Abstract
Social structure influences ecological processes such as dispersal and invasion, and affects survival and reproductive success. Recent studies have used static snapshots of social networks, thus neglecting their temporal dynamics, and focused primarily on a limited number of variables that might be affecting social structure. Here, instead we modelled effects of multiple predictors of social network dynamics in the spotted hyena, using observational data collected during 20 years of continuous field research in Kenya. We tested the hypothesis that the current state of the social network affects its long-term dynamics. We employed stochastic agent-based models that allowed us to estimate the contribution of multiple factors to network changes. After controlling for environmental and individual effects, we found that network density and individual centrality affected network dynamics, but that social bond transitivity consistently had the strongest effects. Our results emphasise the significance of structural properties of networks in shaping social dynamics.
Keywords: Cooperation, network dynamics, social network, spotted hyena
INTRODUCTION
Animals interact socially in a range of contexts such as hunting, detecting predators and raising offspring. Successive interactions between individuals can be described as relationships; these may vary with time, and also affect relationships of other individuals (Hinde 1976). The set of relationships in a group or population comprises its social structure, which plays a fundamental role in facilitating the propagation of information (Fewell 2003) and pathogens (Hamede et al. 2009; Drewe 2010) among individuals. Social structure also influences key ecological and evolutionary processes, such as the evolution of cooperation (Fehl et al. 2011), coevolution, dispersal and invasion (Kurvers et al. 2014). Studies of the consequences of social structure in a range of species have demonstrated its effects on mate choice (McDonald 2007; Oh & Badyaev 2010), survival (Silk et al. 2010; Barocas et al. 2011), reproduction (Gilby et al. 2013) and resource exploitation (Atton et al. 2014). Elucidating the processes and factors that determine the structure of animal societies is therefore essential for understanding cooperation patterns and the consequences of sociality. Current theoretical explanations as to why two individuals should form a social bond include preference for kin and patchiness of resources. Importantly, these approaches overlook the current state of all social bonds in a population at a given time, as a potential factor determining future patterns of social bonding. This is comparable to attempting to explain traits of extant species while ignoring any phylogenetic signal (Pienaar et al. 2013).
Social relationships are dynamic in their nature, affected both by changes in the relationships among individuals comprising the network, and by individuals joining or leaving the population. Most studies of animal social networks have nevertheless taken a static approach, overlooking the temporal dynamics integral to any social system. This approach is restrictive for two reasons. First, constructing social networks from observations performed during a limited time interval may generate a biased picture of the social structure, one that is not representative of the typical situation. Second, a static approach does not allow us to understand how or why the network changes over time (Blonder et al. 2012), or to isolate the factors that shape the social structure. For example if two individuals are associated, and they are both close kin and of high social rank, we cannot tell which of these factors is behind their social connection. In contrast, a dynamic approach that uses longitudinal data can solve these problems by tracing the dynamics of social preferences, thereby facilitating understanding of the social organisation while considering temporal variation in its structure (Pinter-Wollman et al. 2014; Shizuka et al. 2014). However, only a handful of studies, often limited in their scope, have begun to explore the dynamics of animal social networks (Henzi et al. 2009; Blonder & Dornhaus 2011; Kerth et al. 2011; Holekamp et al. 2012; Ilany et al. 2013; Bierbach et al. 2014).
The application of social network analysis to behavioural studies (Krause et al. 2007) has been instrumental in revealing key factors determining social structures. We classify these factors into three categories. The first category includes environmental and seasonal factors, such as rainfall, resource availability and competition with other species. Examples include environmental effects on social structure in equids (Sundaresan et al. 2007) and season-dependent network structure in Bechstein’s bat (Myotis bechsteinii) colonies (Kerth et al. 2011). The second category encompasses social preferences based on individual traits. Examples include sex- and age-related associations in bottlenose dolphins (Tursiops spp.) (Lusseau & Newman 2004), space use in Galápagos sealions, Zalophus wollebaeki (Wolf et al. 2007), personality differences in three-spined sticklebacks, Gasterosteus aculeatus (Pike et al. 2008) and the preference for kin in yellow-bellied marmots, Marmota flaviventris (Wey & Blumstein 2010). The third category has received less attention from biologists, and includes social associations that result from the topological structure of the network itself. Examples of such propensities include the maximum number of relationships individuals can maintain due to species-specific limitations on cognitive capacity (David-Barrett & Dunbar 2013), and the tendency to form associations with one’s other associates, also known as clustering or transitivity, as was found in the rock hyrax, Procavia capensis (Ilany et al. 2013). Importantly, the tendency to cluster is detectable in human hunter-gatherer societies, such as the Hadza (Apicella et al. 2012) and Bushmen (Hage 1976), and also in online social networks such as Facebook (Lewis et al. 2012). These examples suggest that the social structure, which is a summary of social relationships, may in turn determine how these relationships unfold. Nevertheless, the few studies that highlight structural properties of social networks in non-human animals have not controlled properly for other processes, such as the tendency to bond with kin.
Hinde (1976) introduced a classic framework to study sociality at three levels, in which social interactions accumulate into social relationships, which in turn comprise the social structure. In this study, we test the interdependence between social relationships and social structure. We formulate and test the topological effects hypothesis of social dynamics. We propose that social structures are affected by their previous state, limiting the range of possibilities for temporal dynamics. On the basis of previous studies of network models, and in particular human social networks (Jackson & Rogers 2007), we predicted that (1) current topological properties of the social network would predict its future state, (2) the more social bonds with others two individuals share, the more likely they would be to form a social bond (also known as clustering or bond transitivity) and (3) individuals would tend to bond with those individuals that were already central in their social network, as predicted by the preferential attachment model of network dynamics (Barabási & Albert 1999).
