Abstract
Background: X-linked adrenoleukodystrophy (X-ALD) is a disorder caused by mutations in the ABCD1 gene. The commonest phenotype of X-ALD is adrenomyeloneuropathy (AMN), which is characterised by involvement of the spinal cord and peripheral nerves. The aim of this study was to evaluate bladder and bowel symptoms in men with AMN and female X-ALD carriers.
Methods: In this cross-sectional study, patients with confirmed mutation of the ABCD1 gene attending a tertiary care service were approached about bladder and bowel complaints and completed the Urinary Symptom Profile (USP), Qualiveen Short Form (SF-Qualiveen), International Prostate Symptom Score (IPSS) and Neurogenic Bowel Dysfunction (NBD) questionnaires. Neurological disability was assessed using the Expanded Disability Status Scale (EDSS).
Results: Forty-eight patients participated, 19 males (mean EDSS score (n = 16) 5.0 (95% CI ± 1.03)) and 29 females (mean EDSS score (n = 25) 3.2 (95% CI ± 0.98)). Overactive bladder (OAB) symptoms were reported in both males (100%, n = 19) and females (86.2%, n = 25). There was no significant gender difference in severity of OAB symptoms (P = 0.35) and impact on quality of life (P = 0.13). Furthermore, there was no significant difference in OAB severity when symptoms were compared between female carriers and a cohort of women (n = 17) with spinal cord damage due to multiple sclerosis (P = 0.27). Twenty-one percent (n = 4) of males and 10% (n = 3) of females had moderate to severe bowel dysfunction.
Conclusions: Bladder and bowel complaints are common in both men with AMN and female carriers. They have a significant impact on the quality of life yet are under-reported and under-treated. Though having an X-linked pattern of inheritance, female carriers may experience overactive bladder symptoms which are as severe as in male patients and are likely to be neurological in origin.
Introduction
X-linked adrenoleukodystrophy (ALD) (OMIM #300100) is a disorder caused by mutations in the ABCD1 (ATP-binding cassette, subfamily D, member 1) gene which encodes a peroxisomal membrane protein. This results in an accumulation of saturated very long-chain fatty acids (VLCFAs) in plasma and tissues as a consequence of impaired peroxisomal β-oxidation. The disorder primarily affects the adrenal cortex and the central nervous system (Moser et al 2007). There are two main phenotypes of this X-linked condition in males: adrenomyeloneuropathy (AMN), which is the most frequent phenotype, and cerebral ALD. In contrast to cerebral ALD, which is characterised by severe inflammatory demyelination within the brain and typically presents in young boys with rapidly progressive neurological disability, AMN usually develops in the third or fourth decade as a syndrome of slowly progressive spastic paraparesis with sensory disturbances and results from noninflammatory axonopathy involving the ascending and descending tracts of the spinal cord (Moser et al 2007; Kemp et al 2012). AMN has an X-linked pattern of inheritance and typically affects males. Female X-ALD carriers are either asymptomatic or may develop a milder, more slowly progressive neurological disability (O'Neill et al 1984; Jangouk et al 2012; Lourenco et al 2012).
Bladder and bowel dysfunction has been reported in men with AMN in case reports or short series (Griffin et al 1977; O'Neill et al 1981; Tezuka et al 1991; Walther and Cutler 1997; Sakakibara et al 1998; Silveri et al 2004). To some extent, urinary and faecal incontinence have recently been reported in female X-ALD carriers (Engelen et al 2014) but have not been evaluated in greater detail using validated questionnaires. The early identification of the carrier status because of better availability of screening facilities for family members has uncovered a wide spectrum of symptoms and signs amongst female carriers (Jangouk et al 2012). The objective of this study was therefore to evaluate and compare the prevalence, severity and impact on health-related quality of life with regard to bladder and bowel symptoms in a cohort of men and women carrying the ABCD1 mutation.
Methods
This was a cross-sectional study of male patients with AMN and female X-ALD carriers attending a dedicated unit for inherited metabolic diseases at a tertiary-level neurology centre. Of 77 patients (32 males, 45 females) attending the unit, 76 were contacted by telephone. One male patient developed cerebral ALD and was therefore not included (Fig. 1). In this service evaluation, patients agreeing to take part were asked about the extent and duration of their neurological symptoms, as well as bladder and bowel complaints.
