Abstract
This is a descriptive analysis of a cohort of 59 Taiwanese patients with Fabry disease and either classical Fabry or cardiac variant IVS4+919G>A (IVS4) mutations from a disease registry, the Fabry Outcome Survey (FOS; sponsored by Shire). Most of our classical Fabry patients were symptomatic and were identified upon seeking medical advice at our clinics, whereas most of our IVS4 patients attended our clinics after newborn screening identified this mutation in their grandsons. The objective was to determine differences in cardiac manifestations between patients with classical Fabry or IVS4 mutations by comparing age at onset of selected cardiac symptoms. Data were extracted in August 2013 and analyzed retrospectively. Fifty-nine Taiwanese patients (median age at extract 60.7 years [range 15.0–86.9]; n = 36 [61%] male) with proven IVS4 (n = 41 [69%]) or classical Fabry mutations (n = 18 [31%]) had available data on cardiac symptoms. Of 55 (93%) patients with reported left ventricular hypertrophy (LVH), mean [SD] age (years) at first symptom was lower in classical Fabry males (30.0 [15.1]; n = 4) than classical Fabry females (49.6 [8.9]; n = 11; p < 0.05), but not in IVS4 females (57.4 [13.7]; n = 10) compared with IVS4 males (55.9 [11.3]; n = 30). Mean age at first LVH diagnosis was significantly lower in classical Fabry males versus IVS4 males (p < 0.05). No significant difference in age at onset of arrhythmia or conductive abnormality, chest pain, or palpitations or cardiac syncope was found between the groups. The most noteworthy finding of this study is the lack of a significant gender sex difference in age at onset of cardiac symptoms in IVS4 patients.
Introduction
Fabry disease is caused by the deficiency or absence of alpha-galactosidase A (α-Gal A) activity, leading to progressive deposition of glycosphingolipids, mainly globotriaosylceramide (Gb3), in the lysosomes of multiple tissues and organs. Originally thought to be less severe in females (Desnick et al. 2001), more recent evidence indicates that symptoms of this X-linked disorder can manifest as severely in females as in males (Mehta et al. 2004; Wilcox et al. 2008), although they generally occur later in life and show greater variation in severity among female patients (Deegan et al. 2006). The frequency of classic Fabry disease has been estimated as one in 40,000, and its symptoms typically manifest during childhood, including acroparesthesias, angiokeratoma, corneal opacities, and anhidrosis (Desnick et al. 2001; Ries et al. 2005). Atypical, late-onset phenotypes have been reported that lack these classic symptoms but instead present with cardiac (Nakao et al. 1995), renal (Nakao et al. 2003), or cerebrovascular disease (Brouns et al. 2010). The frequency of atypical Fabry disease is unknown, but it has been suggested to be more common than previously believed (Nakao et al. 1995). In Taiwan, our team first revealed a surprisingly high incidence (approximately one in 1,600 males) of a cardiac variant GLA splicing mutation, IVS4+919G>A, in our newborn population (Chong et al. 2008) and subsequently identified this mutation in a number of Taiwan Chinese adult patients with idiopathic hypertrophic cardiomyopathy (Lin et al. 2009, 2010, 2013). Thereafter, another newborn screening center in Taiwan also revealed a very similar incidence (one in 1,460 males) of this mutation in their study (Hwu et al. 2009). In addition to Taiwan, this mutation has also been found in Japan (Ishii et al. 2002), China, and in Han populations from Singapore, Malaysia, the Philippines, and Vietnam by our team (Niu, unpublished data).
Our previous study (Lin et al. 2010) showed that a high proportion of adults (>40 years of age) carrying the IVS4+919G>A mutation experienced microalbuminuria and retinal vessel tortuosity, but symptoms involving these organs were very mild and did not cause significant morbidity. However, a high frequency of severe cardiac symptoms causing significant morbidity was also found among these adults. More recently, DNA-based newborn screening for this mutation revealed a higher incidence (one in 875 males and one in 399 females) than our previous enzyme-based Fabry newborn screening in Taiwan (Chien et al. 2012).