Here, we examine the stability of social bonds and comprehensively test our topological effects hypothesis with respect to long-term social network dynamics in a wild population of a mammalian carnivore, the spotted hyena (Crocuta crocuta), using data from 20 years of continuous field observations. These predators live in large stable groups, called ‘clans’, that are far more complex than those of other mammalian carnivores, and that are, in fact, more similar in size and structure to the societies of Old World primates such as baboons or macaques (Holekamp et al. 2012). The size of hyena clans depends on local prey abundance, and may vary from only a few individuals to more than 100 (Holekamp et al. 2012). Hyena clans usually contain several matrilineal kin groups spanning multiple generations, with low average relatedness among clan members (Van Horn et al. 2004). Wild spotted hyenas live up to 22 years (Boydston et al. 2005). They can discriminate both maternal and paternal kin from unrelated hyenas. They compete with their clan mates for access to killed prey, but high-ranking individuals enjoy priority of access to food (Holekamp et al. 2012). High-ranking females tend to form stronger social bonds than do low-ranking females (Holekamp et al. 1997).
Our analytical approach used a stochastic agent-based modelling framework (Snijders et al. 2010), proposed as a solution to two limitations of relational data. First, by definition a network is a set of relationships among nodes, implying that measures of node properties are not independent. Second, while networks are usually constructed for specific time intervals, the behavioural processes underlying their structure are continuous in time (Lewis et al. 2012). The framework used here, developed originally for the social sciences, models network dynamics as dependent on the current network state, the traits of its members and changes in environmental conditions. Thus, we were able to inquire how network traits affect temporal network dynamics while controlling for environmental factors, and individual traits. Due to the different life-history trajectories of male and female hyenas, we tested the effects of the factors listed above on three types of networks: all hyenas, only females and only males. We tested the effect of several structural parameters, including network density, transitivity (the tendency to associate with ‘the friend of my friend’, also known as clustering) and centrality (Table 1). We controlled for two categories of effects that are known to influence spotted hyena sociality. The first category includes environmental factors, of which we tested rainfall and the abundance of prey. The second category includes individual traits. Here, we controlled for the effect of social rank, dispersal status (natal or immigrant), sex and genetic relatedness.
Table 1.
Description of effects used in the stochastic agent-based models
| Category | Effect | Description |
|---|---|---|
| Network topology | Density | The baseline tendency to form a random social tie. |
| Distance two | The tendency to form an unclosed triad, i.e. a structure where A is connected to both B and C, but B and C are not connected. A positive value indicates individuals avoid forming cohesive clusters. | |
| Triadic closure (transitivity; clustering) | The tendency to form a tie with another individual with whom one shares a tie. For example if A is connected to both B and C, and then B and C also form a tie. A positive value implies a tendency to form cohesive clusters. | |
| Degree of alter | The tendency to form a tie with an individual depending on its degree of centrality within the network. A positive value indicates preferential attachment, where more central individuals become even more connected. | |
| Degree assortativity | The tendency to form a tie with individuals of similar degree. | |
| Isolate | The tendency to be isolated and unconnected to any other individual. Note that in our case, a positive value does not mean a tendency to be solitary, but rather that there is a tendency not to form any strong social bonds. | |
| Individual and dyadic traits | Sex | The tendency to prefer ties with one sex or the other. |
| Social rank | The tendency to form a tie with another individual based on its social rank (see Study population section for details on rank assignment). | |
| Dispersal status | The tendency to form ties with individuals that were either natal or immigrants. Immigrants joined the clan as adults, after dispersing from other clans. | |
| Sex, social rank and dispersal status assortativity | These effects indicate whether there is a tendency to form ties with individual similar in these traits to the focal individual. For example a negative social rank assortativity value suggests that highly ranked hyenas form ties with lower ranked ones. | |
| Relatedness | The tendency to form ties with genetically related individuals. | |
| Ecology | Rainfall | The tendency to connect to more individuals as average daily rainfall increases. |
| Prey abundance | The tendency to connect to more individuals as the average number of prey animals increases per prey census (counted in bi-weekly runs of multiple 4-km transects within the territory of the Talek clan). |
We found that, after taking into account environmental and individual influences, topological effects were crucial in shaping social network dynamics. Specifically, the tendency toward transitivity was essential to explain long-term strong social bonding. In other words, hyenas were more likely to form bonds with conspecifics with whom they share other connections.
METHODS
Study population
The analysis used observations of the Talek clan, conducted daily since 1988, in the Masai Mara National Reserve, Kenya. The Talek clan occupies a group territory of roughly 70 km2. To control for the effects of individual traits, we used data collected along with behavioural observations. Hyenas are individually recognised by their unique spots, and sex is determined by the morphology of the erect phallus. The position of an individual in a matrix ordered by submissive behaviour displayed during agonistic encounters determines its social rank (Holekamp et al. 2012); rank was then standardised for every year to account for variation in clan size. During the 20 years included in our analyses, clan size ranged from 43 to 91, with a mean size of 56 hyenas, but clan size did not affect the network structure (see Supporting Information). Hyenas were assigned a dispersal status, such that males that migrated to the focal clan were considered immigrants, whereas hyenas born in the clan were considered natal. Dyadic relatedness values were assessed using autosomal microsatellites, as described in (Van Horn et al. 2004). To control for effects of environmental variables, we used data from biweekly counts of available prey, as detailed in (Cooper et al. 1999); counts were averaged within years. In addition, we measured rainfall daily, and these values were also averaged within years, eliminating seasonal effects.
Social networks
We could identify all hyenas belonging to the clan. We did not include transient individuals (e.g., visiting the Talek territory to hunt for a day) as clan members. We also excluded individuals from each year of observation who were either born in December or died in January of that year. Hyenas were included in the analysis only if they were observed at least 20 times in at least 1 year, and were considered absent in a given year if observed < 20 times during that year. The fraction of hyenas included in the analysis out of all clan members was 0.85 ± 0.02 (mean ± SE over the 20 years). Hyenas that were included were observed an average ± SE of 94.4 ± 4.3 times per year. Cubs, defined as young hyenas that were still residing at dens (Holekamp et al. 2012), were not included. Observations at dens were also excluded to eliminate bias towards lactating females. Hyenas were assigned to a single observation session if they were found together, separated from other hyenas by at least 200 m, but usually at least 1 km (Smith et al. 2008). As done previously (Holekamp et al. 2012), from these observations we calculated the twiceweight association index (Cairns & Schwager 1987), describing the strength of the relationship within each pair of hyenas during each year: (A+Btogether) / [(Awithout B) + (Bwithout A) + (A+Btogether)] where (A+Btogether) is the number of observation sessions in which A and B are present together, (Awithout B) is the number of sessions in which A was present without B, and (Bwithout A) is the number of sessions in which B was present without A. We defined strong associations as those where the association index was in the upper quartile, which for both decades were values above 0.065 (see Supporting Information for further details, including an analysis of triadic closure in weighted networks).
