Abstract
Dithiomaleimide-based camptothecin-containing nanoparticles are designed to have exceptionally high drug loading and capable of reduction-responsive, FRET-indicated drug release.
Nanomedicine, with size around 20–200 nm, has emerged as a promising modality for cancer treatment due to its capability of improving the pharmacological and pharmacokinetic profiles of anticancer agents.1–4 Various types of nanostructural delivery vehicles have been developed, including lipid vesicles (mostly liposomes), micelles, nanoparticles (NPs) and drug-polymer conjugates. Among these nanomedicine platforms, lipid vesicles composed of a bilayer lipid structure feature in their superior stability and unique interactions with cell membrane, with multiple drug products being approved by FDA for clinical cancer treatment.5–9 However, one key drawback of conventional lipid vesicles is their low drug loading (usually much less than 10%) due to the limited interior volume for drug encapsulation and formulation challenges.10–13 Much effort has been devoted to improving the drug loading of lipid vesicles by changing the lipid materials and formulation methods. Often, marginally improved drug loading is accompanied with compromised vesicle structural stability.14–17
Apart from excellent stability and high drug loading, an ideal lipid vesicle should also be able to actively control the release of carried drugs. A large variety of lipid vesicles subject to controlled structural decomposition and drug release in response to various intracellular triggers have been reported.18–27 The huge concentration gradient of glutathione (GSH) between the intracellular (~ 10 mM) and extracellular environment (~ 0.002 mM) has presented a unique trigger for the design of reduction-responsive lipid vesicles.28–33 However, the design of the GSH-responsive systems has been largely based on the incorporation of disulfide bond. Despite its GSH-responsiveness, the disulfide bond can also be non-specifically cleaved at elevated temperature or by intense light irradiation, which could potentially lead to severe intermolecular cross-linking with biological materials, such as proteins that have multiple disulfide