Fig. 1.
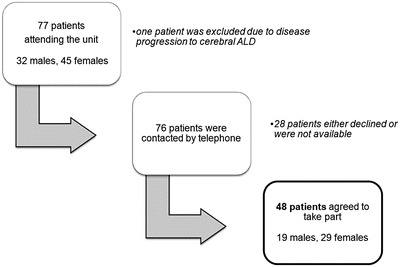
Flow chart illustrating the recruitment process of patients with AMN attending a tertiary unit for inherited metabolic diseases
Four validated self-administered questionnaires were sent by post, including the Urinary Symptom Profile (USP), International Prostate Symptom Score (IPSS), Qualiveen Short Form (SF-Qualiveen) and the Neurogenic Bowel Dysfunction (NBD) score. These were intended to evaluate specific domains of bladder and bowel complaints and the effects these had on quality of life. The USP questionnaire provides a comprehensive evaluation of urinary symptoms and their severity in males and females, assessing three domains: overactive bladder (OAB) (score range 0 to 21), stress urinary incontinence (SUI) (score range 0–9) and low stream (LS) (score range 0–9). A higher score indicates greater severity (Haab et al 2008). The IPSS questionnaire is used to assess bladder symptoms and provides an indicator of severity (Barry et al 1992). Even though the questionnaire was initially developed to assess voiding symptoms in men with benign prostatic hyperplasia (BPH), it is neither prostate nor gender specific (Chai et al 1993; Chancellor and Rivas 1993; Lepor and Machi 1993). The SF-Qualiveen questionnaire is a health-related quality of life questionnaire (HRQoL) for urinary disorders. The questionnaire is composed of 8 items distributed in four domains: “bother with limitations” (two items), “frequency of limitations” (two items), “fears” (two items) and “feelings” (two items). Patients are asked to recall their experience and respond to each question on a 5-point scale (ranging from 0 indicating no impact on HRQoL to 4 indicating high impact on HRQoL). Each Qualiveen domain score is calculated as an average of the scores for the items in each domain (Bonniaud et al 2008). The Neurogenic Bowel Dysfunction (NBD) questionnaire covers bowel complaints including both constipation and faecal incontinence, weighing each symptom of NBD according to impact on the quality of life, and provides a symptom-based score (maximum score 47) (Krogh et al 2006). Completed questionnaires were sent back in self-stamped envelopes.
Additionally, overactive bladder (OAB) scores from the USP questionnaire were compared to the scores from a cohort of women with another disorder affecting the spinal cord, multiple sclerosis (MS) (n = 17, mean age 46.5 years [range 24–65]), who had completed the questionnaire separately.
Neurological disability was assessed from the medical records using the Expanded Disability Status Scale (EDSS) (Kurtzke 1983).
Categorical data were compared using chi-square tests or Fisher’s exact tests depending on the frequency. Numerical data was examined and compared between groups using either an independent t-test or Mann–Whitney U test depending on the distribution. The correlation between EDSS and OAB scores was measured using Pearson’s correlation coefficient (0 = no agreement, 0.01–0.20 = slight, 0.21–0.40 = fair, 0.41–0.60 = moderate, 0.61–0.80 = substantial and 0.81–1 almost perfect agreement). All analyses were performed using Stata 11.2, StataCorp LP, College Station, Texas. Differences were considered statistically significant at P < 0.05. For the individual comparisons of the SF-Qualiveen scores, Bonferroni correction was applied considering a P-value <0.01 to be significant.
Results
Forty-eight patients (19 males, mean age 47.5 years [range 21–68], and 29 females, mean age 46.8 years [range 20–69]) completed the questionnaires, giving an overall response rate of 63.2%. The mean EDSS score (n = 41) was 3.9 (95% CI ± 0.77): 5.0 (95% CI ± 1.03) for males (n = 16) and 3.2 (95% CI ± 0.98) for females (n = 25).