Stochastic agent-based models
Stochastic agent-based models can simultaneously integrate a number of different mechanisms mediating change in social networks over time (Snijders et al. 2010; Ripley et al. 2014). Empirical data for these models must include two or more observations of a social network on a given set of individuals, assuming that relations between individuals represent longterm bonds and not events. These models accommodate cases where individuals join or leave the network (e.g. due to birth, death or dispersal). We used undirected networks based on spatial proximity in the fission–fusion societies of spotted hyenas, where individuals change subgroups several times each day (Smith et al. 2008). Here network ties are binary, representing the presence or absence of a social bond, after filtering out weak bonds (see above). In our model of hyena social network dynamics we included various effects suggesting possible reasons why two hyenas might form and maintain a strong social relationship (Table 1). Details on the assumptions of our models, estimation and validation procedures, can be found in the Supporting Information.
RESULTS
High-quality data were available from a single large hyena clan for two decades, 1989–1998 and 2002–2011, referred to hereafter as decade 1 and decade 2, respectively. Data from 1999–2001 were omitted due to a period of social instability, during which the clan eventually split into two new clans. This rare split event was omitted so we could study network dynamics during stable periods. We used 55,689 observation sessions to construct a weighted social network representing associations between individuals during each year (see Methods). The two decades included 186 and 194 hyenas, respectively. Descriptive statistics documenting variation in hyena social networks, and demographic and ecological factors, are provided in Table 2.
Table 2.
Descriptive statistics of the social networks of spotted hyenas, demographic and ecological factors in the Talek area, Masai Mara National Reserve, Kenya
| Year | N | Network density |
Mean degree |
Clustering coefficient |
Sex ratio (M/F) |
Dispersal status ratio (immigrants/ natal) |
Rainfall* | Prey† |
|---|---|---|---|---|---|---|---|---|
| 1989 | 66 | 0.39 | 25.30 | 0.65 | 1.17 | 0.27 | 3.23 | 359.06 |
| 1990 | 50 | 0.37 | 18.08 | 0.63 | 1.45 | 0.28 | 2.86 | 687.03 |
| 1991 | 57 | 0.16 | 9.12 | 0.53 | 1.48 | 0.36 | 2.58 | 414.66 |
| 1992 | 51 | 0.18 | 9.10 | 0.56 | 1.13 | 0.28 | 2.82 | 637.45 |
| 1993 | 50 | 0.33 | 15.96 | 0.70 | 0.92 | 0.26 | 1.64 | 546.52 |
| 1994 | 47 | 0.15 | 7.02 | 0.56 | 0.52 | 0.24 | 2.44 | 613.85 |
| 1995 | 53 | 0.11 | 5.70 | 0.49 | 0.77 | 0.26 | 3.25 | 617.03 |
| 1996 | 58 | 0.18 | 10.07 | 0.60 | 1.07 | 0.38 | 3.68 | 1104.89 |
| 1997 | 58 | 0.17 | 9.79 | 0.62 | 1.23 | 0.38 | 3.76 | 485.00 |
| 1998 | 57 | 0.14 | 7.75 | 0.60 | 1.19 | 0.36 | 3.69 | 861.20 |
| 2002 | 49 | 0.16 | 7.59 | 0.55 | 1.04 | 0.29 | 3.73 | 336.25 |
| 2003 | 43 | 0.37 | 15.53 | 0.67 | 1.05 | 0.23 | 2.61 | 253.89 |
| 2004 | 48 | 0.33 | 15.33 | 0.67 | 1.18 | 0.41 | 3.86 | 123.70 |
| 2005 | 64 | 0.24 | 15.25 | 0.70 | 1.30 | 0.33 | 3.12 | 189.71 |
| 2006 | 53 | 0.31 | 16.00 | 0.67 | 1.30 | 0.26 | 3.43 | 284.99 |
| 2007 | 44 | 0.29 | 12.68 | 0.68 | 0.87 | 0.22 | 2.81 | 444.51 |
| 2008 | 51 | 0.24 | 12.24 | 0.64 | 0.89 | 0.28 | 2.91 | 516.15 |
| 2009 | 59 | 0.23 | 13.29 | 0.58 | 0.64 | 0.18 | 2.26 | 590.10 |
| 2010 | 70 | 0.14 | 9.91 | 0.44 | 0.53 | 0.19 | 3.44 | 664.10 |
| 2011 | 91 | 0.21 | 18.51 | 0.58 | 0.57 | 0.14 | 3.12 | 310.81 |
Mean daily rainfall during a year in mm.
The mean number of individual prey animals per km2 counted in bi-weekly runs of multiple 4-km transects.