bonds.34–40 An interesting chemistry was reported recently involving the rapid, clean reaction of 2,3-dibromomaleimide and thiols which gives dithiomaleimide in quantitative yields.41–44 The maleimide thioether bonds in the resulting dithiomaleimide conjugate could be substituted by excess thiols,45,46 suggesting its potential for being used as a GSH-responsive moiety. Compared to disulfide bond, the maleimide thioether bond has much better thermal- and photo-stability. Besides its reactivity towards thiols, dithiomaleimides were also reported to have strong green fluorescence,47,48 a property that can potentially be taken advantage for designing functional drug delivery vehicles.
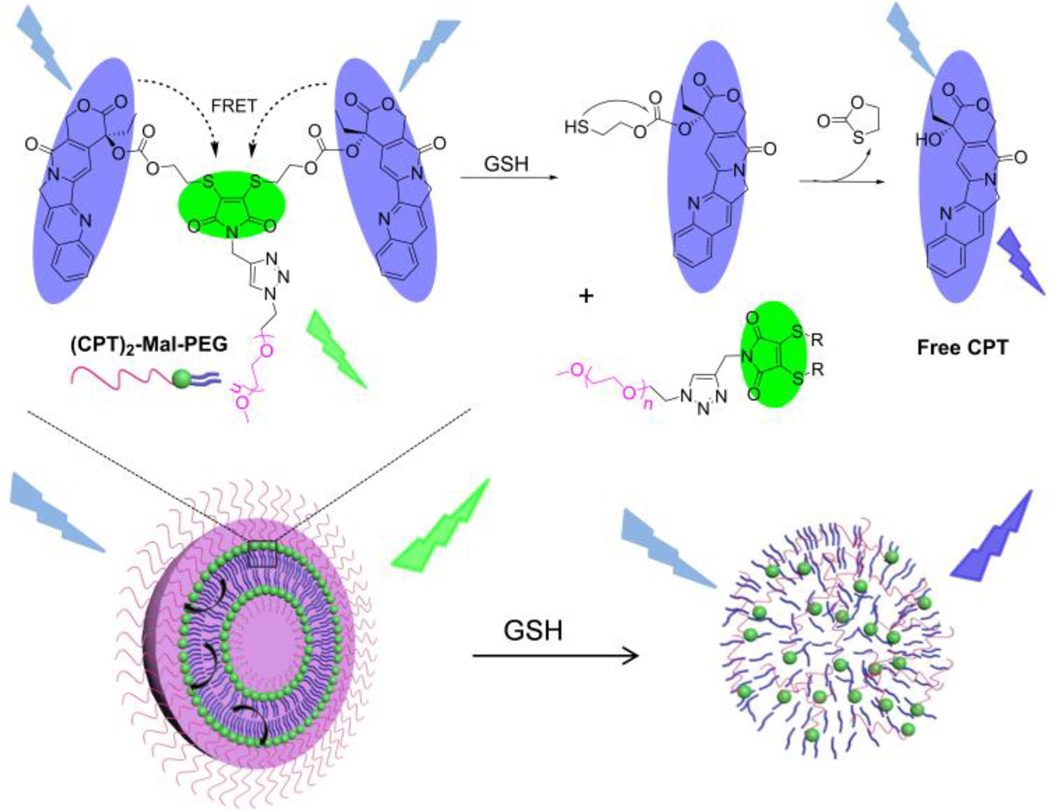
Here, we report a multifunctional dithiomaleimide-based drug delivery nanomedicine with very high drug loading, excellent stability, GSH responsiveness, and drug release self-reporting capability. In our design, camptothecin (CPT)-thiols as the hydrophobic moiety were conjugated to N-propargyl-2,3-dibromomaleimide, followed by conjugation of poly(ethylene glycol) (PEG) via click chemistry to yield an AB2-type amphiphilic structure ((CPT)2-Mal-PEG, Scheme 1 and 2), resembling the building block structure of liposomes with one hydrophilic head group and two hydrophobic hydrocarbon tails. (CPT)2-Mal-PEG NPs with drug loading as high as ~60% can be easily prepared via diffusion method. Interestingly, when excited at 370 nm, (CPT)2-Mal-PEG NPs showed a significant emission peak at 550 nm (dithiomaleimide emission wavelength) instead of at 438 nm (CPT emission wavelength), indicating the existence of Förster resonance energy transfer (FRET) phenomenon between CPT and maleimide thioether bonds. In the presence of GSH, the maleimide thioether bonds could be rapidly cleaved, followed by cyclization reactions to release CPT and disruption of NP structure. A reduction of FRET signal was observed along with the release of free CPT, which provided a non-invasive tool to follow drug release from the (CPT)2-Mal-PEG NPs.
Scheme 1.
Schematic Illustration of GSH-Mediated Drug Release and FRET Inactivation of (CPT)2-Mal-PEG NPs.
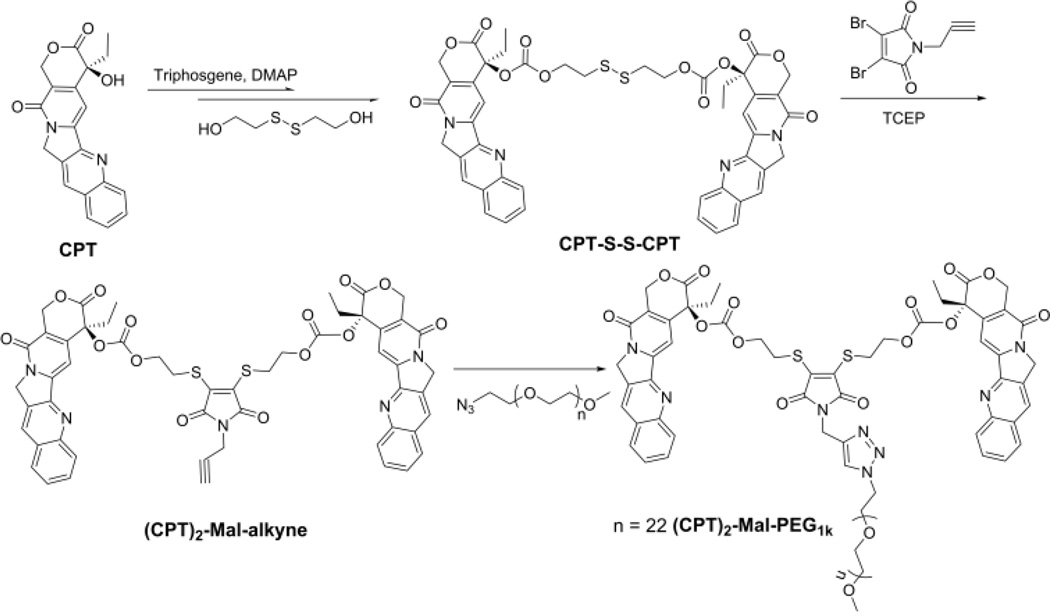
Scheme 2.