Although the hotspot IVS4+919G>A mutation is now being observed with greater frequency, understanding of the natural course of cardiac variant Fabry disease with this specific mutation remains limited. The objective of this study was to determine differences in cardiac manifestations between patients with the IVS4 and classical Fabry mutations by comparing age at first manifestation of selected cardiac symptoms in Taiwanese patients with data recorded in the Fabry Outcome Survey (FOS). The FOS is an international registry, sponsored by Shire, for the long-term collection of data on the natural history of Fabry disease in patients who are either untreated or treated with agalsidase alfa enzyme replacement therapy (ERT).
Patients and Methods
Entry of data from Taiwanese patients into the FOS database began in July 2012. All Taiwanese patients with the IVS4+919G>A mutation (IVS4 patients) or classical mutations (classical Fabry patients; Table 1) are eligible for inclusion in FOS, whether or not they have received ERT with agalsidase alfa. Fabry disease diagnosis in all patients was confirmed by enzyme assay measuring α-Gal A activity (males) and/or α-Gal A gene mutation analysis (males and females). All of the procedures undertaken in this study were approved by the institution review board, and all patients gave written, informed consent prior to data entry.
Table 1.
Classical Fabry mutations
| Classical Fabry mutation |
|---|
| • c.274G>T(D92Y) |
| • c.319C.T(p.Q107X) |
| • c.394G>A(p.G132R) |
| • c.612G>A(p.W204X) |
| • c.901C>T(p.R301X) |
| • c.1034C>G(p.5345X) |
| • c.1066C>G(p.R356W) |
| • c.1081G>C(p.G361X) |
| • c.1087C>T(p.R363C) |
| • c.1095delT(p.Y365X) |
| • c.1194delA(p.E398DfsX6) |
| • c.1228A>G(p.T410A) |
Anonymous data for analysis are submitted electronically to the central FOS database. Each patient’s medical history is documented by a physician or nurse specialist, including the year of Fabry disease diagnosis, signs and symptoms of the disease, treatment, demographic details, and family history.
All measurements routinely performed in clinical practice are entered into the database. Echocardiographic data are collected in accordance with pre-specified guidelines contained within FOS. Measurements were performed according to the American Society of Echocardiography recommendations (Nagueh et al. 2009). Left ventricular parameters including diastolic interventricular septal thickness (IVSd), systolic and diastolic left ventricular internal diameter (LVIDd and LVIDs), and diastolic left ventricular posterior wall thickness (LVPWd) were measured by two-dimensional guided M-Mode echocardiography. Left ventricular mass (LVM) was calculated according to the formula published by the American Society of Echocardiography (Lang et al. 2005): LVM (g) = 0.8 × (1.04 × [(LVIDd) +(IVSd) + (LVPWd)]3 − [LVIDs]3) + 0.6. Left ventricular mass was normalized to height in meters2.7 (LVMI = LVM/height2.7). Left ventricular hypertrophy (LVH) is defined as left ventricular mass indexed to height2.7 (g/m2.7) of >51 g/m2.7 in males and >48 g/m2.7 in females (de Simone et al. 1992).
In this study, we examine the baseline data of cardiac manifestations in Taiwanese patients with IVS4+919G>A or classical Fabry mutations recorded in the FOS database. Age, sex, genetic mutation, LVMI, and age at first symptoms/signs, including arrhythmia or conductive abnormality, chest pain, left ventricular hypertrophy, and palpitations or cardiac syncope, were analyzed. Data for this study were extracted from the FOS database in August 2013. Data extraction and analysis in FOS are supported by Shire.
Statistical Analyses
Descriptive statistics were calculated, and statistical significance to compare age at onset of the selected cardiac symptoms by mutation group (IVS4 or classical Fabry) and sex was evaluated using the full interaction analysis of variance (ANOVA) model methodology. Fisher’s exact test was used to compare the prevalence of cardiac symptoms between the IVS4 and classical mutation groups. Statistical significance was set at 5%, and SAS version 9.2 software was used to perform the tests.
