Abstract
Serotonin (5-hydroxytryptamine; 5-HT) neurons are widely considered to play an important role in central respiratory chemoreception. Although many studies in the past decades have supported this hypothesis, there had been concerns about its validity until recently. One recurring claim had been that 5-HT neurons are not consistently sensitive to hypercapnia in vivo. Another belief was that 5-HT neurons do not stimulate breathing; instead, they inhibit or modulate respiratory output. It was also believed by some that 5-HT neuron chemosensitivity is dependent on TASK channels, but mice with genetic deletion of TASK-1 & TASK-3 have a normal hypercapnic ventilatory response (HCVR). This review explains why these principal arguments against the hypothesis are not supported by existing data. Despite repeated challenges, a large body of evidence now supports the conclusion that at least a subset of 5-HT neurons are central chemoreceptors.
Keywords: 5-HT neurons, chemoreceptors, control of breathing, hypercapnia, acidosis, raphé
Introduction
The primary purpose of breathing is to maintain normal O2 and CO2 levels in the blood. Under resting conditions and at sea level, ventilation is primarily regulated by sensory feedback from central respiratory chemoreceptors (CRCs) as well as by peripheral chemoreceptors (PCRs) to a lesser extent. CRCs, located in the brainstem, are sensitive to parenchymal pH, which is strongly influenced by blood PCO2. PCRs, which will not be discussed in detail in this review, are located in the carotid and aortic bodies and under normal conditions are influenced primarily by blood PO2 and less so by blood PCO2. For more than four decades, central chemoreception has been the subject of extensive research, and much attention has been paid to the ventrolateral medulla (VLM) as the site of CRCs. However, the precise location within the VLM and phenotype of the neurons (or glia) that are CRCs has been disputed. In addition, neurons in other regions of the brainstem have been proposed as CRCs (Richerson 2004, Corcoran et al. 2009, Guyenet et al. 2010). For example, substantial evidence has supported the conclusion that 5-HT neurons are CRCs, only some of which are in the VLM.
In order for any cells to be considered CRCs, they need to display chemosensitivity to physiologically relevant changes in pH/PCO2, and their response must be intrinsic. They also must be connected to the respiratory network in a way that increases respiratory output in response to hypercapnia. Medullary 5-HT neurons possess these properties (Richerson 1995, Wang et al. 2001, Richerson 2004, Richerson et al. 2005, Corcoran et al. 2009). A number of recent studies have strengthened the support for a critical role of 5-HT neurons in central respiratory chemoreception, yet until recently there were still some that remained skeptical about this possibility. Due to rapid changes in this field and new evidence in favor of the 5-HT CRC hypothesis, now is an appropriate time to provide a comprehensive account of research that has occurred in recent years. This review identifies each of the major objections made against the 5-HT central chemoreceptor hypothesis, describes the data that led to these objections, and addresses why the original interpretation of those data have not held up to deeper scrutiny. In the process, we describe evidence that provides support that 5-HT neurons are central chemoreceptors.
Unraveling the Apparent Contradictions
A subset of 5-HT neurons increases their firing rate in vivo in response to hypercapnia
It has been claimed that 5-HT neurons are not central chemoreceptors based on data showing that 5-HT neurons do not respond to CO2 in vivo (Mulkey et al. 2004, Guyenet et al. 2008, DePuy et al. 2011). However, there have been two studies using extracellular recordings in vivo that have shown 5-HT neurons do, in fact, respond to hypercapnia with an appreciable increase in firing rate (Veasey et al. 1995, Veasey et al. 1997, Richerson et al. 2005). In addition, multiple studies have shown that hypercapnia causes c-fos activation in the raphe, with some demonstrating that a subset of activated cells are immunoreactive for markers of serotonin neurons [Reviewed in Richerson et al, 2005].
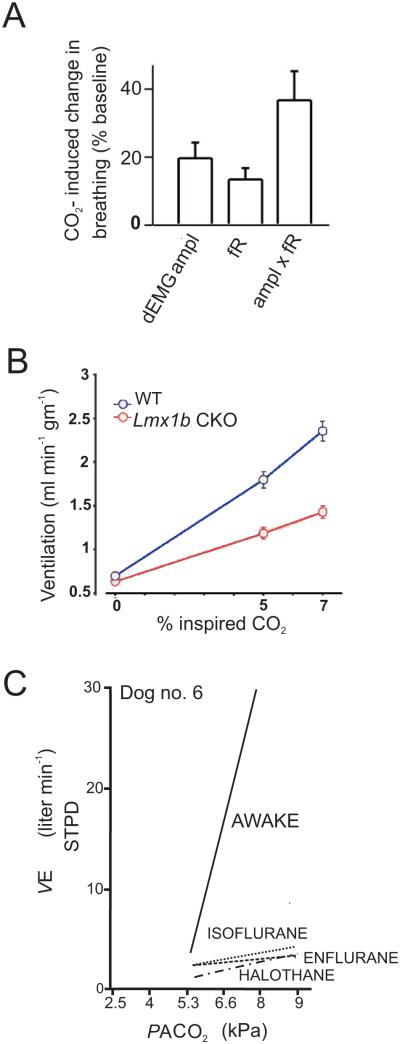
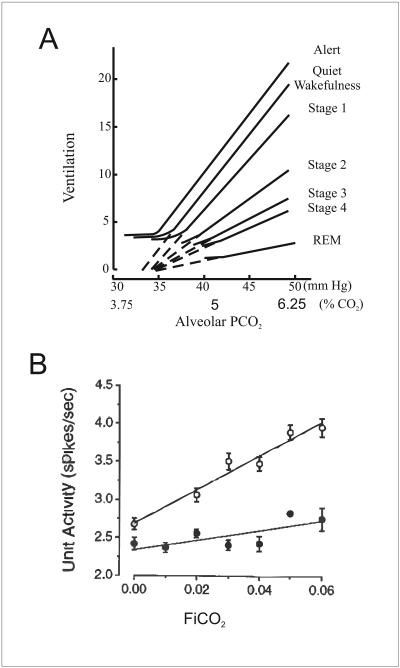
In order to understand why there is inconsistency in the literature on this particular point, it is necessary to identify differences in experimental conditions. 5-HT neurons in the raphé obscurus (DePuy et al. 2011) and VLM surface (Mulkey et al. 2004) of anaesthetized rats in vivo did not increase their firing rate in response to inhalation of 10% CO2 (a non-physiologically high level). It was unclear why 5-HT neurons were not chemosensitive during these in vivo experiments using anesthesia (DePuy et al. 2011) until it was realized that the HCVR was markedly smaller than that seen in other laboratories that did not use anesthesia. Rats studied by DePuy et al (2011) only increased their ventilation by 35% in response to 8% CO2 (Fig. 1a). In contrast, multiple other laboratories have found that rodents have much greater sensitivity, typically responding to 7% CO2 with an increase in ventilation to 250% of control (Fig. 1b) (Taylor et al. 2005, Davis et al. 2006, Hodges et al. 2008). We hypothesized that the blunted HCVR was due to the use of isoflurane or halothane anesthesia. Consistent with this we have obtained preliminary data showing that 1% isoflurane caused profound depression of the HCVR by 81% (n=16; CO2 7%) (Massey et al. 2012). Halogenated anesthetics have also been shown to decrease the HCVR in other species (Fig. 1c) (Hirshman et al. 1977, Dahan and Teppema 2003).
Figure 1. Halogenated anesthetics markedly depress the HCVR.
a) In vivo recordings from 5-HT neurons in isoflurane anesthetized rats show only a 35% increase in ventilation in response to 8% CO2. Reproduced with permission (DePuy et al. 2011). b) Whole-animal plethysmography recordings from unanesthetized WT mice show a greater than 250% increase in ventilation in response to 7% CO2. In mice that lack 5-HT neurons there is a greater than 100% increase in ventilation in response to hypercapnia. Reproduced with permission (Hodges et al. 2008). c) Ventilation in an awake dog increases in response to hypercapnia. However, halogenated anesthetics depress the HCVR in the same dog. Adapted with permission (Hirshman et al. 1977).
The effect of halogenated anesthetics on the HCVR appears to be due in part to an effect on 5-HT neurons. The blunting of chemoreception was accompanied by complete loss of the increase in firing in response to acidosis of 5-HT neurons in culture and in an in situ perfused brain preparation (Johansen et al. 2012, Massey et al. 2012). Inhalational halogenated anesthetics strongly potentiate two-pore domain K+ leak (TASK) channel currents causing membrane hyperpolarization (Patel et al. 1999, Sirois et al. 2000). Although TASK channels are widely expressed in many CNS neurons, 5-HT neurons have one of the highest levels of TASK channel expression (Talley et al. 2001). Halothane and isoflurane would therefore be expected to inhibit 5-HT neurons, which has been shown to decrease respiratory chemoreception (Ray et al., 2011).