Synthesis of Reduction-Responsive Amphiphilic (CPT)2-Mal-PEG.
CPT-S-S-CPT with a disulfide linker was first synthesized through the activation of CPT with triphosgene and further reaction with 2-hydroxylethyl disulfide (Scheme 2). CPT-S-S-CPT was then conjugated to N-propargyl-2,3-dibromomaleimide in the presence of tris(2-carboxyethyl)phosphine (TCEP) to yield (CPT)2-Mal-alkyne. (CPT)2-Mal-PEG was synthesized via Click reaction between (CPT)2-Mal-alkyne and PEG-N3. The overall yield of these three-step reactions was around 35%.
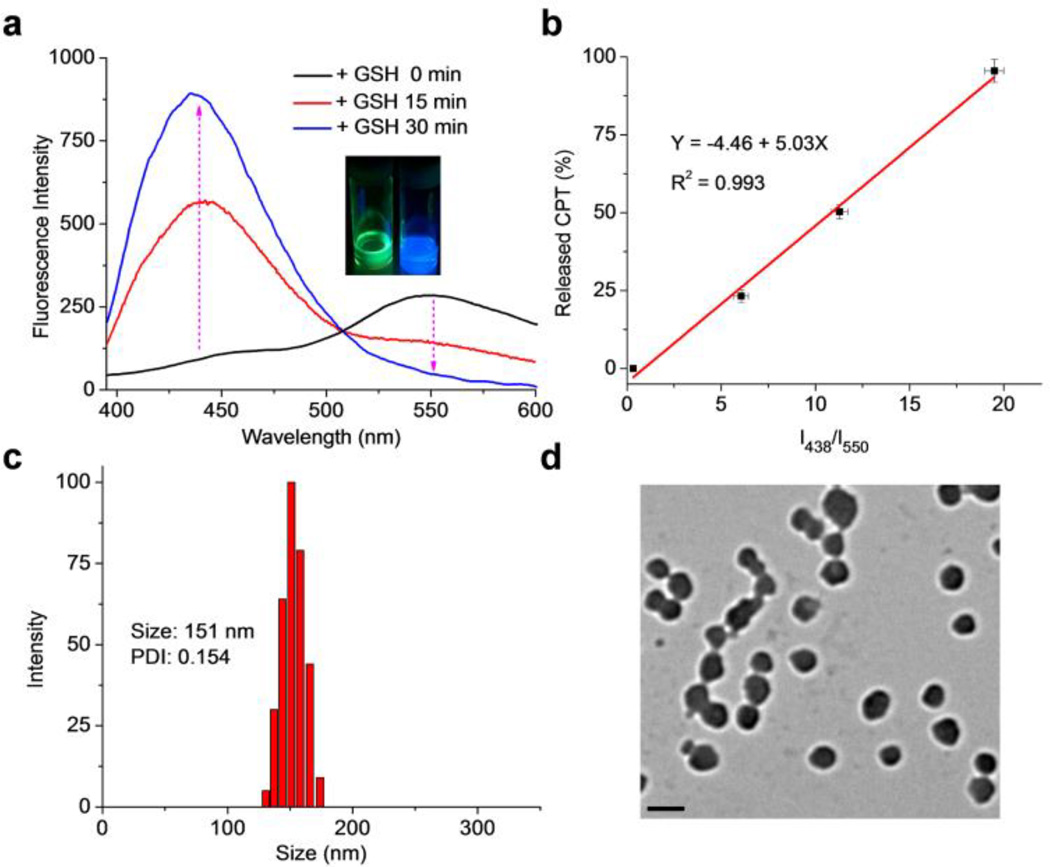
We next selected (CPT)2-Mal-PEG1k to evaluate its GSH-responsiveness and fluorescence property. (CPT)2-Mal-PEG1k in methanol showed strong green fluorescence instead of typical blue fluorescence of CPT under UV irradiation (365 nm) (Inset, Figure 1a). Analysis of (CPT)2-Mal-PEG1k by fluorescence spectrometer also showed a significant emission peak at 550 nm instead of 438 nm (CPT maximum emission wavelength) at an excitation wavelength of 370 nm (Figure 1a, black line), suggesting the existence of FRET phenomenon between CPT and maleimide thioethers. To confirm this, the fluorescence spectra of free CPT and various dithiomaleimides were collected and compared (Figure S6 and Table S1). The maximum excitation and emission wavelengths of CPT are 370 nm and 438 nm, respectively, as compared to the maximum excitation and emission wavelengths of dithiomaleimides at 420–450 nm and 520–560 nm, respectively (Table S1). Given the facts that the emission wavelength of CPT is very close to the maximum excitation wavelength of dithiomaleimides and these two fluorescent moieties are only 5 σ bonds away from each other (<1 nm), it is therefore not surprising to observe the FRET phenomenon (Scheme 1). We then studied whether treatment with an excessive amount of GSH would release CPT from (CPT)2-Mal-PEG1k and how the FRET signal would change over time upon GSH treatment. The emission of (CPT)2-Mal-PEG1k at 438 nm was found to increase significantly, accompanied with the decrease of the emission intensity at 550 nm after treated with 5 mM GSH for 15 min. After 30-min treatment with GSH at the same concentration, the emission intensity at 438 nm further increased while the peak at 550 nm disappeared, indicating the complete degradation