Results
By August 2013, from a total of 71 Taiwanese patients registered in the FOS database, there were 67 Taiwanese patients with proven IVS4+919G>A mutation or classical Fabry mutations. Fifty-nine had cardiac symptoms recorded and were included in this analysis (Fig. 1). The median age at the time of data extraction was 60.7 years (range 15.0–86.9). Two of the patients with classical Fabry mutations were younger than 18 years. Thirty-six patients (61%) were male, and 23 patients (39%) were female. A total of 41 patients (69%) had IVS4+919G>A mutations, which is twice as many as had classical Fabry mutations (18 patients, 31%). Of the IVS4 patients, there were more males (30 patients, 73%) than females (11 patients, 27%). The ratio of male to female patients with the IVS4+919G>A mutation was 2.73:1, compared with 1:2 with classical Fabry mutations (six male patients, 33%; 12 female patients, 67%). The demographic characteristics of the study population are shown in Table 2.
Fig. 1.
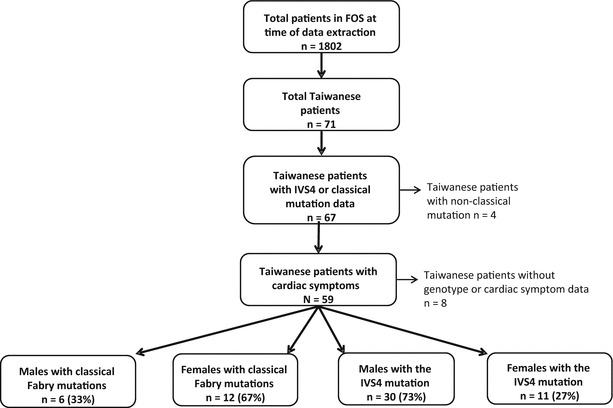
Flow diagram of patients included in this analysis
Table 2.
Demographic characteristics and sex and mutation status of Taiwanese patients in FOS with cardiac symptoms and IVS4 or classical Fabry mutations (n = 59)
| Characteristic | N = 59 |
|---|---|
| Median (range) age at extract, years | 60.7 (15.0–86.9) |
| Aged <18 years at extract, n (%) | 2 (3) |
| Male, n (%) | 36 (61) |
| Female, n (%) | 23 (39) |
| Residual α-Gal A activity, % of midpoint of normal range, mean (SD) | |
| IVS4 overall (n = 41) | 21.6 (21.0) |
| IVS4 male (n = 30) | 10.4 (4.5) |
| IVS4 female (n = 11) | 52.2 (17.4) |
| Classical Fabry overall (n = 16)a | 31.4 (25.0) |
| Classical male (n = 4)a | 2.9 (3.5) |
| Classical female (n = 12) | 41.0 (21.2) |
| Sex and Fabry mutation status, n (%) | |
| IVS4 overall | 41 (69)b |
| IVS4 male | 30 (73) |
| IVS4 female | 11 (27) |
| Classical overall | 18 (31)b |
| Classical male | 6 (33) |
| Classical female | 12 (67) |
a n = 2 missing
bPrevalence, IVS4 overall vs. classical overall; p < 0.05
The prevalence, mean, and median age at first cardiac symptoms in Taiwanese patients in FOS are shown in Table 3. Left ventricular hypertrophy was the most common sign of cardiac manifestation in both IVS4 and classical Fabry patients. Fifty-five (93%) patients, including 40 (97.6%) with the IVS4+919G>A mutation (100% in males and 90.9% in females) and 15 (83.3%) with classical Fabry mutations (66.7% in males and 91.7% in females), were found to have LVH. The mean [SD] age at first diagnosis of LVH was significantly lower in classical Fabry males (30 years [15.1]; n = 4) than in classical Fabry females (49.6 years [8.9]; n = 11; p < 0.05), but in the IVS4 patients there was no difference between IVS4 males (55.9 years [11.3]; n = 30) and IVS4 females (57.4 years [13.7]; n = 10). Also, a significantly lower age at first LVH diagnosis was found for classical Fabry males compared with IVS4 males (p < 0.05); however, no significant difference was found in mean age at first LVH diagnosis between classical Fabry females and IVS4 females. Furthermore, age at LVH diagnosis was significantly lower in classical Fabry males compared with classical Fabry females and IVS4 males and females combined (p < 0.01).