The possibility that inhalational anesthetics introduced an artifact into in vivo recordings (Mulkey et al. 2004, DePuy et al. 2011) is supported by recordings from unanesthetized cats. A subset of 5-HT neurons in the medullary raphé (Veasey et al. 1995) and the dorsal raphé (Veasey et al. 1997) is sensitive to hypercapnia in vivo. There are numerous additional studies performed in unanesthetized animals in which 5-HT neurons are stimulated by hypercapnia in vivo. Microdialysis in the hypoglossal (XII) nucleus of unanesthetized mice demonstrated a 2.4–2.6-fold increase in extracellular 5-HT levels during inhalation of 7% CO2 (Kanamaru and Homma 2007). C-fos staining of some 5-HT neurons increases in the medullary raphé of cats (Larnicol et al. 1994), rats (Sato et al. 1992, Pete et al. 2002, Johnson et al. 2005), and mice (Haxhiu et al. 2001) when exposed to hypercapnia. Recent studies have examined Lmx1bf/f/p mice, in which Lmx1b (the gene for a transcription factor necessary in the differentiation of 5-HT neurons) is excised from genomic DNA selectively in Pet-1 expressing cells (i.e. 5-HT neurons). This results in specific deletion during embryonic development of >99% of 5-HT neurons in the CNS (Zhao et al. 2006). As adults, Lmx1bf/f/p mice exhibit an HCVR that is 50% smaller than that of wild type littermates (Fig. 1b) (Hodges et al. 2008). A 50% decrease in chemoreception was also seen when 5-HT neurons were silenced by activation of genetically encoded DREADD receptors selectively expressed in 5-HT neurons (Ray et al. 2011). It is not known which cells contribute to the remaining 50% of the HCVR in these transgenic lines. However, 5-HT neurons make a major contribution to the HCVR, possibly more than 50% of the total response.
It remains to be determined what percentage of 5-HT neurons are chemoreceptors. In cell culture, between 70% & 90% of 5-HT neurons respond to a decrease in pH from 7.4 to 7.2 (Wang et al. 2001). In slices the percentage of 5-HT neurons that are chemosensitive to this range of pH is smaller, but it is not clear if there are more neurons that have no response, if slices buffer the pH changes to mask some responses, or there are some neurons that have very large responses and others that have smaller responses. It is possible that the former project to respiratory neurons and the latter project to other brain regions involved in functions that are less sensitive to pH/CO2.
5-HT neurons stimulate breathing; they do not inhibit it
In order to be considered a chemoreceptor, a neuron is required to have more properties than just chemosensitivity; it must provide excitatory drive to the respiratory network. Only recently has it become widely accepted that 5-HT neurons stimulate respiratory output. Previously, investigators described the respiratory effects of 5-HT as inhibitory, modulatory, “stabilizing” or primarily involved in plasticity (Hodges and Richerson 2008a). However, a number of recent experiments have convincingly demonstrated that 5-HT neurons stimulate respiratory output. Here we describe possible explanations for why it had previously been unclear how 5-HT affects breathing.
Stimulation
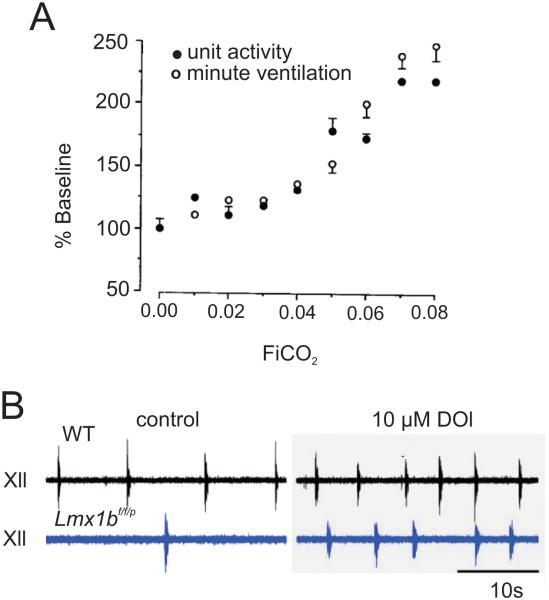
There is now ample evidence that 5-HT neurons stimulate breathing. Early studies of serotonergic raphé neurons in vivo observed a direct correlation between firing rate and the increase in minute ventilation induced by hypercapnia (Fig 2a) (Veasey et al. 1995). An increase in fractional inspired CO2 of as little as 3% led to a statistically significant increase in both 5-HT neuron firing rate and ventilation (Fig 2a). Hodges et al. (2008) also observed increased ventilation after providing exogenous 5-HT given intracerebroventricularly (ICV). Similarly, exogenous application of 1-[2,3-dimethoxy-4-iodophenyl]-2-aminopropane (DOI), a 5-HT2A/2C agonist, increases ventilation in rats (Cayetanot et al. 2002) and neonatal mice in vivo (Hodges et al. 2009) (Fig 2b). Conversely, iontophoretic application of 5-HT2A antagonists such as ketanserin in decerebrate dogs in vivo and unilateral injection of MDL 100,907 into the XII nucleus of rats in vivo decreases respiratory output of hypoglossal motor neurons (Brandes et al. 2006). These antagonists also block the effects of 5-HT microinjected into the ventral aspect of the XII nucleus of rats (Fenik and Veasey 2003) thus supporting the excitatory effect of 5-HT on respiratory motor neurons.
Figure 2. Medullary 5-HT neurons increase their firing in response to hypercapnia in vivo.
a) Relationship between activity of a single medullary 5-HT neuron and minute ventilation as inspired CO2 increases; there is a parallel increase in unit activity and minute ventilation in response to hypercapnia. Reproduced with permission (Veasey et al. 1995). b). Recordings from XII nerve roots show decreased respiratory activity in Lmx1bf/f/p mice (blue) compared to WT mice (black) under control conditions (left traces). DOI bath application (10 μM) increased respiratory activity in Lmx1bf/f/p mice to a comparable level as WT. Reproduced with permission (Hodges et al. 2009).
BIMU8, a 5-HT4A receptor agonist, significantly increases respiratory motor output (Manzke et al. 2003). This stimulatory effect is blocked by the 5-HT4A receptor-specific antagonist GR 113808. In addition, BIMU8 is effective in overcoming fentanyl-induced respiratory depression and apnea (Manzke et al. 2003). Systemic application of the 5-HT1A receptor agonist 8-hydroxy-[N-n-dipropyl-N-(3-iodo-2-propenyl)amino] tetralin (8-OH-DPAT) also restores eupneic respiration after fentanyl-induced apnea (Sahibzada et al. 2000). This latter observation is counterintuitive because 5-HT1A receptors usually inhibit neurons through Gi second messenger pathways. Recent data from rhythmogenic neonatal mouse medullary slices indicate that this is due to disinhibition of the respiratory network via 5-HT1A receptor activation on GABAergic or glycinergic interneurons (Corcoran et al. 2013).
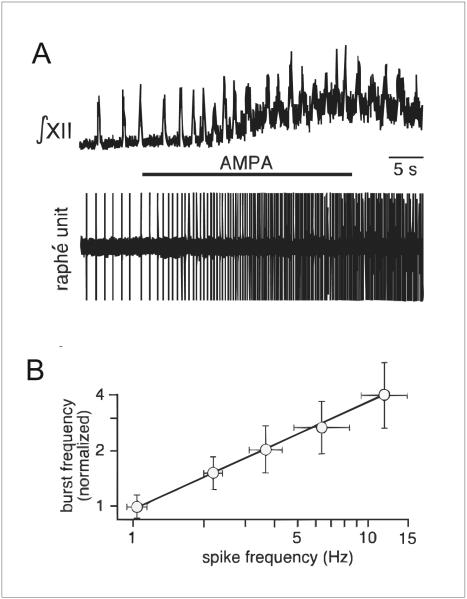
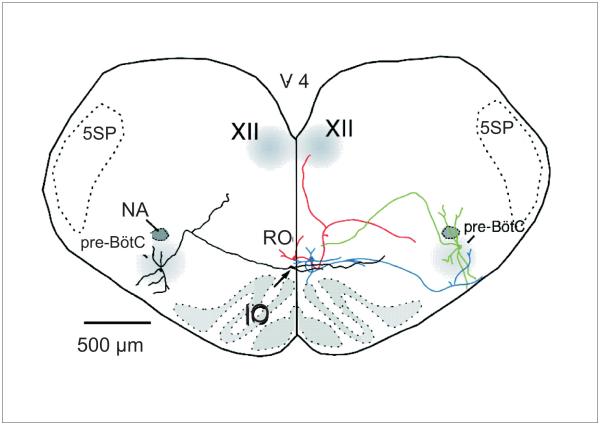
Two sets of experiments were recently performed that demonstrate clearly that increased firing of 5-HT neurons causes increased respiratory output. First, an excitatory projection from 5–HT neurons to the respiratory network was revealed by Ptak et al (2009) in rhythmogenic slices from neonatal mice. Patch clamp recordings were made from raphé obscurus 5-HT neurons. It was found that focal application of the glutamate receptor agonist 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid (AMPA) into the raphe obscurus induces a graded increase in firing rate of 5-HT neurons which leads to a proportional increase in frequency of hypoglossal nerve rootlet bursting (Fig. 3). This stimulation of respiratory motor output is blocked by 5-HT2A receptor antagonists. Some neurons that are tryptophan hydroxylase (TpOH) immunoreactive have axons that project to the pre-Bötzinger complex (pre-BötC), others have axons that project to the XII motor nucleus, and still others have axons that send branches to both of these nuclei (Fig. 4). There are also projections of 5-HT neurons to other respiratory nuclei, including the nucleus of the solitary tract, nucleus ambiguus, RTN and phrenic motor nucleus (Jacobs and Fornal 1997, Lovick 1997, Mason 2001). Together these projections stimulate the respiratory network at multiple sites, via multiple receptors and using multiple mechanisms (Dekin et al. 1985, Richerson 2004, Ptak et al. 2009). Interestingly 5-HT neurons are also embedded within the respiratory network as they receive bursts of excitatory input during inspiration, indicating that there are synapses from the respiratory CPG onto 5-HT neurons (Ptak et al. 2009).