of (CPT)2-Mal-PEG1k which was confirmed by HPLC analysis (Figure S7). A correlation between the intensity ratio (I438/I550) and the percentage of released CPT from (CPT)2-Mal-PEG1k showed a quasi-linear relationship (R2 = 0.993, Figure 1b and S8). These experiments demonstrated that (CPT)2-Mal-PEG1k can release free CPT rapidly in the reductive environment and the release of CPT correlates well with the decrease of FRET signal between CPT and maleimide thioethers.
Figure 1.
(a) Fluorescence spectra of (CPT)2-Mal-PEG1k in methanol treated with 5 mM GSH for 0, 15, and 30 min, respectively. Excitation wavelength was set at 370 nm. Inset: pictures of (CPT)2-Mal-PEG1k in methanol under UV irradiation (365 nm) before (left) and after (right) GSH treatment. (b) Correlation of I438/I550 to the percentage of released CPT from (CPT)2-Mal-PEG1k in the presence of 5 mM GSH. DLS (c) and TEM (d) characterizations of (CPT)2-Mal-PEG1k NPs. The scale bar represents 200 nm.
After demonstrating reduction-responsiveness and the FRET property of (CPT)2-Mal-PEG1k, we next prepared (CPT)2-Mal-PEG1k NPs by adding nanopure water to a DMF solution of (CPT)2-Mal-PEG1k (DMF/H2O, 1/40, v/v). NP with an average hydrodynamic size of 151 nm and a polydispersity (PDI) of 0.154 was obtained (Figure 1c). TEM analysis confirmed the formation of nanostructure with an average size of 140 nm (Figure 1d). As the structure of (CPT)2-Mal-PEG1k is similar to that of (OEG)-DiCPT which was reported to form nanocapsule structure by Shen and coworkers49, (CPT)2-Mal-PEG1k NP likely has similar vesicle-like structure.
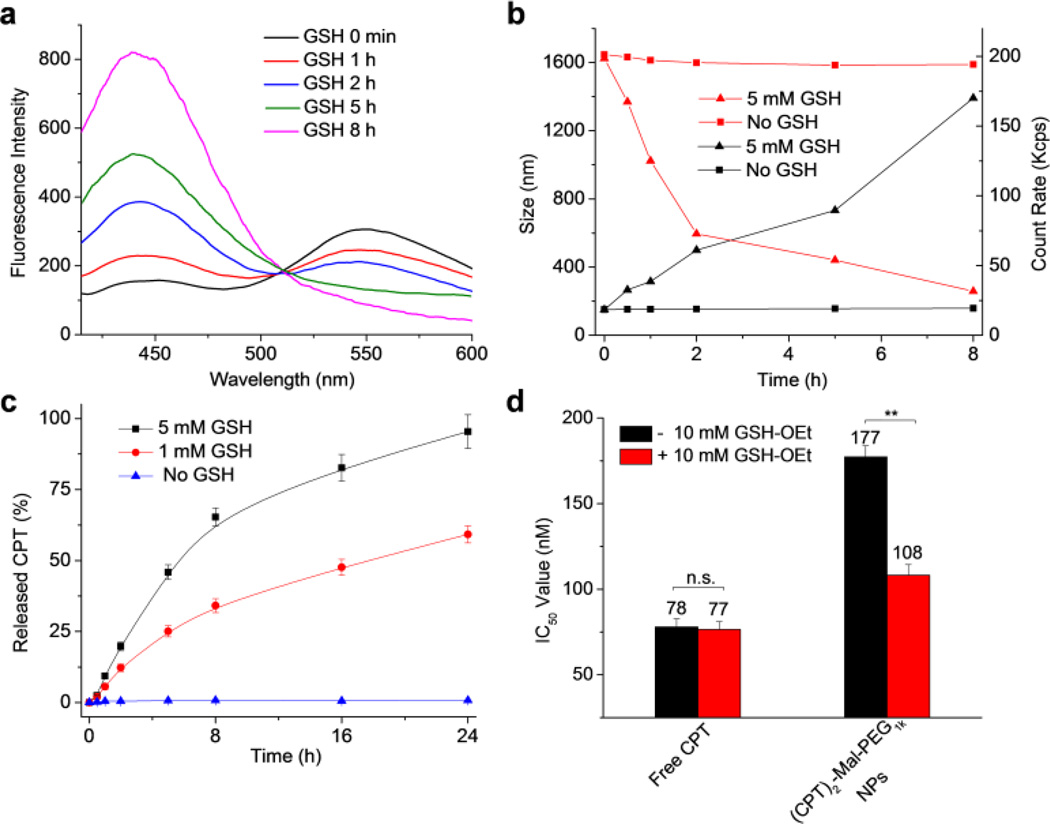
We next studied the GSH-responsive drug release and fluorescence property of the prepared (CPT)2-Mal-PEG1k NPs. As expected, a significant emission peak at 550 nm was observed when the NPs were excited at 370 nm, indicating the existence of FRET signal between CPT and maleimide thioethers in the nanostructure (Figure 2a, black line). Although the analysis here was based on the overall FRET effect, it should be noted that intermolecular FRET may also exist because of the closely packed nanostructures. With the change of the concentration of (CPT)2-Mal-PEG1k NP, the I438/I550 value remained largely unchanged, suggesting that the FRET property of the NP was independent of its