Pattern of Bladder Symptoms and Severity
Overactive bladder (OAB) symptoms were reported in both males (100%, n = 19) and females (86.2%, n = 25) (median duration 4 years [IQR: 3.5–8 years] in males and 2.5 years [IQR: 0–8 years] in females) according to the Urinary Symptom Profile (USP) questionnaire. Less frequently, patients reported stress urinary incontinence or low stream. The pattern of bladder symptoms and scores on the USP questionnaire are presented in Table 1A and 1B, respectively. Notably, all males and 17 (58.6%) females with overactive bladder symptoms also reported problems with mobility (median duration 9 years [IQR: 5.5–16 years] in males and 1 year [IQR: 0–9 years] in females). There is a fair correlation (r = 0.4044 [22 females, 15 males]) between OAB score and EDSS.
Table 1.
Pattern of bladder symptoms and scores in patients with AMN as assessed by the USP questionnaire
| USP questionnaire domain | A | B | ||
|---|---|---|---|---|
| Number of patients with scores ≥ 1 | Median score and IQR | |||
| Male (n = 19) | Female (n = 29) | Male (n = 19) | Female (n = 29) | |
| Overactive bladder (OAB) | 19 (100%) | 25 (86.2%) | 9 (5–14) | 8 (3–10) |
| Stress urinary incontinence (SUI) | 8 (42.1%) | 19 (65.5%) | 0 (0–3) | 2 (0–4) |
| Low stream (LS) | 16 (84.2%) | 16 (55.2%) | 2 (1–3) | 2 (0–2) |
Possible score range for USP domains: OAB (0–21), SUI (0–9), LS (0–9)
USP Urinary Symptom Profile, IQR interquartile range
When the OAB subscores were compared between males and females, there was no significant difference between the groups (P = 0.35) (Fig. 2). The OAB subscores in the female cohort were compared with scores in a cohort of women with spinal cord damage due to multiple sclerosis (MS) (n = 17), and no significant difference was noted between the two groups (P = 0.27) (Fig. 2).
Fig. 2.
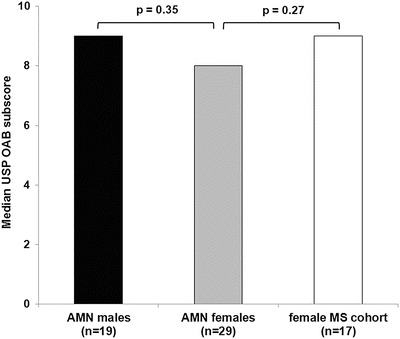
Comparison of median OAB scores between different cohorts. A P-value <0.05 is considered to be statistically significant
The mean score on the IPSS for males was 16.3 (95% CI ± 4.8) and 9.1 (95% CI ± 2.7) for females. The severity of bladder symptoms, as determined by the IPSS score, is shown in Fig. 3. Eighty-four percent (n = 16) of males and 55% (n = 16) of females reported moderate or severe bladder symptoms of which 6 male (32%) and 4 female patients (14%) had bladder symptoms graded as severe. Despite this, only 42.1% (n = 8) of male and 24.1% (n = 7) of female patients were receiving treatment for bladder symptoms at the time of the study. There is a moderate correlation (r = 0.4094 [22 females, 15 males]) between IPSS and EDSS.
Fig. 3.
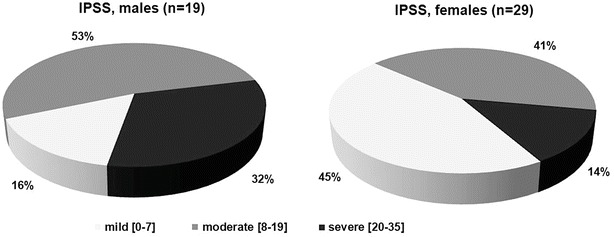
Severity of bladder symptoms in patients with AMN, graded mild [0–7], moderate [8–19] or severe [20–35] according to the IPSS
Impact of Bladder Symptoms on Health-Related Quality of Life
The impact on health-related quality (HRQoL) of life as assessed by the SF-Qualiveen questionnaire is presented in Table 2. The overall mean score for males was 1.9 (95% CI ± 0.5), whilst it was 1.4 (95% CI ± 0.4) for the female cohort, suggesting an impact on HRQoL but no significant difference between groups (P = 0.13).