Figure 3. 5-HT neuron activity proportionally stimulates hypoglossal nerve rootlet bursting.
a) XII output (top) recorded concurrently with extracellular potential of a 5-HT neuron (bottom trace). AMPA (5 μM) microinfusion into the raphé obscurus increased both firing rate of the 5-HT neuron and XII activity. b) XII nerve bursting activity increased proportionally with increased spike frequency of raphe unit. Reproduced with permission (Ptak et al. 2009).
Figure 4. 5-HT neurons project to neurons in the major respiratory nuclei.
Reconstruction of two biocytin-filled raphé obscurus 5-HT neurons (red, blue) and two pre-Bötzinger complex neurons (green, black) illustrating dendrites and axonal projections. Reproduced with permission from (Ptak et al. 2009).
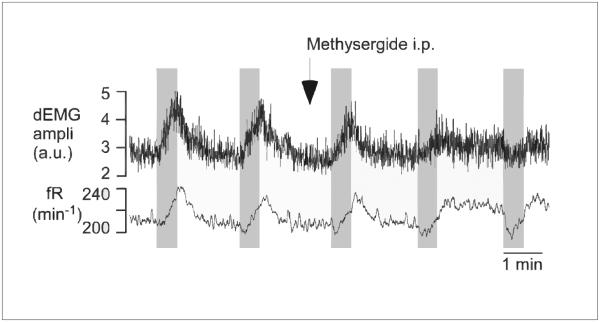
The second study employed optogenetics to selectively stimulate 5-HT neurons in the medullary raphé in vivo. This induces a large increase in frequency and amplitude of phrenic nerve output. This increase in phrenic nerve activity is blocked by methysergide, a non-selective antagonist of 5-HT receptors (Fig. 5) (DePuy et al. 2011). The use of optogenetics was important, because it allowed stimulation to be isolated specifically to 5-HT neurons. Previous experiments stimulating the raphe in adults in vivo were not able to isolate the effect of 5-HT neurons alone, and often got mixed effects that were likely to be due to stimulation of non-5-HT neurons.
Figure 5. 5-HT neurons stimulate respiratory output.
Raphé obscurus 5-HT neurons from rats expressing channelrhodopsin (ChR) stimulate breathing when activated by photostimulation. Intraperitoneal methysergide administration blocks this response. Reproduced with permission (DePuy et al. 2011).
Inhibition
Early studies led to the conclusion that 5-HT neurons normally inhibit breathing. These studies included the use of systemic inhibitors of TpOH, such as p-chlorophenylalanine (PCPA), 6-fluorotryptophan (6-FT), and para-chloroamphetamine (PCA). Each of these drugs reduces 5-HT levels in the brain and systemically, and also induces hyperventilation (Olson et al. 1979, Mitchell et al. 1983). However, one of these studies, as well as others performed around the same time, also reported that specific lesions of 5-HT neurons with the toxin 5,7-dihydroxytryptamine (5,7-DHT) cause significant hypoventilation (Olson et al. 1979, Mueller et al. 1984). A possible explanation for these contradictory results could be that 5,7-DHT does not cross the blood-brain barrier, unlike PCA, 6-FT, and PCPA, and thus must be administered ICV (Olson et al. 1979). Respiratory control is regulated by both central and peripheral neuronal inputs and the pharmacological agents that caused hyperventilation were given intraperitoneally. Beyond depletion of central 5-HT, PCPA can also alter or deplete peripheral 5-HT and other neurotransmitters (Olson et al. 1979, Reader and Gauthier 1984). Another explanation is that when PCPA is given at a dose sufficient to deplete central 5-HT by 90%, there is no change in the post-synaptic response to stimulation of ascending 5-HT fibers to the hippocampus, indicating that there is considerable compensatory reserve to prevent failure of neurotransmission at 5-HT synapses (Chaput et al. 1990). Finally, 5,7-DHT is neurotoxic, and can cause death of 5-HT neurons. The result is not just reduced release of 5-HT, as would occur with the other agents, but also reduced release of neuropeptides that are co-localized in 5-HT neurons, such as thyrotropin-releasing hormone (TRH) and substance P (SP). Conversely, PCPA, PCA and 6-FT deplete 5-HT, but the neurons remain intact and would continue to release TRH and SP. The decrease in extracellular 5-HT levels would lead to loss of 5-HT1A receptor-mediated autoinhibition, which could increase release of TRH and SP, both of which have powerful stimulatory effects on breathing (Yamamoto et al. 1981, Hedner et al. 1983, Nink et al. 1991). The end result of 5-HT depletion might be greater stimulation of the respiratory network rather than less.
There has been relatively little other direct evidence that 5-HT neurons inhibit respiratory output. There are regions of the midline medulla that when stimulated electrically induce inhibition of breathing (Lalley et al. 1997). Some of these responses are mediated by 5-HT release as they are blocked by methysergide. However, what is not known is whether the “inhibitory 5-HT neurons” project directly to the respiratory network. Alternatively, they may project to and inhibit another pool of 5-HT neurons, and this other pool might normally stimulate the respiratory network. Interconnections between different subsets of 5-HT neurons are not well understood.
Some neurons of the pre-BötC are inhibited by 5-HT1A receptors (Lalley et al. 1997, Richter et al. 2003). The identity of these neurons is unknown, but if they were inhibitory interneurons then inhibition by 5-HT would lead to disinhibition of respiratory output. Direct projections have never been demonstrated from 5-HT neurons that inhibit output from the respiratory network.
Modulation
5-HT neurons have also been proposed to play a neuromodulatory role in control of breathing (Hodges and Richerson 2008a). However, it has not always been clear how neuromodulation is defined in this context, since this term has had many different definitions. According to Kaczmarek and Levitan (1987) it is “the ability of neurons to alter their electrical properties in response to intracellular biochemical changes resulting from synaptic or hormonal stimulation.” It can often refer to changes in neuronal excitability induced through second messenger pathways upon activation of G protein-coupled receptors (GPCRs) (Hodges and Richerson 2010, Bocchiaro and Feldman 2004). In that sense, most actions of 5-HT are neuromodulatory, because all but one of the 5-HT receptor subtypes (5-HT3) are GPCRs (Bockaert et al. 2006). Therefore, the effects of 5-HT on all of the GPCRs are neuromodulatory. However, they can still be stimulatory. As an example, 5-HT2A receptors are GPCRs and therefore neuromodulatory. However, they stimulate respiratory output in slices (Pena and Ramirez 2002; Ptak et al. 2009) and in vivo (Hodges and Richerson 2008a, Doi and Ramirez 2010). 5-HT2A receptor activation in neurons of the pre-BötC enhances a leak Na+ current, which in turn promotes bursting pacemaker activity and strengthens respiratory motor output (Ptak et al. 2009).
Plasticity
Serotonin is necessary for some forms of neural plasticity observed in the respiratory network. A well-studied example is long-term facilitation (LTF), which is characterized by a prolonged increase in respiratory motor output after specific patterns of intermittent hypoxic challenges (Mitchell et al. 2001). LTF has most frequently been observed in anesthetized rats as increased phrenic nerve burst amplitude and a long-lasting increase in frequency (Karen B. Bach 1996, Baker and Mitchell 2000, Fuller et al. 2000, Mitchell et al. 2001). Methysergide (Baker-Herman and Mitchell 2002) and ketanserin (Kinkead and Mitchell 1999) block LTF. This effect of 5-HT could be viewed as a more complex form of respiratory stimulation on a longer time domain that is superimposed on the immediate and short-term forms described above. The end result is an increase in respiratory motor output, so it could be viewed as a complementary form of stimulation mediated by chemoreceptors.