concentration (Figure S9). We then studied the degradation of (CPT)2-Mal-PEG1k NPs in the presence of GSH. Compared to GSH-mediated degradation of (CPT)2-Mal-PEG1k, it took much longer time for GSH to completely degrade the maleimide thioether structure of the (CPT)2-Mal-PEG1k NPs, presumably because of the well-packed (CPT)2-Mal-PEG1k nanostructure. After the NPs were incubated with 5 mM GSH for 2 h, the emission peak at 550 nm was largely reduced while the emission at 438 nm increased significantly, which could be explained by GSH-induced cleavage of maleimide thioether bonds, release of CPT, and disruption of the presumed nanocapsule structure. After the NPs were treated with 5 mM GSH for 8 h, I438/I550 reached a plateau, indicating the complete degradation of maleimide thioether structure. To further demonstrate the disruption of nanostructures upon GSH treatment, we monitored the size and the count rate of NPs in PBS over time (Figure 2b). In the absence of GSH, the change of size and count rate was negligible over 5 days (Figure 2b and S10), demonstrating the excellent stability of (CPT)2-Mal-PEG1k NPs. In the presence of 5 mM GSH, however, the size of NPs increased from 151 nm to 733 nm at 5 h, and further increased to ~1300 nm at 8 h, presumably due to the aggregation of the released CPT (Figure 2b and S11). Decreased count rate of the NP solution also suggested the disruption of nanostructure over GSH treatment (Figure 2b).
Figure 2.
(a) Fluorescence spectra of (CPT)2-Mal-PEG1k NPs in PBS (pH 7.4) after treated with 5 mM GSH for 0 min, 1 h, 2 h, 5 h, and 8 h, respectively. Excitation wavelength was set at 370 nm. (b) Changes of the size (black line) and the count rate (red line) of (CPT)2-Mal-PEG1k NPs over time in the presence (▲) or absence (■) of 5 mM GSH. (c) CPT release profiles of (CPT)2-Mal-PEG1k NPs in the presence of 0 mM, 1mM, and 5 mM GSH, respectively. (d) IC50 values of free CPT and (CPT)2-Mal-PEG1k NPs against LS174T colon cancer cells with or without GSH-OEt pretreatment. Data were presented as average ± standard deviation, n = 3. Statistical significance analysis was assessed by Two-Sample Unpaired Student’s t-test; 0.01 < p ≤ 0.05 and p ≤ 0.01 were considered statistically significant and highly significant and were denoted as “*” and “**” respectively.