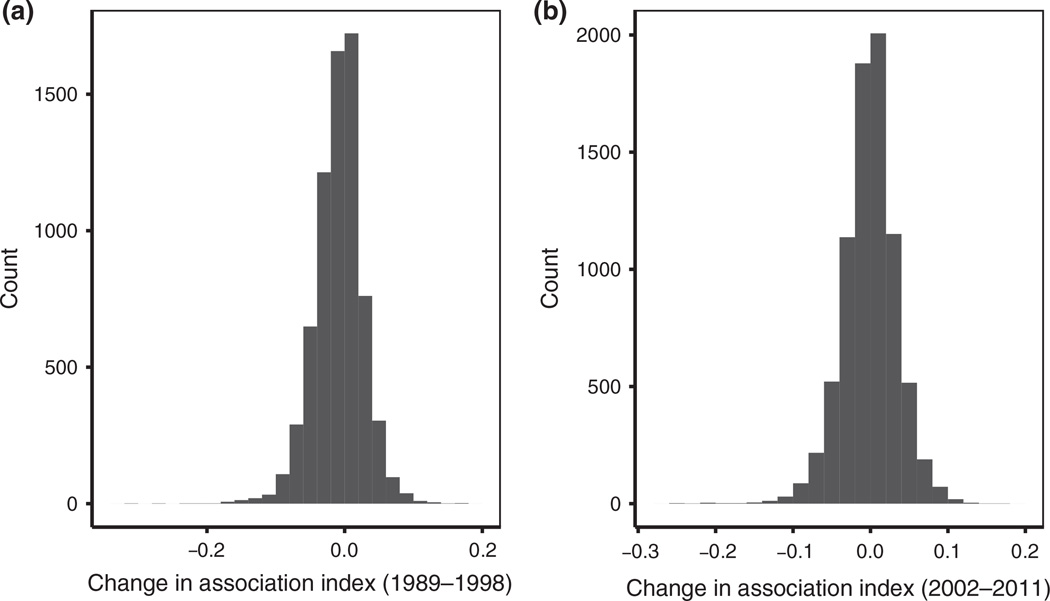
Comparing the strength of association within the same pairs of hyenas over consecutive years revealed a slight negative trend in both decades (Fig. 1; mean change and SE in association index across consecutive years: decade 1: −0.0089 ± 0.0012; decade 2: −0.0012 ± 0.0003), indicating that, on average, the strength of relationships within dyads weakens slightly over time. Investigating the change in association strength revealed that male–male associations were slightly less stable than female–female associations (mean change and SE in association index across consecutive years: decade 1 males: −0.0096 ± 0.0009; decade 1 females: −0.0092 ± 0.0006; decade 2 males: −0.0015 ± 0.0009; decade 2 females: −0.0001 ± 0.0006).
Figure 1.
The distribution of changes in association index strength (see Methods for definition) across consecutive years, in decades 1 (a) and 2 (b). Only cases where both hyenas were present in two consecutive years were included.
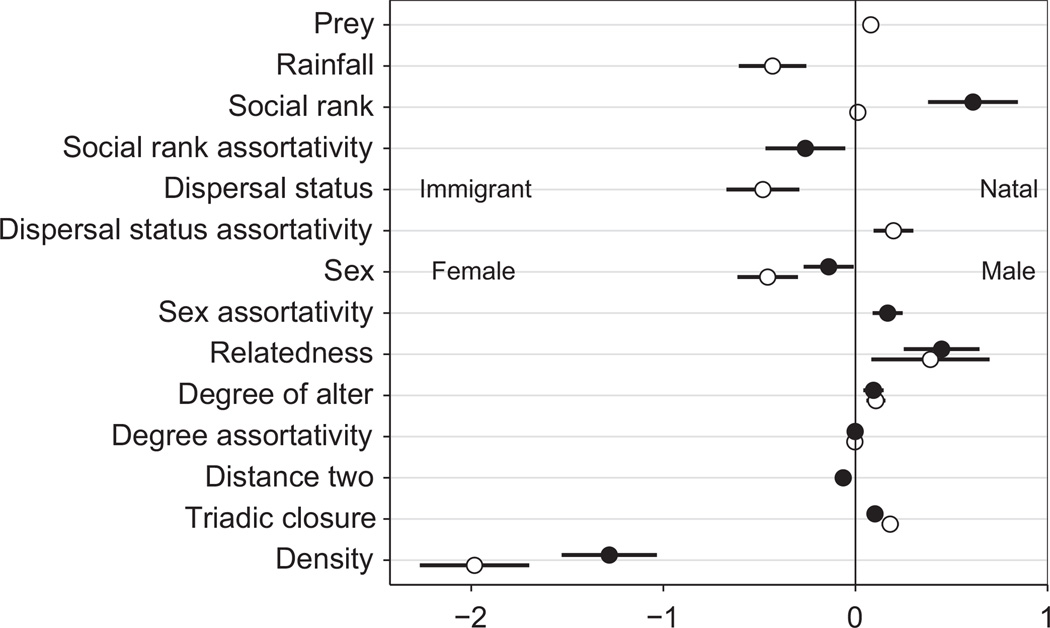
We then investigated the causal factors generating year-toyear variation in the social network (Movie S1). For this analysis we used binary networks in which we retained only strong social bonds (see Methods). Fig. 2 presents parameter estimates and 95% confidence intervals for two models of hyena network dynamics estimated over 20 years, in two separate decades. Although effects of some predictor variables were significant in only one of the two decades studied, in no case did the direction of any effect change between decades from positive to negative or vice versa. Density, which is the current number of social bonds in the network, had a strongly negative influence on social bond dynamics, confirming that hyenas do not form bonds with every other hyena; instead they are selective in their social choices. Triadic closure (transitivity) had a positive influence; sharing a contact in common increased the log-odds of two hyenas bonding or retaining their bond by 0.14, on average. Additional shared contacts multiplied this effect. To verify that triadic closure was not merely a by-product of the data collection method (using the ‘gambit of the group’), we performed an additional analysis in which only two individuals were randomly chosen from each observed group. This showed a similar trend (Tables S4 and S5). Hyenas tended to associate with central individuals in the network (high degree of alter), and degree assortativity was slightly negative, suggesting preferential attachment. These topological effects were evaluated while controlling for other factors that influenced network dynamics. Relatedness had a positive influence on social bonding, such that hyenas formed more ties with their kin than with non-kin. Females had a higher probability than males of forming associations, as did hyenas of lower social rank (by convention, the highest rank individual is assigned a rank of 1, etc). Prey abundance, rainfall, social rank assortativity, dispersal status and distance two (i.e. forming an unclosed triad) affected social dynamics only in one of the two decades. Importantly, models that did not include triadic closure could not reproduce the observed social dynamics and did not provide acceptable goodness-of-fit.