Chemosensitivity of adult 5-HT neurons is not due to TASK channels
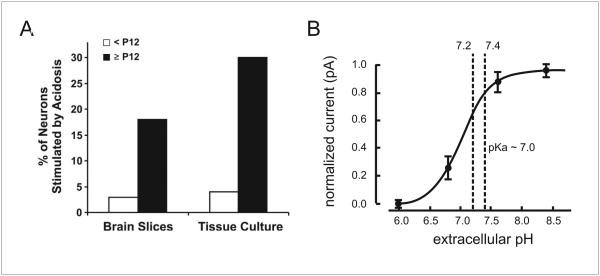
It has previously been stated that 5-HT neurons are not central chemoreceptors, because of data from TASK knockout mice (Mulkey et al. 2007b). Dorsal raphé 5-HT neurons in brain slices from WT mice increase firing rate two-fold when pH decreases from 7.5 to 6.9. This in vitro chemosensitivity is abolished if either TASK-1 or TASK-3 channels are genetically deleted alone or together. However, the in vivo HCVR is normal in mice with genetic deletion of either or both TASK channels. The authors concluded that TASK-1/3 channels are necessary for chemosensitivity of 5-HT neurons, but that respiratory chemoreception is not dependent on 5-HT neurons in adult rodents. However, one must be cautious in accepting those conclusions for several reasons. First, the 5-HT neurons that have been proposed to be respiratory chemoreceptors are in the medulla, not the dorsal raphe of the midbrain, although those in the midbrain may be important for arousal and anxiety/panic to hypercapnia (Buchanan and Richerson 2009, Buchanan and Richerson 2010). Second, the data obtained to make these conclusions were all from neurons in brain slices from P7-P12 mice, which is too young for a mature response. At this age 5-HT neurons require a very large decrease in pH to induce only a small increase in firing rate. Chemosensitivity of 5-HT neurons of the degree that is likely to be relevant to adult chemoreception does not begin to develop until P12 (Fig 6a) and is not mature until after P21 (Wang and Richerson 1999, Wu et al. 2008). This age dependence of 5-HT neuron chemosensitivity is seen in vitro in slices and culture and parallels the age-dependent increase in the HCVR in rodents in vivo (Serra et al. 2001, Davis et al. 2006). Similarly, inhibition of medullary raphé 5-HT neurons by microdialysis of 8-OH-DPAT does not affect the HCVR in piglets younger than P10, but does decrease it in older animals (Messier et al. 2004). Penatti et al. (2006) did not observe any change in the HCVR in conscious piglets at P4–P12 after lesioning medullary raphé 5-HT neurons using a SERT antibody-saporin conjugate. Additionally, 5,7-DHT increases the HCVR in neonatal rats (Cummings and Frappell 2008), whereas it causes hypoventilation in adult rats (Olson et al. 1979). In contrast, neurons of both the locus coeruleus and nucleus tractus solitarius (NTS) are chemosensitive early in development in vitro (Stunden et al. 2001, Conrad et al. 2009, Nichols et al. 2009). These data indicate that the HCVR in neonates is very small and depends primarily on non-5-HT neurons. During adulthood the HCVR becomes much larger and 5-HT neurons play a more significant role, contributing at least 50% to central chemoreception (Hodges and Richerson 2008b, Ray et al. 2011).
Figure 6. Chemosensitivity of 5-HT neurons is age-related, but not TASK-dependent.
a) 5-HT neuron chemosensitivity does not mature until after P12. Recordings from Sprague-Dawley rat raphé neurons recorded in slices and culture are not chemosensitive until after P12. Reproduced with permission (Corcoran et al. 2009). b) A change in TASK channel conductance does not occur over the physiological pH range. Voltage-clamp recordings were made from dorsal raphé 5-HT neurons. Currents measured under pH variations from 6.0 to 8.4 in halothane were normalized to the maximum and minimum current. These were fitted to a logistic equation that predicted a pK of ~7.0. Dashed lines indicate the pH range over which 5-HT neurons are typically tested. Altered with permission (Washburn et al. 2002).
Another reason the conclusions from Mulkey et al (2007b) are not relevant to respiratory chemoreception is that the pH changes used to elicit chemosensitivity were not physiologically relevant. 5-HT neurons did not respond to a decrease in pH from 7.5 to 7.3, but instead required a decrease in pH from 7.5 to 6.9 in order to induce a response. Such a large pH change is not consistent with the well-established observation that 5-HT neurons respond to a pH decrease from 7.4 to 7.2 with an average increase in firing rate to 300% of baseline (Wang et al. 1998, Wang et al. 2001, Wang et al. 2002). It is unlikely a living mammal would have a blood pH of 6.9 unless it is in extremis. In the case of a pure respiratory acidosis, the PCO2 would have to rise above 100 mmHg in order for the pH to drop below 7.1 (Katsura et al. 1992a, Katsura et al. 1992b), which greatly surpasses the range of 50-70 mmHg that might occur in a critically ill patient with chronic obstructive pulmonary disease, and would likely only occur when a patient goes into respiratory failure, cardiopulmonary arrest or other medical emergency (Koo et al. 1975). When studying putative chemoreceptors, it is imperative to keep in vitro experimental conditions within a physiological range. Any cellular response to changes in PCO2/pH beyond 80 mmHg/ 7.0 are unlikely to be relevant to normal respiratory control, since a PCO2 above that range is not seen in healthy individuals, and is only seen in patients with severe illness such as cardiopulmonary arrest, diabetic ketoacidosis or other serious illness. Even in those cases CSF pH is maintained within a much tighter range, typically between 7.4 and 7.25, than arterial pH (Fencl, 1971). Another reason to not study such an extreme level of pH is that the HCVR plateaus above a very high level of PaCO2 (Lambertsen 1980).
There are other reasons why it is unlikely that TASK channels mediate the response of adult 5-HT neurons to pH. For example, TASK channels have a relatively small response to changes in pH between 7.6 and 7.2 (Fig. 6b) (Washburn et al. 2002), whereas 5-HT neurons have a very large firing rate response over that pH range. TASK channels are sensitive to extracellular pH (Rajan et al. 2000, Morton et al. 2003), whereas 5-HT neuron firing rate is sensitive to intracellular pH (Wang et al. 2002). In addition, TASK channels are leak channels, and are not gated by Ca2+ or depolarization, whereas 5-HT neurons have a pH sensitive tail current that is activated by both depolarization and Ca2+ (Richerson et al. 2009). TASK channels are selective for K+ whereas the pH sensitive channel in 5-HT neurons is permeable to both K+ and Na+ (Richerson et al. 2009). These data point to a novel pH sensitive calcium-activated non-selective cation (CAN) current (Richerson 2004, Massey et al. 2013) as mediating the response of mature 5-HT neurons to changes in pH between 7.4 and 7.2. There is no evidence that TASK channels mediate the large changes in firing rate that take place in mature raphé neurons in response to small changes in pH near 7.4 (Corcoran et al. 2009).
Although TASK channels are sensitive to pH (Rajan et al. 2000, Morton et al. 2003), there is other evidence that they are not crucial for central respiratory chemoreception. For example, TASK channels are broadly expressed in the CNS (Talley et al. 2001) and yet not all neurons and brain functions are chemosensitive to hypercapnia (Richerson 1998, Richerson et al. 2001). Hippocampal principal neurons and essentially all motor neurons express high levels of TASK channels (Talley et al. 2001, Taverna et al. 2005); however, hypercapnic acidosis does not stimulate these neurons, but rather has no effect or inhibits them (Richerson 1998, Wang and Richerson 2000, Somjen and Tombaugh 1998).
For all of the above reasons, the fact that a normal HCVR was seen in TASK knockout mice does not refute the hypothesis that 5-HT neurons are respiratory chemoreceptors.
Chemosensitivity of 5-HT neurons is reduced during sleep
It has previously been suggested that 5-HT neurons are not chemoreceptors because they decrease their firing rate and become less responsive to hypercapnia during sleep. A study performed on unanesthetized cats found that a subset of neurons in the medullary raphe (identified as serotonergic by validated criteria) are activated by hypercapnia during wakefulness (Veasey et al. 1995), but the response to CO2 disappeared during sleep. This was interpreted by others as showing that activation by hypercapnia was due to arousal rather than to intrinsic chemosensitivity (Guyenet et al. 2005). However, we disagree with this conclusion. It has long been known that there is a decrease in the HCVR during sleep, but the mechanism has never been defined. 5-HT neurons in vivo usually fire fastest during wakefulness, slower during non-rapid eye movement sleep (NREM), and are nearly silent during rapid eye movement sleep (REM) (McGinty and Harper 1976, Trulson and Jacobs 1979). The arousal state-dependent discharge pattern of 5-HT neurons is congruous with their ability to regulate respiration as central chemoreceptors. Arousal in response to hypercapnia is dependent on 5-HT neurons (Buchanan and Richerson 2010), presumably acting as CO2 sensors within the midbrain. Previously it has been proposed that state-dependent control of upper airway tone is partly mediated by state-dependent changes in 5-HT neuron firing (Kubin et al. 1992, Buchanan 2008). There is also a progressive reduction in the slope of the HCVR in deeper stages of sleep (Fig. 7a) (Bulow 1963). The mechanisms of this effect are not known, but the firing rate of 5-HT neurons decreases during sleep, presumably due to inhibition from sleep generating centers such as the ventrolateral preoptic nucleus in the hypothalamus (Saper et al. 2005). Since generation of bursting activity in respiratory centers is enhanced by 5-HT and TRH input (Dekin et al. 1985, Pena and Ramirez 2002, Ptak et al. 2009), the decrease in firing of 5-HT neurons might contribute to the decrease in baseline ventilation that occurs during sleep. There is also a progressive decrease in chemosensitivity of 5-HT neurons during sleep (Fig. 7b), which may represent the cellular mechanism that underlies the decrease in the HCVR (Fig. 7a).