Table 3.
Prevalence (%) of cardiac symptoms and mean (SD) and median (range) age at onset in Taiwanese patients in FOS
| IVS4 mutation | Classical Fabry mutation | |||
|---|---|---|---|---|
| Age at first symptom | Male (N = 30) | Female (N = 11) | Male (N = 6) | Female (N = 12) |
| LVHa | n = 30 (100%) | n = 10 (90.9%) | n = 4 (66.7%) | n = 11 (91.7%) |
| Mean (SD) | 55.9 (11.3)b | 57.4 (13.7) | 30.0 (15.1)b,c | 49.6 (8.9)c |
| Median (range) | 58.9 (19.9–68.3) | 57.8 (34.7–86.0) | 28.1 (14.3–49.4) | 51.7 (32.6–59.7) |
| Chest pain | n = 9 (30%) | n = 5 (45.5%) | n = 1 (16.7%) | n = 8 (66.7%) |
| Mean (SD) | 48.6 (12.7) | 40.9 (10.8) | 43.5 | 47.8 (13.6) |
| Median (range) | 47.4 (27.1–66.6) | 43.2 (24.4–52.1) | 43.5 (43.5–43.5) | 48.8 (29.2–68.2) |
| Arrhythmia or conductive abnormality | n = 25 (83.3%)d | n = 6 (54.5%) | n = 0 (0%)d | n = 7 (58.3%) |
| Mean (SD) | 58.0 (7.3) | 63.3 (19.6) | – | 49.2 (11.5) |
| Median (range) | 60.3 (39.8–67.5) | 60.5 (37.1–86.0) | – | 51.2 (32.7–65.7) |
| Palpitations or syncope | n = 10 (33.3%) | n = 5 (45.5%) | n = 1 (16.7%) | n = 6 (50%) |
| Mean (SD) | 54.7 (10.2) | 42.4 (11.9) | 43.5 | 51.6 (13.2) |
| Median (range) | 58.8 (39.8–66.6) | 47.0 (24.4–54.8) | 43.5 (43.5–43.5) | 52.5 (29.2–68.2) |
aAge at first diagnosis of LVH
bAge, IVS4 male vs. classical Fabry male; p < 0.05
cAge, classical Fabry male vs. classical Fabry female; p < 0.05
dPrevalence, IVS4 male vs. classical Fabry male; p < 0.001
Regarding arrhythmia or conductive abnormality, none of our classical Fabry males had these signs; however, more than half of the IVS4 females (54.5%), classical Fabry females (58.3%), and most of the IVS4 males (83.3%) did. Interestingly, no significant difference in age at onset of arrhythmia or conductive abnormality was found between these three groups. Regarding chest pain, 30% of IVS4 males, 45.5% of IVS4 females, 16.7% of classical Fabry males, and 66.7% of classical Fabry females had this symptom. No significant difference in age at onset of this cardiac symptom was found among these four groups. Similarly, there was no significant difference between these four groups in age at onset of palpitations or cardiac syncope, which occurred in 33.3% IVS4 males, 45.5% IVS4 females, 16.7% classical Fabry males, and 50% classical Fabry females.