Figure 2.
Parameter estimates and 95% confidence intervals for stochastic agent-based models of social network dynamics in a large spotted hyena group over two decades. Empty and filled points represent decade 1 (1989–1998) and decade 2 (2002–2011), respectively. Coefficients represent the change in log-odds of the probability of forming a strong social bond depending on a given factor (e.g. there is a higher probability of forming a bond if it will close a triad in the network or if it will connect genetically related individuals). Parameter estimates are presented only if the parameter was part of the best model for a given decade. See Table 1 for parameter details and Table S1 for full model results.
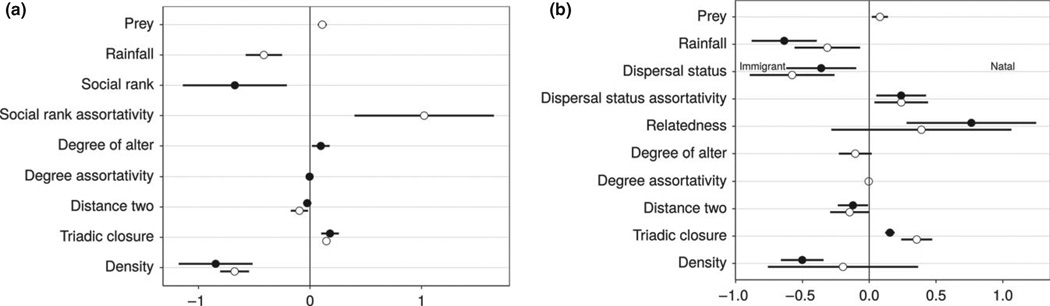
Because male and female hyenas differ with respect to their life history traits, we next examined subsets of the data that included only members of one sex (Fig. 3 and Tables S2–S3; 82 females and 104 males in decade 1; 91 females and 103 males in Decade 2). A smaller number of factors determined social dynamics in each sex than in the full network model. Parameter estimates for the male networks were more consistent across decades than those for females. In the female networks only triadic closure (positive) and density (negative) showed similar trends in both decades. In contrast, five different parameters were consistent across decades in influencing male social bonding. As was the case for females, two males had a higher chance of associating if they shared one or more associates. They also avoided unclosed triads (‘distance two’ effect). Immigrant males were likelier to form social ties than were natal males, and males preferred to associate with others of the same dispersal status as their own. Rainfall had a negative influence on social dynamics, where drier years caused denser networks. Among males, two additional factors showed similar patterns in the two studied decades, but in decade 1 were not significant: males tended to form ties with their kin, and density had a negative influence, as was observed in the full and female networks.
Figure 3.
Parameter estimates and 95% confidence intervals for stochastic agent-based models of the social network dynamics of females (a) and males (b) in a spotted hyena clan over two decades. Empty and filled points represent decade 1 (1989–1998) and decade 2 (2002–2011), respectively. Coefficients represent the change in log-odds of the probability of a strong social bond depending on a given factor (e.g. there is higher probability of forming a bond if it will close a triad in the network or if it will connect genetically related individuals). Parameter estimates are presented only if the parameter was part of the best model for a given decade. In the female networks, relatedness was not part of the selected models. In the male networks, social rank and social rank assortativity were not part of the selected models. See Tables S2 and S3 for full model results.
DISCUSSION
This study shows how multiple factors affect long-term social network dynamics in the spotted hyena. Remarkably, the results from the two different decades we modelled (1989–1998 and 2002–2011) show similar social trends, with not even a single effect contradicting the result from the previous decade. Importantly, our new methods yield results that are strongly consistent with previous results from the same study system obtained using more traditional methods (Holekamp et al. 1997, 2012; Smale et al. 1997; Cooper et al. 1999; Szykman et al. 2001; Wahaj et al. 2004; Smith et al. 2008). In fact, no conflicts at all were detected between our modelling results and earlier findings from this study system. This implies that stochastic agent-based models such as Siena can potentially be useful for modelling how multiple factors of various types affect social dynamics in other species as well. These models should also facilitate the study of processes occurring within networks, such as disease and cultural transmission (Snijders et al. 2010). They can also potentially be used to study how individual traits, such as reproductive success, and relational traits such as social rank, are temporally affected by network dynamics, opening many new avenues of research.
The factors found to influence hyena social dynamics include rainfall, prey availability, sex, social rank, dispersal status and topological effects such as network density and preferences based on the centrality of individual hyenas in their social networks. Importantly, our analysis identifies the tendency towards triadic closure (transitivity) in all modelled networks and in both decades. In other words, hyenas tend to associate with the ‘friends of their friends’. This tendency was found to be consistent over time, and models that did not include this effect could not explain the observed dynamics. Thus, the current structure of the social network strongly affects its future state. The most consistent topological effect, triadic closure, results in clustering of the network or the formation of cohesive groups that may facilitate efficient cooperation leading to fitness maximisation (Lion & van Baalen 2008). Interestingly, network clustering is also a feature of human hunter-gatherer societies (Hage 1976; Apicella et al. 2012) and contemporary humans using Facebook (Lewis et al. 2012), suggesting structural similarity between humans and hyenas in that respect. Importantly, our dynamic approach permits the identification of triadic closure, which would be impossible using static networks. Whereas in static network bonds in closed triads might result merely from spatial proximity among members of the triad, a dynamic approach can test whether two individuals sharing a mutual bond would be likely to form a direct new bond between them.
Theory suggests that social structure can play an important role in the evolution of cooperation, with clusters of cooperators required to maintain cooperation (Nowak 2006). Our results demonstrate the strong tendency for spotted hyenas to form clusters; this tendency is present even after controlling for relatedness and other factors. Hence the tendency to cluster may not be just a by-product of kin selection or patchy distribution of resources, but instead may itself be adaptive, thus potentially facilitating cooperation among both kin and non-kin. Whereas our results are consistent with the notion that hyenas prefer to associate with their kin (e.g. Holekamp et al. 1997, 2012), they also demonstrate how multiple other factors, including bonds with third parties, influence social dynamics at the dyadic level.