Table 2.
Scores on the SF-Qualiveen questionnaire, comparing between males and females
| SF-Qualiveen domain | Mean score (95% CI) | ||
|---|---|---|---|
| Male (n = 19) | Female (n = 29) | P-value | |
| Bother with limitations | 2.1 (1.5–2.7) | 1.3 (0.9–1.7) | 0.06 |
| Frequency of limitations | 2.1 (1.5–2.7) | 1.5 (1.1–1.9) | 0.10 |
| Fears | 1.8 (1.2–2.4) | 1.5 (1.1–1.9) | 0.42 |
| Feelings | 1.7 (1.1–2.3) | 1.2 (0.8–1.6) | 0.17 |
| Overall score | 1.9 (1.4–2.4) | 1.4 (1.0–1.8) | 0.13 |
For the individual comparisons, Bonferroni correction was applied considering a P-value <0.01 to be statistically significant
CI confidence interval
Pattern of Bowel Complaints
During the telephone interview, 11 (57.9%) male and 10 (34.5%) female patients reported bowel complaints, the predominant symptom being constipation. Most patients, 13 (68.4%) males and 22 (75.9%) females, had none or “very minor” bowel symptoms on the NBD questionnaire. However, four males (21%) and three females (10%) reported moderate to severe bowel symptoms (Fig. 4).
Fig. 4.
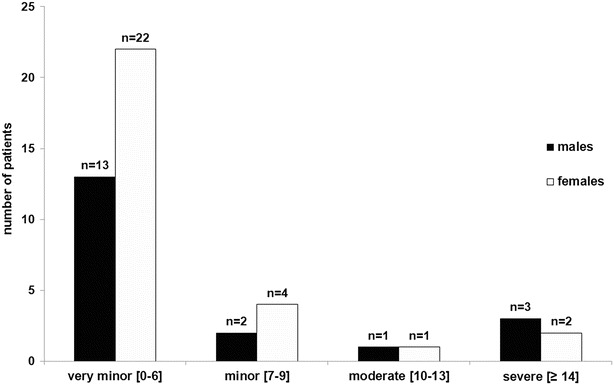
Bowel dysfunction in males (n = 19) and females (n = 29) with AMN. Patients categorised into standardised severity grades (very minor [0–6], minor [7–9], moderate [10–13] and severe [≥14]) according to their NBD score
Discussion
Lower urinary tract dysfunction has been described in the ALD spectrum of disorders. Patients most often report overactive bladder symptoms, and urodynamic testing demonstrates detrusor overactivity (Griffin et al 1977; O'Neill et al 1981; Tezuka et al 1991; Walther and Cutler 1997; Sakakibara et al 1998; Shinbo et al 2001; Silveri et al 2004). Adrenomyeloneuropathy (AMN) represents a more slowly progressive form of the condition and presents predominantly as a spinal cord syndrome with spastic paraparesis and sensory disturbances in men. There have been a few case reports of bladder dysfunction in AMN, and urodynamics demonstrate the presence of detrusor overactivity and detrusor sphincter dyssynergia (Griffin et al 1977; Tezuka et al 1991; Walther and Cutler 1997; Sakakibara et al 1998), a pattern distinctive of spinal cord dysfunction.
Through a series of standardised validated questionnaires, we have established that lower urinary tract symptoms are common in male patients with AMN and in X-ALD carriers. Urinary urgency, frequency, nocturia and incontinence, collectively known as overactive bladder symptoms, were most commonly reported, and 67% of patients reported symptoms to be of moderate or severe grade and having a significant impact on the quality of life. Despite this high figure, only a third of patients were receiving treatment for managing bladder symptoms at the time of the study.
Adrenoleukodystrophy, respectively, adrenomyeloneuropathy, has an X-linked pattern of inheritance and therefore is classically a disorder affecting only males, whereas females carrying only one copy of the mutation should not express the phenotype. However, it is now recognised that female carriers of the mutation also manifest with symptoms (O'Neill et al 1984; Jangouk et al 2012; Engelen et al 2014). Females may develop a range of neurological deficits including hyperreflexia, impaired vibration sense and also spastic paraparesis, deficits reported also in our cohort, and may erroneously be diagnosed initially as having some other neurological condition such as multiple sclerosis before being found to be carrying the ABCD1 gene mutation (Dooley and Wright 1985; Stockler et al 1993; Krenn et al 2001; Di Filippo et al 2011).