Discussion
The most noteworthy finding in this study is that no significant difference in age at onset of cardiac manifestations was found between IVS4 males and females. This is quite different to the situation in classical Fabry patients, where males show a trend toward a more severe clinical course and earlier disease onset age than females (Mehta et al. 2009). It is generally thought that cardiac manifestations of Fabry disease, such as arrhythmia, angina, and LVH, are caused by Gb3 accumulation in sinus nodes, the conduction system, vascular endothelium, and myocardiocytes. Therefore, the more severe the α-Gal A defect, the greater the accumulation of Gb3 and the more severe the clinical manifestations and earlier the disease onset will be. Fabry disease is believed to be an X-linked disease, where female patients usually have two X chromosomes containing two α-Gal A genes. After random X-chromosome inactivation, a heterozygote Fabry female becomes mosaic, with two cell populations, one of which expresses the normal α-Gal A gene and the other the abnormal α-Gal A gene. As a consequence, except in rare cases of skewed X-chromosome inactivation, random X-chromosome inactivation usually leads to greater residual enzyme activity, lower levels of Gb3 accumulation, a milder clinical and biochemical phenotype, and a later Fabry disease onset age in females compared with males, especially when they have the same α-Gal A gene mutation. Since this phenomenon can be observed in classical Fabry patients, we found the lack of a significant difference in age at onset of cardiac manifestations between IVS4 males and females in the current study surprising. Interestingly, we also found that females with classical Fabry mutations appeared to have a similar age at onset of the selected cardiac symptoms as male and female IVS4 patients. Currently, we do not have a good explanation for this finding. It could mean that the amount of Gb3 accumulation is not the main factor inducing cardiac manifestations in heterozygous females and patients with a milder form of Fabry disease. Indeed, there is some evidence to suggest that increased levels of urinary and plasma Gb3 are not correlated with clinical Fabry symptoms (Vedder et al. 2007). Perhaps there are other contributing pathogenic factors, such as degree of the inflammatory reaction (Biancini et al. 2012; De Francesco et al. 2013), the vulnerability of cardiomyocytes (modified by some cardiomyopathic genes or other unknown genes) (Desnick and Doheny 2014), or cell non-autonomous phenomena.
Recently, a new hypothesis of cross-induction by globotriaosylsphingosine (lyso-Gb3) was proposed in order to explain damage to cells containing active non-mutated X chromosomes in heterozygous Fabry females (Pinto et al. 2010). This hypothesis is based on increased levels of plasma lyso-Gb3, but not Gb3, found in most symptomatic female heterozygotes, which appears to be positively correlated with the severity of the clinical picture (Aerts et al. 2008). This widely diffusible lyso-Gb3 might have the capacity to induce cell damage via some mechanism that we do not yet understand well. Interestingly, in a previous study on our IVS4 patients, we also found only lyso-Gb3 levels, not Gb3, elevated in our symptomatic male and female patients, and these lyso-Gb3 levels also appeared to positively correlate with the severity of cardiac manifestations (Liao et al. 2013). Since we found that serum lyso-Gb3 can be elevated in both male and female infants, does this mean that cardiac manifestations are induced after a long and insidious course of elevated lyso-Gb3?
We believe that it is too soon to propose any alternative hypotheses regarding the pathogenesis of cardiac manifestations in Fabry disease, especially with such inconclusive evidence. Although this study is the first and largest analysis comparing age at onset of cardiac manifestations between male and female IVS4 cardiac variant and classical Fabry disease patients, the sample size is not large enough to reach any definitive conclusions. However, we hope that this report will encourage investigators in the field of Fabry disease to collect more data to help clarify whether or not a significant difference exists in age at onset of cardiac manifestations between cardiac variant Fabry male and female patients or even among classical Fabry female patients. If there is, it will be interesting to investigate the possible alternative pathogenesis of cardiac manifestations in these patients.
There are some limitations in this study. This analysis is retrospective in nature and uses data from FOS (a physician-driven registry for patient data in real-world healthcare settings); thus, the criteria for patient selection were not as stringent as in a clinical trial. As previously noted, the sample size was small (zero in one of the cardiac symptom groups); this is not unusual in rare disease registries, but for this analysis some of the potentially eligible patients could not be included due to incomplete database entries at data extraction. However, of the 71 Taiwanese patients registered in FOS at the time of data extraction, the majority (n = 59; 83.1%) met the inclusion criteria. Due to the small sample size, only descriptive statistics were used, and the possibility of a confounding effect, or that some of these analyses may have lacked the statistical power to detect a significant effect, cannot be ruled out without further follow-up to confirm the trends observed. There is also the possibility of selection bias in this analysis, since most of our classical Fabry patients came to our clinics owing to their symptoms/signs of Fabry disease (acroparesthesia, renal insufficiency, or hypertrophic cardiomyopathy). In contrast, most of our IVS4 patients attended our clinics as a result of newborn screening identifying the IVS4+919G>A mutation in their grandsons. Some of these IVS4 grandparents did not have significant clinical manifestations of the heart, but they were found to have hypertrophic cardiomyopathy; thus, the prevalence of hypertrophic cardiomyopathy was high in these patients. Furthermore, the patients of the IVS4 group tended to be older than those of the classical Fabry group, and we do not know how long LVH had been present in these patients; therefore, the analysis of age at onset of symptoms such as chest pain and palpitations might be subject to recall bias.