Our prediction that hyenas should tend to bond with central individuals in their social network was supported by the data from the full network; in both decades hyenas were more likely to form strong social bonds with group-mates that were more central in the network. However, examining degree centrality in the sex-specific networks shows a more complex pattern. In females, this effect was positive in decade 2, but not part of the selected model in decade 1. In males, this effect was negative in decade 1 and not part of the selected model in decade 2. This suggests that males do not prefer to bond with other central males, and even in the female–female networks this tendency was limited to only one decade. It follows that the significant tendency to connect to central individuals in the networks including both sexes is probably a result of such preference in male–female bonds, but less so in same-sex bonds. Our analysis does not take into account the type of observed social associations, but it is possible that males follow central females because they prefer them as mates, as shown previously (Szykman et al. 2001).
We found that males were more consistent across decades than females with respect to the factors determining their social affiliations. Because adult males disperse, leaving their natal clans and later joining new ones, their social tendencies may be more generic, facilitating social integration in a range of social circumstances. In contrast, females usually spend their entire lives in one clan, and thus may be more responsive to variation in their local physical and social environments. Whereas social dynamics among male and female hyenas are influenced by different sets of factors, both sexes in both studied decades were influenced by the tendency toward triadic closure, and avoidance of the distance-two structure. This further supports the inherent affinity for clustering as a defining rule of social structure. Although female hyenas attain their social ranks from their mothers (Holekamp & Smale 1991) and retain their ranks throughout their lives (Holekamp et al. 2012), when male hyenas disperse and join new clans they suffer an inevitable decrease in social rank (Smale et al. 1997). Our finding that immigrant males form more strong social bonds than natal males, and tend to associate with other immigrants, suggests that these bonds might compensate for the disadvantages of their low rank.
Whereas our results highlight the importance of topological effects of network structure, they also support many of the previous findings regarding social preferences in spotted hyenas (Holekamp et al. 2012). We found that females were more strongly connected than males. Because females’ social ranks are higher than those of immigrant males, females have priority of access to kills, allowing them to stay in prey-rich areas of the clan territory. In contrast, male hyenas often need to travel to more remote areas to feed, where competition with clan members is reduced, leading to their lower levels of connectedness. Nevertheless, we found that, after controlling for sex, relatedness, and degree centrality, lower ranked individuals in the full model tended to form more social ties than higher ranked ones, possibly as a means to offset the negative impact of their rank. In the females-only networks we found an opposite trend, in which higher-ranked females formed more ties than lower-ranked females, suggesting that the result in the full networks may be attributed to ties between low-ranked females and low-ranked males. This result reaffirms earlier findings (Holekamp et al. 1997), which showed that high-ranked female hyenas form stronger social associations than low-ranked ones. Our data also revealed that rainfall negatively affects the social network density. As rain causes prey to scatter, wetter years feature lower density networks among hyenas. Indeed, analysis of change in hyena networks between seasons within years reveals that periods with more prey indeed cause denser hyena networks (Holekamp et al. 2012).
Our data reveal that hyenas tend to maintain the strength of social ties across years, suggesting that the initial association strength is important in determining the nature of a relationship. Similarly, in a study of the social structure of wintering migrant sparrows, Shizuka et al. (2014) found only minor temporal changes in the strength of social ties among individuals. Interestingly, bond stability in hyenas is maintained despite environmental changes and demographic fluctuations, such as the birth, death or dispersal of individuals, changes in overall clan size and changes in the sex ratio (Hinde 1976). Furthermore, in most hyena networks the density effect was negative, suggesting that there is no tendency to associate with every member of the clan. Strong social relationships are formed in response to a range of factors, but in a selective manner, as has also been shown in primates (e.g. Mitani 2009). We note, however, that our definition of social bonds limits the number of possible edges in the network and this limitation was also essential to achieve model convergence. Thus, whereas the negative density effect in our results shows trends similar to those found in analyses of other social networks, care should be taken in their interpretation. Nevertheless, taken together with the results of Shizuka et al. (2014), it appears that individual animals tend to maintain existing social ties but also respond to a range of variables by altering their relationships.
Our study includes a number of limitations. First, our data come from one large clan of spotted hyenas in southwestern Kenya. The generalisability of the social preferences seen here to other populations remains to be determined. Second, our modelling approach uses binary networks, reducing the subtle levels of social association, and ignoring weak ties, while focusing on numbers of strong bonds rather than strictly on variation in bond strength. Nevertheless, it revealed the factors affecting social dynamics among strongly bonded individuals. Furthermore, our results confirm previous results from the same population where the strength of associations was taken into account, reaffirming our method of defining the binary networks (see Supporting Information). Future development of the methodology may allow us to consider temporal changes in the strength of associations rather than merely their number.