To our knowledge, bladder and bowel symptoms have never been evaluated in greater detail using validated questionnaires in female carriers of the ABCD1 gene mutation, and the results of this study suggest that these symptoms are common. As in male counterparts, overactive bladder symptoms were most often reported. The results of previous studies evaluating urodynamic findings in patients with AMN (Griffin et al 1977; Tezuka et al 1991; Walther and Cutler 1997; Sakakibara et al 1998) suggest that these symptoms are due to detrusor overactivity. Overactive bladder symptoms are prevalent in the general population (Milsom et al 2001); however, there is reason to believe that symptoms were neurological in origin. Firstly, the severity of overactive bladder symptoms was similar between males and females, with comparable degrees of neurological disability between the two cohorts. The severity of bladder symptoms correlated with disability on the EDSS. Secondly, when symptoms were compared to that reported in a cohort of women with a known spinal cord disorder (MS), where lower urinary tract dysfunction is well described (Fingerman and Finkelstein 2000; Fowler et al 2009), overactive bladder scores were found to be not significantly different. Stress incontinence was reported less often and is likely to be multifactorial in AMN (Chaudhry et al 1996) though this was not further studied. Symptoms of bowel dysfunction were apparent in males and females; however, they were less common than bladder symptoms. Some patients did however experience severe symptoms, and these should therefore be specifically enquired about during the clinical evaluation, as effective treatments are available to address these (Preziosi and Emmanuel 2009).
Bladder dysfunction in the setting of X-linked adrenoleukodystrophy remains a poorly addressed problem. The routine workup of patients with progressive neurological disorders, involving a focussed history and non-invasive tests, is often sufficient to initiate treatment for the neurogenic bladder. The treatment options available for managing overactive bladder symptoms are many, including lifestyle modifications, antimuscarinic medications and intradetrusor injections of botulinum toxin (Fowler et al 2009; Panicker and Fowler 2010).
We present possibly the largest cohort of X-ALD patients reporting bladder and bowel symptoms and use validated questionnaires providing an accurate assessment of symptoms. The study was hospital based, and therefore the prevalence of bladder and bowel symptoms observed might not accurately reflect that of men and women with an ABCD1 mutation in general. Patients with minimal symptoms who might not report to a tertiary centre are likely to be underrepresented. Nevertheless, the study shows that bladder and bowel complaints are common in patients with X-ALD, notably in female carriers, and have a significant impact on the quality of life yet are under-reported and under-treated. It is therefore important to enquire about these symptoms, as they are eminently treatable with gratifying results. Though having an X-linked pattern of inheritance, female carriers may experience overactive bladder symptoms which are as severe as in male patients and are likely to be neurological in origin.
Acknowledgements
This project received a proportion of funding from the NIHR Biomedical Research Centre. Matthew D. Smith received a research bursary from the Wellcome Trust. K I Tudor is a recipient of the EFNS grant named “Department–Department Co-operation Programme”.
Take-Home Message (Synopsis)
Bladder and bowel dysfunction is common in both men and women with mutation of the ABCD1 gene for X-linked adrenoleukodystrophy, notably in female carriers, and has a significant impact on the quality of life yet are under-reported and under-treated.
Compliance with Ethical Guidelines
Conflict of Interest
Johann Hofereiter, Matthew D. Smith, Jai Seth, Katarina Ivana Tudor, Zoe Fox, Anton Emmanuel, Elaine Murphy, Robin H. Lachmann and Jalesh Panicker declare that they have no conflict of interest.
Ethics Approval
This was an evaluation of current care provided by a clinical service and, according to the guidance from the National Research Ethics Service of the United Kingdom, would fall under the remit of a service evaluation, which does not require ethics review.
Details of the Contributions of Individual Authors
Johann Hofereiter (first author) was involved in the conception and design of the study, collecting data, analysis and drafting and revising the manuscript.