Conclusions
This retrospective study on Taiwanese patients with Fabry disease found no significant difference in onset age of cardiac manifestations between males and females with the cardiac variant IVS4+919G>A mutation.
Acknowledgments
This analysis was funded by Shire. The authors would like to thank Dr HF Chen, Dr TH Chu, YH Wang, WT Su, and CC Yeh for their contributions to data collection. Medical writing support was provided by Tina Rose of Excel Scientific Solutions, which was funded by Shire.
Compliance with Ethics Guidelines
Conflict of Interest
Dau-Ming Niu has received research support, reimbursement for travel, and speaker honoraria from Shire and Genzyme.
Wen-Chung Yu has received travel grants and speaker honoraria from Shire and Genzyme.
Hao-Chuan Liu, Ting-Rong Hsu, Chia-Feng Yang, and Hsiang-Yu Lin declare that they have no conflicts of interest.
Amandine Perrin is an employee of Shire.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Details of the Contributions of Individual Authors
All authors contributed to the planning and conduct of the study. Amandine Perrin performed the statistical analyses. All authors drafted the manuscript and approved the final version.
Footnotes
Competing interests: None declared
Contributor Information
Wen-Chung Yu, Email: wcyu@vghtpe.gov.tw.
Dau-Ming Niu, Email: dmniu1111@yahoo.com.tw.
Collaborators: Johannes Zschocke
References
- Aerts JM, Groener JE, Kuiper S, et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008;105(8):2812–2817. doi: 10.1073/pnas.0712309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancini GB, Vanzin CS, Rodrigues DB, et al. Globotriaosylceramide is correlated with oxidative stress and inflammation in Fabry patients treated with enzyme replacement therapy. Biochim Biophys Acta. 2012;1822(2):226–232. doi: 10.1016/j.bbadis.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Brouns R, Thijs V, Eyskens F, et al. Belgian Fabry study: prevalence of Fabry disease in a cohort of 1000 young patients with cerebrovascular disease. Stroke. 2010;41(5):863–868. doi: 10.1161/STROKEAHA.110.579409. [DOI] [PubMed] [Google Scholar]
- Chien YH, Lee NC, Chiang SC, Desnick RJ, Hwu WL. Fabry disease: incidence of the common later-onset alpha-galactosidase A IVS4+919G->A mutation in Taiwanese newborns–superiority of DNA-based to enzyme-based newborn screening for common mutations. Mol Med. 2012;18:780–784. doi: 10.2119/molmed.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong KW, Lu YH, Hsu JH, Lo MY, Hsiao CY, Niu DM (2008) High incidence of cardiac variant of Fabry disease in Taiwanese revealed by newborn screening Conference High incidence of cardiac variant of Fabry disease in Taiwanese revealed by newborn screening, Hualien, Taiwan, 2008, pp 92–98
- De Francesco PN, Mucci JM, Ceci R, Fossati CA, Rozenfeld PA. Fabry disease peripheral blood immune cells release inflammatory cytokines: role of globotriaosylceramide. Mol Genet Metab. 2013;109(1):93–99. doi: 10.1016/j.ymgme.2013.02.003. [DOI] [PubMed] [Google Scholar]
- de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20(5):1251–1260. doi: 10.1016/0735-1097(92)90385-Z. [DOI] [PubMed] [Google Scholar]
- Deegan PB, Baehner AF, Barba Romero MA, Hughes DA, Kampmann C, Beck M. Natural history of Fabry disease in females in the Fabry Outcome Survey. J Med Genet. 2006;43(4):347–352. doi: 10.1136/jmg.2005.036327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnick RJ, Doheny DO. Targeted sequencing of over 4000 hypertrophic cardiomyopathy (HCM) patients for mutations causing HCM and Fabry disease: HCM mutations frequent in patients with GLA later-onset mutations, polymorphisms, and variants. Mol Genet Metab. 2014;111(2):S36–S37. [Google Scholar]