We next studied CPT release kinetics from (CPT)2-Mal-PEG1k NPs in the presence or absence of GSH. In the absence of GSH, negligible CPT release was observed over 24 h. In comparison, over 59% and 95% of CPT were released from (CPT)2-Mal-PEG1k NPs after the NPs were treated with 1 mM GSH and 5 mM GSH, respectively for 24 h (Figure 2c). CPT release profile of (CPT)2-Mal-PEG1k NPs in the presence of 5 mM GSH determined by HPLC correlated well with FRET-indicated release kinetics in the first 5 h (Figure S12). Different from the unchanged I438/I550 value after 8 h, release of free CPT increased continuously, which was probably resulted from the difference between cleavage of maleimide thioether struture and release of free CPT. Despite the complete cleavage of maleimide thioether bonds, CPT might be entrapped in the dialysis tube and slowly diffuse into the release medium.
To demonstrate the proliferation inhibition capability of (CPT)2-Mal-PEG1k NPs, we investigated the cytotoxicity of NPs against LS174T colon cancer cells via MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay. LS174T cells were incubated with (CPT)2-Mal-PEG1k NPs or free CPT at various CPT concentrations for 48 h, and the cell viability results were shown in Figure 2d. (CPT)2-Mal-PEG1k NPs and free CPT showed an IC50 value of 177 nM (CPT equivalent) and 78 nM, respectively. To further demonstrate the reduction-responsive cytotoxicity of (CPT)2-Mal-PEG1k NPs, we investigated the viability of NP-treated LS174T cells with the addition of cellular GSH level regulators. GSH-OEt has been widely used to increase the GSH level via its hydrolyzation after entering cells.50–53 Prior to treatment with NPs, cells were pretreated with GSH-OEt for 4 h. As shown in Figure 2d, GSH-OEt significantly decreased the IC50 value of (CPT)2-Mal-PEG1k NPs from 177 nM (CPT equivalent) to 108 nM (CPT equivalent). In comparison, free CPT showed negligible change in IC50 with GSH-OEt pretreatment. This comparison well demonstrated the reduction-responsive cytotoxicity of (CPT)2-Mal-PEG1k NPs.
Conclusions
In conclusion, we developed a multifunctional dithiomaleimide-based CPT-containing NP with very high drug loading (up to 60%). (CPT)2-Mal-PEG1k NPs showed great stability under physiological condition while underwent rapid disruption in the presence of GSH. Release of CPT from (CPT)2-Mal-PEG1k NPs in the presence of GSH was accompanied with a reduction of the FRET signal between CPT and maleimide thioethers, suggesting the drug-release self-reporting property of (CPT)2-Mal-PEG1k NPs. In vitro anticancer efficacy study demonstrated GSH-responsive cancer inhibitory effect of (CPT)2-Mal-PEG1k NPs. Further studies of (CPT)2-Mal-PEG1k NP, such as in vivo efficacy and structural analysis, are underway.