Figure 7. Respiratory CO2 chemoreception decreases during sleep.
a) The HCVR is robust in humans who are alert or in quiet wakefulness. It progressively decreases as subjects move from wakefulness to deeper sleep. REM sleep, when 5-HT neurons are silent, shows the lowest HCVR. Adapted with permission (Bulow 1963). b) State-dependent responses in neuronal activity for a 5-HT neuron responsive to a hypercapnia challenge during quiet waking (QW) and slow-wave sleep (SWS). Open circles, QW data; closed circles, SWS data. Reproduced with permission (Veasey et al. 1995).
Recordings from unanesthetized, behaving cats identified neurons that responded to hypercapnia during wakefulness, but were less responsive or unresponsive during sleep (Veasey et al. 1995). This led to the conclusion that these neurons were simply responding to an increase in arousal induced by CO2 (DePuy et al. 2011). Exposure to CO2 during sleep induces arousal (Buchanan and Richerson 2010), which is an important protective reflex. We have proposed that this is mediated by chemosensitivity of midbrain 5-HT neurons (Richerson 2004). If true, the effect of CO2 on arousal would occur at the same time as that on ventilation. As CO2 increases, 5-HT neurons fire at a faster rate, inducing both arousal and increased ventilation. The arousal would also cause an increase in slope of the HCVR. If a 5-HT neuron increases its firing rate when an animal wakes up, the increase in neuronal firing would be ascribed to arousal. If all 5-HT neurons were excluded from analysis just because there was coexisting arousal, then many chemosensitive 5-HT neurons would go undetected.
The role of 5-HT in control of sleep and wakefulness has long been controversial. Early experiments led to the persistent belief that 5-HT causes sleep (Jouvet 1999). This conclusion was based on experiments using PCPA to deplete 5-HT levels. However, as with experiments on respiratory control using PCPA (see above), this approach led to complicated effects that opposed those obtained using other approaches. Although this view of 5-HT still continues to be perpetuated in the literature, it has been acknowledged to be incorrect (Jouvet 1999). The first experiments revealing that PCPA gave invalid results used single unit recordings and found that 5-HT neurons fire fastest during wakefulness, as described above (McGinty and Harper 1976, Trulson and Jacobs 1979). This is the opposite one would expect if these neurons caused sleep. It is now accepted by the majority of sleep neuroscientists that 5-HT neurons are part of the ascending arousal system (Steriade et al. 1993, Monckton and McCormick 2002, Saper et al. 2005) and activation of these neurons causes arousal instead of sleep.
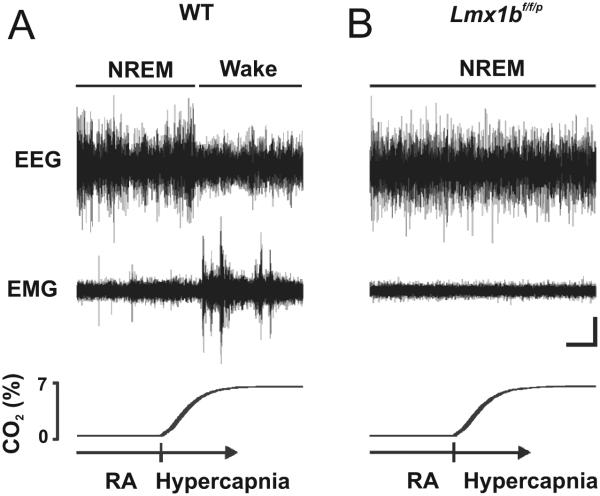
What is now emerging is a picture where 5-HT neurons induce arousal, but rather than doing so in an ill-defined way, they do so specifically in response to hypercapnia. It has been hypothesized that midbrain 5-HT neurons initiate the arousal response to hypercapnia (Washburn et al. 2002, Severson et al. 2003, Richerson 2004, Buchanan 2008). Functional MRI studies show that hypercapnia induced by brief apneic episodes leads to an increase in activity within the thalamus and cerebral cortex (Kannurpatti et al. 2003). 5-HT release within the thalamus changes thalamocortical rhythms from a bursting pattern to a tonic pattern (Monckton and McCormick 2002). This correlates with a change from high-voltage, low-frequency, synchronized activity in the cortical EEG compatible with slow wave sleep to low-voltage, high-frequency, desynchronized activity consistent with wakefulness (Steriade et al. 1993). Furthermore, arousal to 10% CO2 is completely absent in Lmx1bf/f/p mice (Fig 8) (Buchanan and Richerson 2010), indicating that 5-HT neurons are required for the potentially life-saving response that should normally occur when hypercapnia occurs during sleep.
Figure 8. Genetic deletion of 5-HT neurons prevents arousal to hypercapnia.
Four-minute EEG (top), EMG (middle), and PCO2 (bottom) recordings showing an arousal response to 7% CO2 in WT mice, but its absence in Lmx1bf/f/p mice. Reproduced with permission (Buchanan and Richerson 2010).
The relationship between 5-HT neurons and central arteries is important for accuracy, not speed, of blood PCO2 measurement
Most 5-HT neurons in both the medullary and midbrain raphé are adjacent to large cerebral arteries (Severson et al. 2003, Bradley et al. 2002). This is an ideal position for 5-HT neurons to accurately monitor changes in arterial PCO2. However, the functional significance of this anatomical specialization has been questioned. Data from dogs in vivo shows a prolonged delay in the ventilatory response to central chemoreceptor stimulation (Smith et al. 2006). The authors concluded that this favored a site for CO2 reception distant from blood. However, the delay for a change in ventilatory output depends not only on how long it takes for a change in arterial PCO2 to induce a change in 5-HT neuron firing rate, but also how long it takes for neurotransmitters released from 5-HT neurons to induce changes in activity of the downstream respiratory network. The purpose of respiratory chemoreceptors is to monitor how efficiently the lungs are oxygenating the blood. For this reason, they need to be positioned where they have the ability to faithfully measure arterial PCO2, the physiological parameter that best reflects alveolar ventilation. This parallels the functional purpose of peripheral chemoreceptors, which are closely associated with the carotid arteries and the aorta - locations where they can monitor arterial blood as soon as it exits the left ventricle of the heart. 5-HT neurons in the medullary raphé are immediately adjacent to the basilar artery and its large midline branches (Fig. 9). This region is highly perfused by large arteries and is relatively devoid of veins, which would lead to a local tissue PCO2 that more accurately reflects changes in lung ventilation and arterial blood PCO2 compared to any other site within the brain such as locations near distal venous blood, parenchymal tissue or bulk CSF.
Figure 9. 5-HT neurons are located adjacent to large arteries in the medulla.
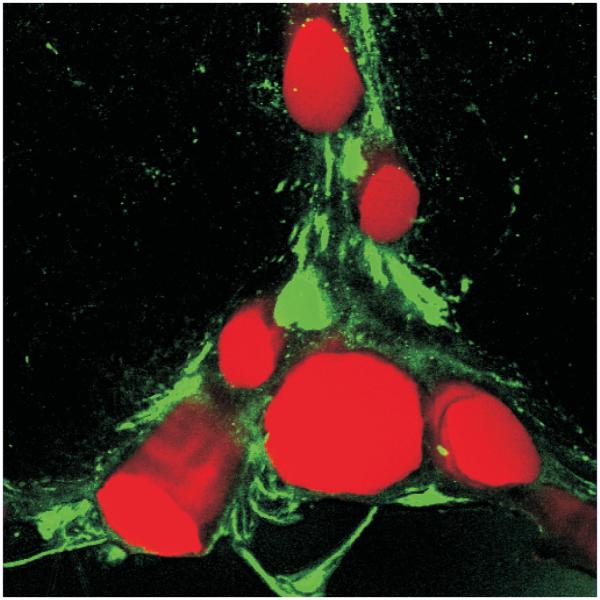
5-HT neurons (green) are found in close approximation to the basilar and its associated arteries (red) in the medulla. Reproduced with permission (Fiske 2002).