- Desnick RJ, Ioannou YA, Eng CM. α-Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 3733–3774. [Google Scholar]
- Hwu WL, Chien YH, Lee NC, et al. Newborn screening for Fabry disease in Taiwan reveals a high incidence of the later-onset GLA mutation c.936+919G>A (IVS4+919G>A) Hum Mutat. 2009;30(10):1397–1405. doi: 10.1002/humu.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Nakao S, Minamikawa-Tachino R, Desnick RJ, Fan JQ. Alternative splicing in the alpha-galactosidase A gene: increased exon inclusion results in the Fabry cardiac phenotype. Am J Hum Genet. 2002;70(4):994–1002. doi: 10.1086/339431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the american society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Liao HC, Huang YH, Chen YJ, et al. Plasma globotriaosylsphingosine (lysoGb3) could be a biomarker for Fabry disease with a Chinese hotspot late-onset mutation (IVS4+919G>A) Clin Chim Acta. 2013;426:114–120. doi: 10.1016/j.cca.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Lin HY, Chong KW, Hsu JH, et al. High incidence of the cardiac variant of Fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet. 2009;2(5):450–456. doi: 10.1161/CIRCGENETICS.109.862920. [DOI] [PubMed] [Google Scholar]
- Lin HY, Huang CH, Yu HC, et al. Enzyme assay and clinical assessment in subjects with a Chinese hotspot late-onset Fabry mutation (IVS4+919G->A) J Inherit Metab Dis. 2010;33(5):619–624. doi: 10.1007/s10545-010-9166-7. [DOI] [PubMed] [Google Scholar]
- Lin HY, Liu HC, Huang YH et al (2013) Effects of enzyme replacement therapy for cardiac-type Fabry patients with a Chinese hotspot late-onset Fabry mutation (IVS4+919G>A). BMJ Open 3(7) [DOI] [PMC free article] [PubMed]
- Mehta A, Ricci R, Widmer U, et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest. 2004;34(3):236–242. doi: 10.1111/j.1365-2362.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- Mehta A, Clarke JT, Giugliani R, et al. Natural course of Fabry disease: changing pattern of causes of death in FOS - Fabry Outcome Survey. J Med Genet. 2009;46(8):548–552. doi: 10.1136/jmg.2008.065904. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Nakao S, Takenaka T, Maeda M, et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333(5):288–293. doi: 10.1056/NEJM199508033330504. [DOI] [PubMed] [Google Scholar]
- Nakao S, Kodama C, Takenaka T, et al. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int. 2003;64(3):801–807. doi: 10.1046/j.1523-1755.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- Pinto LL, Vieira TA, Giugliani R, Schwartz IV. Expression of the disease on female carriers of X-linked lysosomal disorders: a brief review. Orphanet J Rare Dis. 2010;5:14. doi: 10.1186/1750-1172-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries M, Gupta S, Moore DF, et al. Pediatric Fabry disease. Pediatrics. 2005;115(3):e344–e355. doi: 10.1542/peds.2004-1678. [DOI] [PubMed] [Google Scholar]
- Vedder AC, Linthorst GE, van Breemen MJ, et al. The Dutch Fabry cohort: diversity of clinical manifestations and Gb3 levels. J Inherit Metab Dis. 2007;30(1):68–78. doi: 10.1007/s10545-006-0484-8. [DOI] [PubMed] [Google Scholar]
- Wilcox WR, Oliveira JP, Hopkin RJ, et al. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab. 2008;93(2):112–128. doi: 10.1016/j.ymgme.2007.09.013. [DOI] [PubMed] [Google Scholar]