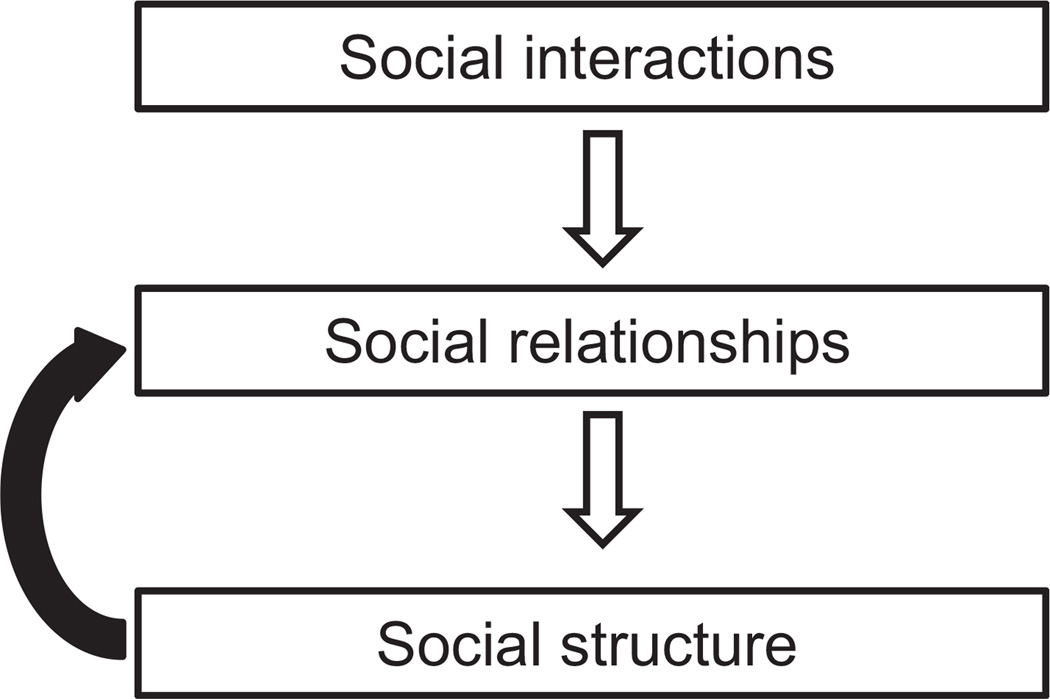
The current results demonstrate the value of network analysis in incorporating population structure into social decision-making, where individuals take into account not only the traits and social roles of their focal partners, but also third parties and their social relationships. We provide strong evidence regarding how the social structure that is a summary of social relationships between individuals (Hinde 1976) also affects the dynamics of these relationships (Fig. 4). Furthermore, this study expands the number of variables known to affect long-term social associations. We demonstrate that, although the social network structure is highly variable across time, consistent rules can be inferred that determine network dynamics over long periods, as has been shown in humans (Lewis et al. 2012). We show that the most consistent rules are topological effects that impose limitations on network dynamics, but also create opportunities for novel interactions. Thus, the previous state of the social network can be seen as being analogous to its ‘phylogenetic signal’; the history of the network affects its temporal dynamics, and may be stronger than the effects of environmental or individual variables. The methods we used can be extended to assess the implications of social structure for individual traits and reproductive success, and as such should be valuable for biologists interested in the causes and consequences of social dynamics. Our results suggest that we should strive for a dynamic approach to better understand social structure. They also call for theoretical and empirical studies of the advantages of specific social tendencies, to better understand the evolutionary origins of the observed effects.
Figure 4.
A modification of Hinde’s (1976) classic framework of social structure. Our data show how the social structure that is the summary of social relationships in turn affects the temporal dynamics of these relationships (black arrow). These topological effects limit the influence of environmental variables and individual traits.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Office of the President of Kenya and the Kenyan National Commission for Science, Technology and Innovation for permission to conduct this research. We also thank the Kenya Wildlife Service, the Narok County government and the Senior Warden of the Masai Mara National Reserve for their cooperation. The research is described in Animal Research Protocol AUF 05/05-064-00, and complies with Kenyan laws. We thank P. S. Bills for database management and assistance. We also acknowledge valuable input from three anonymous reviewers. A. I. was a Postdoctoral Fellow at the National Institute for Mathematical and Biological Synthesis, an Institute sponsored by the National Science Foundation, the U.S. Department of Homeland Security, the U.S. Department of Agriculture and NSF Awards EF-0832858 and DBI-1300426, with additional support from The University of Tennessee, Knoxville. Work by A.S.B. & K.E.H. was facilitated by NSF grants IOS-1121474 and DEB-1353110, and NIH grant R01GM105042.
Footnotes
AUTHOR CONTRIBUTIONS
AI and KEH designed the study. ASB performed the genetic analysis. AI performed all other analyses. AI wrote the paper, with contributions from all authors.
Additional Supporting Information may be downloaded via the online version of this article at Wiley Online Library (www.ecologyletters.com).
REFERENCES
- Apicella CL, Marlowe FW, Fowler JH, Christakis NA. Social networks and cooperation in hunter-gatherers. Nature. 2012;481:497–501. doi: 10.1038/nature10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atton N, Galef BJ, Hoppitt W, Webster MM, Laland KN. Familiarity affects social network structure and discovery of prey patch locations in foraging stickleback shoals. Proc. Biol. Sci. 2014;281:20140579. doi: 10.1098/rspb.2014.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabási A-L, Albert R. Emergence of scaling in random networks. Science. 1999;286:509–512. doi: 10.1126/science.286.5439.509. [DOI] [PubMed] [Google Scholar]
- Barocas A, Ilany A, Koren L, Kam M, Geffen E. Variance in centrality within rock hyrax social networks predicts adult longevity. PLoS ONE. 2011;6:e22375. doi: 10.1371/journal.pone.0022375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierbach D, Oster S, Jourdan J, Arias-Rodriguez L, Krause J, Wilson AM, et al. Social network analysis resolves temporal dynamics of male dominance relationships. Behav. Ecol. Sociobiol. 2014;68:935–945. [Google Scholar]
- Blonder B, Dornhaus A. Time-ordered networks reveal limitations to information flow in ant colonies. PLoS ONE. 2011;6:e20298. doi: 10.1371/journal.pone.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonder B, Wey TW, Dornhaus A, James R, Sih A. Temporal dynamics and network analysis. Methods Ecol. Evol. 2012;3:958–972. [Google Scholar]
- Boydston EE, Kapheim KM, Van Horn RC, Smale L, Holekamp KE. Sexually dimorphic patterns of space use throughout ontogeny in the spotted hyena (Crocuta crocuta) J. Zool. 2005;267:271–281. [Google Scholar]
- Cairns SJ, Schwager SJ. A comparison of association indices. Anim. Behav. 1987;35:1454–1469. [Google Scholar]
- Cooper SM, Holekamp KE, Smale L. A seasonal feast: long-term analysis of feeding behaviour in the spotted hyaena (Crocuta crocuta) Afr. J. Ecol. 1999;37:149–160. [Google Scholar]