Supplementary Material
Acknowledgements
This work was supported by National Science Foundation (DMR-1309525) and National Institute of Health (NIH Director’s New Innovator Award 1DP2OD007246-01).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details, including the synthesis and characterizations of N-propargyl-2,3-dibromomaleimide, CPT-S-S-CPT, (CPT)2-Mal-alkyne, and (CPT)2-Mal-PEG, degradation of (CPT)2-Mal-PEG1k and (CPT)2-mal-PEG1k NPs, MTT assay. See DOI 10.1039/c000000x/
Notes and references
- 1.Moghimi SM, Hunter AC, Murray JC. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 2.Wagner V, Dullaart A, Bock A-K, Zweck A. Nat. Biotechnol. 2006;24:1211–1218. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 3.Farokhzad OC, Langer R. Adv. Drug Deliv. Rev. 2006;58:1456–1459. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Angew. Chem. Int. Edit. 2009;48:872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein J, Ralston E, Leserman L, Klausner R, Dragsten P, Henkart P, Blumenthal R. Liposome technology. 1984;3:183–204. [Google Scholar]
- 6.Winterhalter M, Lasic D. Chem. Phys. Lipids. 1993;64:35–43. doi: 10.1016/0009-3084(93)90056-9. [DOI] [PubMed] [Google Scholar]
- 7.Gabizon A, Chemla M, Tzemach D, Horowitz A, Goren D. J. Drug Target. 1996;3:391–398. doi: 10.3109/10611869608996830. [DOI] [PubMed] [Google Scholar]
- 8.Barenholz YC. J. Control. Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Meyerhoff A. Clin. Infect. Dis. 1999;28:42–48. doi: 10.1086/515085. [DOI] [PubMed] [Google Scholar]
- 10.Zhigaltsev IV, Maurer N, Akhong Q-F, Leone R, Leng E, Wang J, Semple SC, Cullis PR. J. Control. Release. 2005;104:103–111. doi: 10.1016/j.jconrel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Adv. Drug Deliv. Rev. 2004;56:1177–1192. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Sofou S, Sgouros G. Exp. Opin. Drug Deliv. 2008;5:189–204. doi: 10.1517/17425247.5.2.189. [DOI] [PubMed] [Google Scholar]
- 13.Weiner N, Martin F, Riaz M. Drug Dev. Ind. Pharm. 1989;15:1523–1554. [Google Scholar]
- 14.Mohammed A, Weston N, Coombes A, Fitzgerald M, Perrie Y. Int. J. Pharm. 2004;285:23–34. doi: 10.1016/j.ijpharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Dos Santos N, Cox KA, McKenzie CA, van Baarda F, Gallagher RC, Karlsson G, Edwards K, Mayer LD, Allen C, Bally MB. BBA-BIOMEMBRANES. 2004;1661:47–60. doi: 10.1016/j.bbamem.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Zucker D, Marcus D, Barenholz Y, Goldblum A. J. Control. Release. 2009;139:73–80. doi: 10.1016/j.jconrel.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 17.Johnsson M, Bergstrand N, Edwards K. J. Liposome Res. 1999;9:53–79. [Google Scholar]
- 18.Andresen TL, Jensen SS, Jørgensen K. Prog. Lipid Res. 2005;44:68–97. doi: 10.1016/j.plipres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. J. Control. Release. 2006;116:150–158. doi: 10.1016/j.jconrel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Leroux J-C, Allémann E, De Jaeghere F, Doelker E, Gurny R. J. Control. Release. 1996;39:339–350. [Google Scholar]
- 21.Torchilin V, Zhou F, Huang L. J. Liposome Res. 1993;3:201–255. [Google Scholar]
- 22.Leroux J-C, Roux E, Le Garrec D, Hong K, Drummond DC. J. Control. Release. 2001;72:71–84. doi: 10.1016/s0168-3659(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 23.Meers P. Adv. Drug Deliv. Rev. 2001;53:265–272. doi: 10.1016/s0169-409x(01)00205-8. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Zhang G, Liu S. Chem. Soc. Rev. 2012;41:5933–5949. doi: 10.1039/c2cs35103j. [DOI] [PubMed] [Google Scholar]
- 25.Li M-H, Keller P. Soft Matter. 2009;5:927–937. [Google Scholar]
- 26.Liu L, Wang W, Ju X-J, Xie R, Chu L-Y. Soft Matter. 2010;6:3759–3763. [Google Scholar]
- 27.Wu W, Zhang Q, Wang J, Chen M, Li S, Lin Z, Li J. Polym. Chem. 2014;5:5668–5679. [Google Scholar]