Close proximity to large arteries could also permit detection of changes in lung gas exchange rapidly after they occur (Smith et al. 2006). However, a rapid response would not necessarily be an advantage. For example, when the speed of negative feedback is not matched properly for a particular control system, oscillations in output can occur. The respiratory control system can be prone to oscillations, as seen in Cheyne-Stokes breathing; this can occur when the rate of feedback is poorly matched to the output of the system.
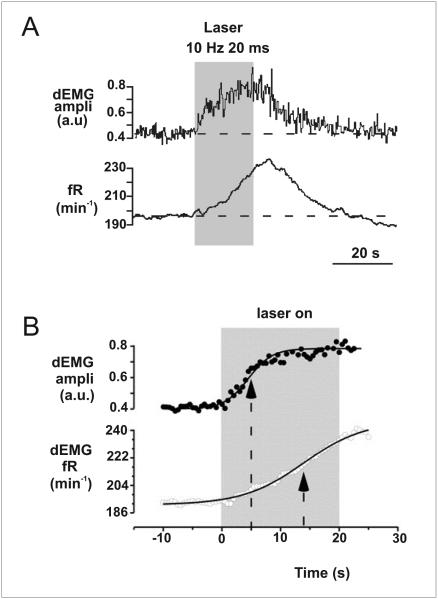
There is also no advantage for central CO2 chemoreceptors to be fast. First, changes in arterial blood PCO2 occur relatively slowly. The respiratory control system also has a number of properties that introduce a delay downstream of CO2/pH sensation. For example, there is a slow cellular response of respiratory neurons to activation of the receptors for the neurotransmitters released by 5-HT neurons (5-HT, TRH and SP) almost all of which (other than 5-HT3) are G-protein coupled receptors. Optogenetic experiments determined the time course for the response of the respiratory system to selective stimulation of 5-HT neurons (Fig 10) (DePuy et al. 2011). A rapid increase in firing of 5-HT neurons caused a half-maximum response of the amplitude of diaphragm EMG to occur in 15.8 s. The increase in respiratory frequency was even slower, continuing for up to 10 seconds after the end of a 20-second burst of 5-HT neuron firing. These delays are consistent with the expected response time following activation of GPCRs. For example, 5-HT2A receptors, which are important for stimulation of respiratory output by 5-HT neurons, activate the IP3 second-messenger system and subsequently increase intracellular Ca2+ levels (Bhattacharyya et al. 2002), processes which both introduce a delay. Thus, downstream motor activity is significantly delayed after 5-HT neurons are stimulated by CO2, so there is no advantage to having a fast response of 5-HT neurons to arterial CO2 changes. Instead, the proximity to arteries is likely to exist so that the measurement of PCO2 can be as accurate as possible.
Figure 10. Respiratory response induced by 5-HT stimulation is delayed.
a) Raphé obscurus 5-HT neurons from rats expressing channelrhodopsin (ChR) stimulate breathing when activated by photostimulation. Reproduced with permission from (DePuy et al. 2011). b) Amplitude and frequency response at onset of 5-HT photostimulation; data points are fitted to a Boltzmann sigmoid equation to determine half-maximal response. These data reveal that respiratory output is significantly delayed following 5-HT neuron stimulation. Reproduced with permission (DePuy et al. 2011).
Other brainstem regions contain neurons that may also be chemoreceptors
There are multiple other regions of the brainstem that contain neurons that may also be chemoreceptors, including the RTN (Mulkey et al. 2004) nucleus tractus solitaries (NTS) (Dean et al. 1990), locus coeruleus (LC) (Pineda & Aghajanian 1997) and hypothalamus (Dillon & Waldrop 1992). Of these regions, the RTN has received the most attention. It contains neurons that express Phox2b and are chemosensitive in vitro and in vivo (Mulkey et al. 2004, Mulkey et al. 2007a, DePuy et al. 2011). These neurons have also recently been found to be chemosensitive after acute dissociation, albeit with a smaller response to pH (Wang et al. 2013).
The RTN projects throughout the respiratory network (Smith et al. 1989) including the Pre-BötC. Interestingly, the RTN receives synaptic input from many other putative chemoreceptors, including 5-HT neurons, peripheral chemoreceptors, and the NTS (Takakura et al. 2006). Therefore, it is possible that the response of RTN neurons to acidosis in vivo is enhanced by synaptic input from these other chemoreceptors. For example, when acidosis causes an increase in firing rate of 5-HT neurons, RTN neurons would respond to the increase in extracellular 5-HT (Mulkey et al. 2007a) at the same time they respond to the acidosis, with both responses being excitatory. It would be hard to differentiate how much of the response of RTN neurons was due to which mechanism. Thus, in addition to being chemosensitive themself, RTN neurons relay information to the respiratory network from multiple other chemoreceptors or are integrators of inputs from multiple sources of chemoreceptor afferents (Nattie et al. 2004, Forster et al. 2010). The very large response of RTN neurons to inhalation of CO2 in vivo (Mulkey et al, 2004) may be due in part to this summation of inputs from many different chemoreceptors.
On top of the complexity that has already been described, 5-HT may also amplify the effects of some chemoreceptors. 5-HT delivered into the lateral ventricles of Lmx1bf/f/p mice, can restore the blunted HCVR response back to normal (Hodges et al. 2008). The mechanism is unknown, but could be due to enhancement of synaptic input from peripheral chemoreceptors within the NTS or other sites, or amplification of chemosensitivity of non-5-HT neurons such as chemoreceptors in the RTN. In the latter case the response of pH sensitive ion channels in RTN neurons, which may include TASK-2 (Wang et al. 2013), might be enhanced by activation of 5-HT receptors.
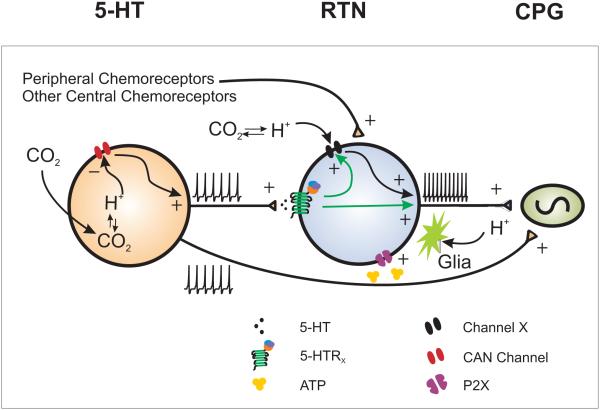
Figure 11 shows a model that includes the interaction between 5-HT neurons and RTN neurons, connections to other elements of the respiratory network, and ATP release from neighboring glia, which has been proposed to be important for the response of RTN neurons as well (Gourine et al. 2010).
Figure 11. 5-HT neuron and RTN chemosensitivity.
A model in which 5-HT neurons and RTN neurons are both chemosensitive to pH changes. In addition, 5-HT neurons enable or enhance RTN neuron chemosensitivity. 5-HT neurons are chemosensitive and stimulate RTN neurons. RTN neurons are chemosensitive and are also activated by chemosensitive glia and by projections from peripheral and other central chemoreceptors. There is evidence for each of these possibilities, as described in the text. Some are still hypotheses, and for others it is not known whether they occur under normal conditions in vivo.
Summary
The field of central respiratory chemoreception has rapidly evolved over the past few years. 5-HT neurons have now met all of the criteria needed for designation as CRCs, and each of the major arguments against their role as CRCs has been found to lack support. Neurons of the RTN and other sites also contain CRC candidates. In addition, the RTN may be a site for integration of signals from CRCs in other locations. In some regions glia may play a critical role as either sensors or modulators of the response to pH. There is considerable work remaining to be done in order to define the importance of each of the putative CRC sites. In doing so it will be necessary to recognize the strengths and weaknesses of every experimental approach that we as a field use, and interpret data cautiously unless the data are concordant across multiple preparations. These considerations will be important as we refine our understanding of these cell that are so critically important for life.
