1111/j.1532-849X.2009.00446.x. [DOI] [PubMed] [Google Scholar]
- 35.Omeroglu H, Ates Y, Akkus O, et al. Biomechanical analysis of the effects of single high-dose vitamin D3 on fracture healing in a healthy rabbit model. Arch Orthop Trauma Surg. 1997;116:271–4. doi: 10.1007/BF00390051. [DOI] [PubMed] [Google Scholar]
- 36.Omeroglu H, Omeroglu S, Korkusuz F, et al. Effect of 25-OH-vitamin D on fracture healing in elderly rats. J Orthop Res. 1999;17:795. doi: 10.1002/jor.1100160604. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T, Kurokawa T, Orimo H. Increased mechanical strength of the vitamin D-replete rat femur by the treatment with a large dose of 24R,25(OH)2D3. Bone. 1989;10:117–23. doi: 10.1016/8756-3282(89)90009-4. [DOI] [PubMed] [Google Scholar]
- 38.Alshahrani F, Aljohani N. Vitamin D: deficiency, sufficiency and toxicity. Nutrients. 2013;5:3605–16. doi: 10.3390/nu5093605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pludowski P, Holick MF, Pilz S, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmunity reviews. 2013;12:976–89. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Maier GS, Horas K, Seeger JB, et al. Is there an association between periprosthetic joint infection and low vitamin D levels? International orthopaedics. 2014 doi: 10.1007/s00264-014-2338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quraishi SA, Bittner EA, Blum L, et al. Association between preoperative 25-hydroxyvitamin D level and hospital-acquired infections following Roux-en-Y gastric bypass surgery. JAMA surgery. 2014;149:112–8. doi: 10.1001/jamasurg.2013.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowery EM, Bemiss B, Cascino T, et al. Low vitamin D levels are associated with increased rejection and infections after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31:700–7. doi: 10.1016/j.healun.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Lutton C, Sugiyama S, Wullschleger ME, et al. Transplanted abdominal granulation tissue induced bone formation--an in vivo study in sheep. Connective tissue research. 2009;50:256–62. doi: 10.1080/03008200902836057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.