- 28.Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. J. Control. Release. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 29.Khorsand B, Lapointe G, Brett C, Oh JK. Biomacromolecules. 2013;14:2103–2111. doi: 10.1021/bm4004805. [DOI] [PubMed] [Google Scholar]
- 30.Meng F, Hennink WE, Zhong Z. Biomaterials. 2009;30:2180–2198. doi: 10.1016/j.biomaterials.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Tang L, Tu C, Song Z, Yin Q, Yin L, Zhang Z, Cheng J. Biomacromolecules. 2013;14:3706–3712. doi: 10.1021/bm401086d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang K, Luo G-F, Liu Y, Li C, Cheng S-X, Zhuo R-X, Zhang X-Z. Polym. Chem. 2012;3:1084–1090. [Google Scholar]
- 33.Tanaka K, Ohashi W, Kitamura N, Chujo Y. Bull. Chem. Soc. Jpn. 2011;84:612–616. [Google Scholar]
- 34.Jakob U, Muse W, Eser M, Bardwell JC. Cell. 1999;96:341–352. doi: 10.1016/s0092-8674(00)80547-4. [DOI] [PubMed] [Google Scholar]
- 35.Donnet C, Arystarkhova E, Sweadner KJ. J. Biol. Chem. 2001;276:7357–7365. doi: 10.1074/jbc.M009131200. [DOI] [PubMed] [Google Scholar]
- 36.Love CS, Ashworth I, Brennan C, Chechik V, Smith DK. Langmuir. 2007;23:5787–5794. doi: 10.1021/la070185o. [DOI] [PubMed] [Google Scholar]
- 37.Shu XZ, Liu Y, Palumbo F, Prestwich GD. Biomaterials. 2003;24:3825–3834. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 38.Zhou S, Mozziconacci O, Kerwin BA, Schöneich C. Pharm. Res. 2013;30:1291–1299. doi: 10.1007/s11095-012-0968-1. [DOI] [PubMed] [Google Scholar]
- 39.Jiang J, Qi B, Lepage M, Zhao Y. Macromolecules. 2007;40:790–792. [Google Scholar]
- 40.Peters K, Richards FM. Annu. Rev. Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- 41.Jones MW, Strickland RA, Schumacher FF, Caddick S, Baker JR, Gibson MI, Haddleton DM. J. Am. Chem. Soc. 2012;134:1847–1852. doi: 10.1021/ja210335f. [DOI] [PubMed] [Google Scholar]
- 42.Smith ME, Schumacher FF, Ryan CP, Tedaldi LM, Papaioannou D, Waksman G, Caddick S, Baker JR. J. Am. Chem. Soc. 2010;132:1960–1965. doi: 10.1021/ja908610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones MW, Strickland RA, Schumacher FF, Caddick S, Baker JR, Gibson MI, Haddleton DM. Chem. Commun. 2012;48:4064–4066. doi: 10.1039/c2cc30259d. [DOI] [PubMed] [Google Scholar]
- 44.Robin MP, Jones MW, Haddleton DM, O'Reilly RK. ACS Macro Lett. 2011;1:222–226. doi: 10.1021/mz200164x. [DOI] [PubMed] [Google Scholar]
- 45.Mabire AB, Robin MP, Willcock H, Pitto-Barry A, Kirby N, O'Reilly RK. Chem. Commun. 2014;50:11492–11495. doi: 10.1039/c4cc04713c. [DOI] [PubMed] [Google Scholar]
- 46.Baldwin AD, Kiick KL. Bioconjugate Chem. 2011;22:1946–1953. doi: 10.1021/bc200148v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robin MP, O'Reilly RK. Chem. Sci. 2014;5:2717–2723. [Google Scholar]
- 48.Robin MP, Wilson P, Mabire AB, Kiviaho JK, Raymond JE, Haddleton DM, O'Reilly RK. J. Am. Chem. Soc. 2013;135:2875–2878. doi: 10.1021/ja3105494. [DOI] [PubMed] [Google Scholar]
- 49.Shen Y, Jin E, Zhang B, Murphy CJ, Sui M, Zhao J, Wang J, Tang J, Fan M, Van Kirk E, Murdoch WJ. J. Am. Chem. Soc. 2010;132:4259–4265. doi: 10.1021/ja909475m. [DOI] [PubMed] [Google Scholar]
- 50.Hong R, Han G, Fernández JM, Kim B-j, Forbes NS, Rotello VM. J. Am. Chem. Soc. 2006;128:1078–1079. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- 51.Morito N, Yoh K, Itoh K, Hirayama A, Koyama A, Yamamoto M, Takahashi S. Oncogene. 2003;22:9275–9281. doi: 10.1038/sj.onc.1207024. [DOI] [PubMed] [Google Scholar]
- 52.Koo AN, Lee HJ, Kim SE, Chang JH, Park C, Kim C, Park JH, Lee SC. Chem. Commun. 2008:6570–6572. doi: 10.1039/b815918a. [DOI] [PubMed] [Google Scholar]
- 53.Wang YC, Li Y, Sun TM, Xiong MH, Wu J, Yang YY, Wang J. Macromol. Rapid Commun. 2010;31:1201–1206. doi: 10.1002/marc.200900863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.