Matthew D. Smith was involved in collecting data, analysis and drafting and revising the manuscript.
Jai Seth was involved in the design of the study, collecting data and revising the manuscript.
Zoe Fox was involved in data analysis and revising the manuscript.
Anton Emmanuel was involved in the design of the study and revising the manuscript.
Elaine Murphy was involved in the design of the study and revising the manuscript.
Robin H. Lachmann was involved in the design of the study and revising the manuscript.
Jalesh Panicker was involved in the conception and design of the study, monitoring data collection and revising the manuscript. He is the guarantor.
Footnotes
Competing interests: None declared
Contributor Information
Johann Hofereiter, Email: johann.hofereiter@med.uni-muenchen.de.
Collaborators: Johannes Zschocke
References
- Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The measurement committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- Bonniaud V, Bryant D, Parratte B, Guyatt G. Development and validation of the short form of a urinary quality of life questionnaire: SF-Qualiveen. J Urol. 2008;180:2592–2598. doi: 10.1016/j.juro.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Chai TC, Belville WD, McGuire EJ, Nyquist L. Specificity of the American Urological Association voiding symptom index: comparison of unselected and selected samples of both sexes. J Urol. 1993;150:1710–1713. doi: 10.1016/s0022-5347(17)35874-3. [DOI] [PubMed] [Google Scholar]
- Chancellor MB, Rivas DA. American Urological Association symptom index for women with voiding symptoms: lack of index specificity for benign prostate hyperplasia. J Urol. 1993;150:1706–1708. doi: 10.1016/s0022-5347(17)35872-x. [DOI] [PubMed] [Google Scholar]
- Chaudhry V, Moser HW, Cornblath DR. Nerve conduction studies in adrenomyeloneuropathy. J Neurol Neurosurg Psychiatry. 1996;61:181–185. doi: 10.1136/jnnp.61.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M, Luchetti E, Prontera P, et al. Heterozygous X-linked adrenoleukodystrophy-associated myelopathy mimicking primary progressive multiple sclerosis. J Neurol. 2011;258:323–324. doi: 10.1007/s00415-010-5726-x. [DOI] [PubMed] [Google Scholar]
- Dooley JM, Wright BA. Adrenoleukodystrophy mimicking multiple sclerosis. Can J Neurol Sci. 1985;12:73–74. doi: 10.1017/s0317167100046631. [DOI] [PubMed] [Google Scholar]
- Engelen M, BarbierM DIM, et al. X-linked adrenoleukodystrophy in women: a cross-sectional cohort study. Brain. 2014;137:693–706. doi: 10.1093/brain/awt361. [DOI] [PubMed] [Google Scholar]
- Fingerman JS, Finkelstein LH. The overactive bladder in multiple sclerosis. J Am Osteopath Assoc. 2000;100:S9–S12. [PubMed] [Google Scholar]
- Fowler CJ, Panicker JN, Drake M, et al. A UK consensus on the management of the bladder in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:470–477. doi: 10.1136/jnnp.2008.159178. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Goren E, Schaumburg H, Engel WK, Loriaux L. Adrenomyeloneuropathy: a probable variant of adrenoleukodystrophy. I. Clinical and endocrinologic aspects. Neurology. 1977;27:1107–1113. doi: 10.1212/WNL.27.12.1107. [DOI] [PubMed] [Google Scholar]
- Haab F, Richard F, Amarenco G, et al. Comprehensive evaluation of bladder and urethral dysfunction symptoms: development and psychometric validation of the urinary symptom profile (USP) questionnaire. Urology. 2008;71:646–656. doi: 10.1016/j.urology.2007.11.100. [DOI] [PubMed] [Google Scholar]