References
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. Journal of Physiology-London. 2000;529(1):215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. Journal of Neuroscience. 2002;22(14):6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Puri S, Miledi R, Panicker MM. Internalization and recycling of 5-HT2A receptors activated by serotonin and protein kinase C-mediated mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(22):14470–14475. doi: 10.1073/pnas.212517999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(12):4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell and Tissue Research. 2006;326(2):553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nature Neuroscience. 2002;5(5):401–2. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Brandes IF, Zuperku EJ, Stucke AG, Jakovcevic D, Hopp FA, Stuth EAE. Serotonergic modulation of inspiratory hypoglossal motoneurons in decerebrate dogs. Journal of Neurophysiology. 2006;95(6):3449–3459. doi: 10.1152/jn.00823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Hodges MR, Richerson GB. Contributions of chemosensitive serotonergic neurons to interactions between the sleep-wake cycle and respiratory control, Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Birkhäuser Verlag; Switzerland: 2008. [Google Scholar]
- Buchanan GF, Richerson GB. Role of chemoreceptors in mediating dyspnea. Respiratory Physiology & Neurobiology. 2009;167(1):9–19. doi: 10.1016/j.resp.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(37):16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulow K. Respiration and wakefulness in man. Acta physiologica Scandinavica. Supplementum. 1963;209:1–110. [PubMed] [Google Scholar]
- Cayetanot F, Gros F, Larnicol N. Postnatal changes in the respiratory response of the conscious rat to serotonin 2A/2C receptor activation are reflected in the developmental pattern of fos expression in the brainstem. Brain Research. 2002;942(1-2):51–57. doi: 10.1016/s0006-8993(02)02690-2. [DOI] [PubMed] [Google Scholar]
- Chaput Y, Lesieur P, Demontigny C. Effects of short-term serotonin depletion on the efficacy of serotonin neurotransmission - electrophysiological studies in the rat central nervous system. Synapse. 1990;6(4):328–337. doi: 10.1002/syn.890060404. [DOI] [PubMed] [Google Scholar]
- Conrad SC, Nichols NL, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitivity in neurons from the nucleus tractus solitarii (NTS) of neonatal rats. Respiratory Physiology & Neurobiology. 2009;166(1):4–12. doi: 10.1016/j.resp.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respiratory Physiology & Neurobiology. 2009;168(1-2):49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Commons KG, Smith JC, Harris MB, Richerson GB. Dual effects of 5-HT1a receptor activation on breathing in neonatal mice. Journal of Neuroscience. 2013. in press. [DOI] [PMC free article] [PubMed]
- Cummings KJ, Frappell PB. Characterizing the breath-to-breath hypercapnic ventilatory response in neonatal rats. Faseb Journal. 2008;22 [Google Scholar]
- Dahan A, Teppema LJ. Influence of anaesthesia and analgesia on the control of breathing. British Journal of Anaesthesia. 2003;91(1):40–49. doi: 10.1093/bja/aeg150. [DOI] [PubMed] [Google Scholar]
- Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. Journal of Applied Physiology. 2006 doi: 10.1152/japplphysiol.00378.2006. [DOI] [PubMed] [Google Scholar]
- Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon-dioxide does not require chemical synaptic input. Neuroscience. 1990;36(1):207–216. doi: 10.1016/0306-4522(90)90363-9. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Richerson GB, Getting PA. Thyrotropin-Releasing-Hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229(4708):67–69. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- DePuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of Breathing by Raphe Obscurus Serotonergic Neurons in Mice. Journal of Neuroscience. 2011;31(6):1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon GH, Waldrop TG. In vitro responses of caudal hypothalamic neurons to hypoxia and hypercapnia. Neuroscience. 1992;51(4):941–950. doi: 10.1016/0306-4522(92)90531-6. [DOI] [PubMed] [Google Scholar]
- Doi A, Ramirez J-M. State-Dependent Interactions between Excitatory Neuromodulators in the Neuronal Control of Breathing. Journal of Neuroscience. 2010;30(24):8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fencl V. Distribution of H+ and HCO3− in cerebral fluids, Ion Homeostasis of the Brain. The Regulation of Hydrogen and Potassium Ion Concentrations in Cerebral Intra- and Extracellular Fluids. 1971:175–185. [Google Scholar]
- Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. American Journal of Respiratory and Critical Care Medicine. 2003;167(4):563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- Fiske B. Putative chemoreceptors get close to arteries. Nature Neuroscience. 2002;5(5):396–396. [Google Scholar]
- Forster HV, Smith CA. Contributions of central and peripheral chemoreceptors to the ventilatory response to CO2/H+ Journal of Applied Physiology. 2010;108(4):989–994. doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respiration Physiology. 2000;121(2-3):135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes Control Breathing Through pH-Dependent Release of ATP. Science. 2010;329(5991):571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Experimental Physiology. 2005;90(3):247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. Journal of Physiology-London. 2008;586(8):2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Central Respiratory Chemoreception. Journal of Comparative Neurology. 2010;518(19):3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare GMT, Kavanagh BP, Mazer CD, Hum KM, Kim SY, Coackley C, Barr A, Baker AJ. Hypercapnia increases cerebral tissue oxygen tension in anesthetized rats. Canadian Journal of Anaesthesia-Journal Canadien D Anesthesie. 2003;50(10):1061–1068. doi: 10.1007/BF03018375. [DOI] [PubMed] [Google Scholar]
- Hedner J, Hedner T, Wessberg P, Lundberg D, Jonason J. Effects of TRH and TRH analogs on the central regulation of breathing in the rat. Acta Physiologica Scandinavica. 1983;117(3):427–437. doi: 10.1111/j.1748-1716.1983.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Hirshman CA, McCullough RE, Cohen PJ, Weil JV. Depression of hypoxic ventilatory response by halothane, enflurane and isoflurane in dogs. British Journal of Anaesthesia. 1977;49(10):957–963. doi: 10.1093/bja/49.10.957. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008a;164(1-2):222–32. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons. Respir Physiol Neurobiol. 2008b;164(3):350–7. doi: 10.1016/j.resp.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. Journal of Applied Physiology. 2010;108(5):1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen Z-F, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. Journal of Neuroscience. 2008;28(10):2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29(33):10341–9. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Current Opinion in Neurobiology. 1997;7(6):820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Johansen SL, Iceman KE, Richerson GB, Harris MB. Society for Neuroscience. New Orleans, LA: 2012. Influence of isoflurane on CO2 sensitive and insensitive raphé neurons. 897.08. [Google Scholar]
- Jouvet M. Sleep and serotonin: An unfinished story. Neuropsychopharmacology. 1999;21(2):S24–S27. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK, Levitan IB. Neuromodulation: The Biochemical Control of Neuronal Excitability. Oxford University Press, Inc.; 200 Madison Avenue, New York, New York 10016: 1987. What is Neuromodulation? pp. 1–17. [Google Scholar]
- Kanamaru M, Homma I. Effects of CO2 inhalation on 5-HT release in the dorso-medial medulla oblongata in infant. Neuroscience Research. 2007;58:S162–S162. [Google Scholar]
- Kannurpatti SS, Biswal BB, Hudetz AG. Baseline physiological state and the fMRI-BOLD signal response to apnea in anesthetized rats. Nmr in Biomedicine. 2003;16(5):261–268. doi: 10.1002/nbm.842. [DOI] [PubMed] [Google Scholar]
- Bach Karen B. Hypoxia-induced LTF of respiratory activity is 5-HT dependent. Respiration Physiology. 1996;104 doi: 10.1016/0034-5687(96)00017-5. G. S. M. [DOI] [PubMed] [Google Scholar]
- Katsura K, Asplund B, Ekholm A, Siesjo BK. Extracellular and intracellular pH in the brain during ischemia, related to tissue lactate content in normocapnic and hypercapnic rats. European Journal of Neuroscience. 1992a;4(2):166–176. doi: 10.1111/j.1460-9568.1992.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Katsura K, Ekholm A, Siesjo BK. Tissue PCO2 in brain ischemia related to lactate content in normocapnic and hypercapnic rats. Journal of Cerebral Blood Flow and Metabolism. 1992b;12(2):270–280. doi: 10.1038/jcbfm.1992.37. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1999;277(3):R658–R666. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Kinney GA, Spain WJ. Synaptically evoked GABA transporter currents in neocortical glia. Journal of Neurophysiology. 2002;88(6):2899–2908. doi: 10.1152/jn.00037.2002. [DOI] [PubMed] [Google Scholar]
- Koo KW, Sax DS, Snider GL. Arterial blood gases and pH during sleep in chronic obstructive pulmonary disease. American Journal of Medicine. 1975;58(5):663–670. doi: 10.1016/0002-9343(75)90502-1. [DOI] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal Motoneurons in the decerebrate cat. Neuroscience Letters. 1992;139(2):243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Lalley PM, Benacka R, Bischoff AM, Richter DW. Nucleus raphe obscurus evokes 5-HT-1A receptor-mediated modulation of respiratory neurons. Brain Research. 1997;747(1):156–159. doi: 10.1016/s0006-8993(96)01233-4. [DOI] [PubMed] [Google Scholar]
- Lambertsen C. Chemical Control of Respiration At Rest. 14th Mosby Company; St. Louis: 1980. [Google Scholar]
- Lovick TA. The medullary raphe nuclei: A system for integration and gain control in autonomic and somatomotor responsiveness? Experimental Physiology. 1997;82(1):31–41. doi: 10.1113/expphysiol.1997.sp004013. [DOI] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301(5630):226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- Massey CA, Wu Y, Richerson GB. Society for Neuroscience. New Orleans, LA: 2012. Isoflurane eliminates serotonin (5-HT) neuron chemosensitivity in vitro and markedly depresses the hypercapnic ventilatory response in vivo. 897.04. [Google Scholar]