- Jangouk P, Zackowski KM, Naidu S, Raymond GV. Adrenoleukodystrophy in female heterozygotes: underrecognized and undertreated. Mol Genet Metab. 2012;105:180–185. doi: 10.1016/j.ymgme.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Kemp S, Berger J, Aubourg P. X-linked adrenoleukodystrophy: clinical, metabolic, genetic and pathophysiological aspects. Biochim Biophys Acta. 2012;1822:1465–1474. doi: 10.1016/j.bbadis.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Krenn M, Bonelli RM, Niederwieser G, Reisecker F, Koltringer P. Adrenoleukodystrophy mimicking multiple sclerosis. Nervenarzt. 2001;72:794–797. doi: 10.1007/s001150170037. [DOI] [PubMed] [Google Scholar]
- Krogh K, Christensen P, Sabroe S, Laurberg S. Neurogenic bowel dysfunction score. Spinal Cord. 2006;44:625–631. doi: 10.1038/sj.sc.3101887. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Lepor H, Machi G. Comparison of AUA symptom index in unselected males and females between fifty-five and seventy-nine years of age. Urology. 1993;42:36–40. doi: 10.1016/0090-4295(93)90332-5. [DOI] [PubMed] [Google Scholar]
- Lourenco CM, Simao GN, Santos AC, Marques W., Jr X-linked adrenoleukodystrophy in heterozygous female patients: women are not just carriers. Arq Neuropsiquiatr. 2012;70:487–491. doi: 10.1590/S0004-282X2012000700003. [DOI] [PubMed] [Google Scholar]
- Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–766. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- Moser HW, Mahmood A, Raymond GV. X-linked adrenoleukodystrophy. Nat Clin Pract Neurol. 2007;3:140–151. doi: 10.1038/ncpneuro0421. [DOI] [PubMed] [Google Scholar]
- O'Neill BP, Marmion LC, Feringa ER. The adrenoleukomyeloneuropathy complex: expression in four generations. Neurology. 1981;31:151–156. doi: 10.1212/WNL.31.2.151. [DOI] [PubMed] [Google Scholar]
- O'Neill BP, Moser HW, Saxena KM, Marmion LC. Adrenoleukodystrophy: clinical and biochemical manifestations in carriers. Neurology. 1984;34:798–801. doi: 10.1212/WNL.34.6.798. [DOI] [PubMed] [Google Scholar]
- Panicker JN, Fowler CJ. The bare essentials: uro-neurology. Pract Neurol. 2010;10:178–185. doi: 10.1136/jnnp.2010.213892. [DOI] [PubMed] [Google Scholar]
- Preziosi G, Emmanuel A. Neurogenic bowel dysfunction: pathophysiology, clinical manifestations and treatment. Expert Rev Gastroenterol Hepatol. 2009;3:417–423. doi: 10.1586/egh.09.31. [DOI] [PubMed] [Google Scholar]
- Sakakibara R, Hattori T, Fukutake T, Mori M, Yamanishi T, Yasuda K. Micturitional disturbance in a patient with adrenomyeloneuropathy (AMN) Neurourol Urodyn. 1998;17:207–212. doi: 10.1002/(SICI)1520-6777(1998)17:3<207::AID-NAU5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Shinbo H, Kageyama S, Hayami S, et al. Voiding dysfunction in a patient with adolescent adrenoleukodystrophy. Int J Urol. 2001;8:144–147. doi: 10.1046/j.1442-2042.2001.00271.x. [DOI] [PubMed] [Google Scholar]
- Silveri M, De Gennaro M, Gatti C, Bizzarri C, Mosiello G, Cappa M. Voiding dysfunction in x-linked adrenoleukodystrophy: symptom score and urodynamic findings. J Urol. 2004;171:2651–2653. doi: 10.1097/01.ju.0000110885.26017.b0. [DOI] [PubMed] [Google Scholar]
- Stockler S, Millner M, Molzer B, Ebner F, Korner E, Moser HW. Multiple sclerosis-like syndrome in a woman heterozygous for adrenoleukodystrophy. Eur Neurol. 1993;33:390–392. doi: 10.1159/000116978. [DOI] [PubMed] [Google Scholar]
- Tezuka H, Tachibana Y, Terashi A. A case of adrenomyeloneuropathy with marked spinal cord atrophy on magnetic resonance imaging. Rinsho Shinkeigaku. 1991;31:461–464. [PubMed] [Google Scholar]
- Walther MM, Cutler GB. Urodynamic abnormalities in two brothers with adrenomyeloneuropathy. World J Urol. 1997;15:262–265. doi: 10.1007/BF01367665. [DOI] [PubMed] [Google Scholar]