- Massey CA, Wu Y, Zaykin A, Wang W, Hodges MR, Wylie CJ, Deneris ES, Richerson GB. A novel pH-sensitive calcium-activated nonselective cation current is responsible for medullary raphe 5-HT neuron chemosensitivity. Society for Neuroscience. 2013;39 [Google Scholar]
- McGinty DJ, Harper RM. Dorsal raphe neurons - Depression of firing during sleep in cats. Brain Research. 1976;101(3):569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- Messier ML, Li AH, Nattie EE. Inhibition of medullary raphe serotonergic neurons has age-dependent effects on the CO2 response in newborn piglets. Journal of Applied Physiology. 2004;96(5):1909–1919. doi: 10.1152/japplphysiol.00805.2003. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB. Physiological and genomic consequences of intermittent hypoxia: Invited review: Intermittent hypoxia and respiratory plasticity. Journal of Applied Physiology. 2001;90(6):2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Smith CA. Effects of p-chlorophenylalanine on ventilatory control in goats. Journal of Applied Physiology. 1983 doi: 10.1152/jappl.1983.54.1.277. H., V. E. [DOI] [PubMed] [Google Scholar]
- Monckton JE, McCormick DA. Neuromodulatory role of serotonin in the ferret thalamus. Journal of Neurophysiology. 2002;87(4):2124–2136. doi: 10.1152/jn.00650.2001. [DOI] [PubMed] [Google Scholar]
- Morton MJ, O'Connell AD, Sivaprasadarao A, Hunter M. Determinants of pH sensing in the two-pore domain K+ channels TASK-1 and-2. Pflugers Archiv-European Journal of Physiology. 2003;445(5):577–583. doi: 10.1007/s00424-002-0901-2. [DOI] [PubMed] [Google Scholar]
- Mueller RA, Towle AC, Breese GR. Supersensitivity to the respiratory stimulatory effect of TRH in 5,7-dihydroxytryptamine-treated rats. Brain Research. 1984;298(2):370–373. doi: 10.1016/0006-8993(84)91440-9. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007a;27(51):14128–38. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nature Neuroscience. 2004;7(12):1360–9. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. Journal of Neuroscience. 2007b;27(51):14049–58. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EELA, Richerson George B, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. The Journal of Physiology. 2004;(556):235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Mulkey DK, Wilkinson KA, Powell FL, Dean JB, Putnam RW. Characterization of the chemosensitive response of individual solitary complex neurons from adult rats. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2009;296(3):R763–R773. doi: 10.1152/ajpregu.90769.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nink M, Krause U, Lehnert H, Heuberger W, Huber I, Schulz R, Hommel G, Beyer J. Thyrotropin-Releasing-Hormone has stimulatory effects on ventilation in humans. Acta Physiologica Scandinavica. 1991;141(3):309–318. doi: 10.1111/j.1748-1716.1991.tb09086.x. [DOI] [PubMed] [Google Scholar]
- Olson EB, Dempsey JA, McCrimmon DR. Serotonin and the Control of Ventilation in Awake Rats. J. Clin. Invest. 1979;64 doi: 10.1172/JCI109510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nature Neuroscience. 1999;2(5):422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. Journal of Neuroscience. 2002;22(24):11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti EM, Berniker AV, Kereshi B, Cafaro C, Kelly ML, Niblock MM, Gao HG, Kinney HC, Li A, Nattie EE. Ventilatory response to hypercapnia and hypoxia after extensive lesion of medullary serotonergic neurons in newborn conscious piglets. Journal of Applied Physiology. 2006;101(4):1177–1188. doi: 10.1152/japplphysiol.00376.2006. [DOI] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience. 1997;77(3):723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29(12):3720–37. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Liu GX, Muller RP, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel - An extracellular histidine as pH sensor. Journal of Biological Chemistry. 2000;275(22):16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333(6042):637–42. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader TA, Gauthier P. Catecholamines and serotonin in the rat central nervous system after 6-OHDA, 5-7-DHT and P-CPA. Journal of Neural Transmission. 1984;59(3):207–227. doi: 10.1007/BF01250009. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. Journal of Neurophysiology. 1995;73(3):933–944. doi: 10.1152/jn.1995.73.3.933. [DOI] [PubMed] [Google Scholar]
- Richerson GB. pH effects on respiratory neurons. In: Kaila K, Ransom BR, editors. pH and Brain Function. John Wiley & Sons; New York, NY: 1998. [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nature Reviews Neuroscience. 2004;5(6):449–61. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Experimental Physiology. 2005;90(3):259–66. doi: 10.1113/expphysiol.2005.029843. discussion 266-9. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang WG, Tiwari J, Bradley SR. Chemo sensitivity of serotonergic neurons in the rostral ventral medulla. Respiration Physiology. 2001;129(1-2):175–189. doi: 10.1016/s0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wu Y, Hodges MR, Wang W, Zaykin A, Deneris ES. Acidosis inhibits a calcium-activated non-selective cation current in mature serotonergic neurons. 2009. J., W. C. translated by Nara, Japan.
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends in molecular medicine. 2003;9(12):542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Ferreira M, Wasserman AM, Taveira-Dasilva AM, Gillis RA. Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine(1A) receptors. Journal of Pharmacology and Experimental Therapeutics. 2000;292(2):704–713. [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Serra A, Brozoski D, Hedin N, Franciosi R, Forster HV. Mortality after carotid body denervation in rats. Journal of Applied Physiology. 2001;91(3):1298–1306. doi: 10.1152/jappl.2001.91.3.1298. [DOI] [PubMed] [Google Scholar]
- Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6(11):1139–40. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Lei QB, Talley EM, Lynch C, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. Journal of Neuroscience. 2000;20(17):6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. Journal of Comparative Neurology. 1989;281(1):69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Smith CA, Rodman JR, Chenuel BJA, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. Journal of Applied Physiology. 2006;100(1):13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- Somjen GG, Tombaugh GC. pH modulation of neuronal excitability and central nervous system functions. In: Kaila K, Ransom BR, editors. pH and Brain Function. Wiley-Liss; New York: 1998. [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical Oscillations in the Sleeping and Aroused Brain. Science. 1993;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respiration Physiology. 2001;127(2-3):135–155. doi: 10.1016/s0034-5687(01)00242-0. [DOI] [PubMed] [Google Scholar]
- Takakura ACT, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. Journal of Physiology-London. 2006;572(2):503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei QB, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. Journal of Neuroscience. 2001;21(19):7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, Tkatch T, Metz AE, Martina M. Differential expression of TASK channels between horizontal interneurons and pyramidal cells of rat hippocampus. Journal of Neuroscience. 2005;25(40):9162–9170. doi: 10.1523/JNEUROSCI.2454-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566(Pt 2):543–57. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit-activity in freely moving cats-correlaton with level of behavioral arousal. Brain Research. 1979;163(1):135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. Journal of Neuroscience. 1995;15(7):5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience. 1997;79(1):161–169. doi: 10.1016/s0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- Vukicevic M, Kellenberger S. Modulatory effects of acid-sensing ion channels on action potential generation in hippocampal neurons. American Journal of Physiology-Cell Physiology. 2004;287(3):C682–C690. doi: 10.1152/ajpcell.00127.2004. [DOI] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience. 1999;90(3):1001–1011. doi: 10.1016/s0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]
- Wang WA, Richerson GB. Chemosensitivity of non-respiratory rat CNS neurons in tissue culture. Brain Research. 2000;860(1-2):119–129. doi: 10.1016/s0006-8993(00)02033-3. [DOI] [PubMed] [Google Scholar]
- Wang WG, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pH(O) and P-CO2. Journal of Physiology-London. 2002;540(3):951–970. doi: 10.1113/jphysiol.2001.013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WG, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. Journal of Physiology-London. 1998;511(2):433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WG, Tiwari JK, Bradley SR, Zaykin AV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. Journal of Neurophysiology. 2001;85(5):2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bevengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Barhanin J, Bayliss DA. TASK-2 Channels Contribute to pH Sensitivity of Retrotrapezoid Nucleus Chemoreceptor Neurons. Journal of Neuroscience. 2013;33(41):16033–16044. doi: 10.1523/JNEUROSCI.2451-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH-and halothane-sensitive K+ conductance. Journal of Neuroscience. 2002;22(4):1256–1265. doi: 10.1523/JNEUROSCI.22-04-01256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Hodges MR, Richerson GB. Society for Neuroscience. Washington, DC: 2008. Stimulation by hypercapnic acidosis in mouse 5-HT neurons is enhanced by age and increased temperature. 383.9. [Google Scholar]
- Wu Y, Wang W, Diez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56(5):851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Lagercrantz H, Voneuler C. Effects of substance P and TRH on ventilation and pattern of breathing in newborn rabbits. Acta Physiologica Scandinavica. 1981;113(4):541–543. doi: 10.1111/j.1748-1716.1981.tb06935.x. [DOI] [PubMed] [Google Scholar]
- Zhao Z-Q, Scott M, Chiechio S, Wang J-S, Renner KJ, Gereau RW, Johnson RL, Deneris ES, Chen Z-F. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. Journal of Neuroscience. 2006;26(49):12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]