- David-Barrett T, Dunbar RIM. Processing power limits social group size: computational evidence for the cognitive costs of sociality. Proc. Biol. Sci. 2013;280:20131151. doi: 10.1098/rspb.2013.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe JA. Who infects whom? Social networks and tuberculosis transmission in wild meerkats. Proc. Biol. Sci. 2010;277:633–642. doi: 10.1098/rspb.2009.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehl K, van der Post DJ, Semmann D. Co-evolution of behaviour and social network structure promotes human cooperation. Ecol. Lett. 2011;14:546–551. doi: 10.1111/j.1461-0248.2011.01615.x. [DOI] [PubMed] [Google Scholar]
- Fewell JH. Social insect networks. Science. 2003;301:1867–1870. doi: 10.1126/science.1088945. [DOI] [PubMed] [Google Scholar]
- Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, et al. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 2013;67:373–381. doi: 10.1007/s00265-012-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage P. Structural balance and clustering in Bushmen kinship relations. Behav. Sci. 1976;21:36–47. [Google Scholar]
- Hamede RK, Bashford J, McCallum H, Jones M. Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecol. Lett. 2009;12:1147–1157. doi: 10.1111/j.1461-0248.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- Henzi SP, Lusseau D, Weingrill T, van Schaik CP, Barrett L. Cyclicity in the structure of female baboon social networks. Behav. Ecol. Sociobiol. 2009;63:1015–1021. [Google Scholar]
- Hinde RA. Interactions, relationships and social-structure. Man. 1976;11:1–17. [Google Scholar]
- Holekamp KE, Smale L. Dominance acquisition during mammalian social development: the “inheritance” of maternal rank. Am. Zool. 1991;31:306–317. [Google Scholar]
- Holekamp KE, Cooper SM, Katona CI, Berry NA, Frank LG, Smale L. Patterns of association among female spotted hyenas (Crocuta crocuta) J. Mammal. 1997;78:55–64. [Google Scholar]
- Holekamp KE, Smith JE, Strelioff CC, Van Horn RC, Watts HE. Society, demography and genetic structure in the spotted hyena. Mol. Ecol. 2012;21:613–632. doi: 10.1111/j.1365-294X.2011.05240.x. [DOI] [PubMed] [Google Scholar]
- Ilany A, Barocas A, Koren L, Kam M, Geffen E. Structural balance in the social networks of a wild mammal. Anim. Behav. 2013;85:1397–1405. [Google Scholar]
- Jackson MO, Rogers BW. Meeting strangers and friends of friends: how random are social networks? Am. Eco. Rev. 2007;97:890–915. [Google Scholar]
- Kerth G, Perony N, Schweitzer F. Bats are able to maintain long-term social relationships despite the high fission-fusion dynamics of their groups. Proc. Biol. Sci. 2011;278:2761–2767. doi: 10.1098/rspb.2010.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, Croft DP, James R. Social network theory in the behavioural sciences: potential applications. Behav. Ecol. Sociobiol. 2007;62:15–27. doi: 10.1007/s00265-007-0445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurvers RHJM, Krause J, Croft DP, Wilson ADM, Wolf M. The evolutionary and ecological consequences of animal social networks: emerging issues. Trends Ecol. Evol. 2014;29:326–335. doi: 10.1016/j.tree.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Lewis K, Gonzalez M, Kaufman J. Social selection and peer influence in an online social network. Proc. Natl Acad. Sci. USA. 2012;109:68–72. doi: 10.1073/pnas.1109739109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lion S, van Baalen M. Self-structuring in spatial evolutionary ecology. Ecol. Lett. 2008;11:277–295. doi: 10.1111/j.1461-0248.2007.01132.x. [DOI] [PubMed] [Google Scholar]
- Lusseau D, Newman MEJ. Identifying the role that animals play in their social networks. Proc. Biol. Sci. 2004;271:S477–S481. doi: 10.1098/rsbl.2004.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DB. Predicting fate from early connectivity in a social network. Proc. Natl Acad. Sci. USA. 2007;104:10910–10914. doi: 10.1073/pnas.0701159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani JC. Male chimpanzees form enduring and equitable social bonds. Anim. Behav. 2009;77:633–640. [Google Scholar]
- Nowak MA. Five rules for the evolution of cooperation. Science. 2006;314:1560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KP, Badyaev AV. Structure of social networks in a passerine bird: consequences for sexual selection and the evolution of mating strategies. Am. Nat. 2010;176:E80–E89. doi: 10.1086/655216. [DOI] [PubMed] [Google Scholar]
- Pienaar J, Ilany A, Geffen E, Yom-Tov Y. Macroevolution of life-history traits in passerine birds: adaptation and phylogenetic inertia. Ecol. Lett. 2013;16:571–576. doi: 10.1111/ele.12077. [DOI] [PubMed] [Google Scholar]
- Pike TW, Samanta M, Lindstrom J, Royle NJ. Behavioural phenotype affects social interactions in an animal network. Proc. Biol. Sci. 2008;275:2515–2520. doi: 10.1098/rspb.2008.0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter-Wollman N, Hobson EA, Smith JE, Edelman AJ, Shizuka D, de Silva S, et al. The dynamics of animal social networks: analytical, conceptual, and theoretical advances. Behav. Ecol. 2014;25:242–255. [Google Scholar]
- Ripley RM, Snijders TAB, Boda Z, Voros A, Preciado P. Manual for RSiena. Oxford: University of Oxford; 2014. [Google Scholar]
- Shizuka D, Chaine AS, Anderson J, Johnson O, Laursen IM, Lyon BE. Across-year social stability shapes network structure in wintering migrant sparrows. Ecol. Lett. 2014;17:998–1007. doi: 10.1111/ele.12304. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, et al. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Smale L, Nunes S, Holekamp KE. Sexually dimorphic dispersal in mammals: patterns, causes, and consequences. Adv. Study Beh. 1997;26:181–250. [Google Scholar]
- Smith JE, Kolowski JM, Graham KE, Dawes SE, Holekamp KE. Social and ecological determinants of fission-fusion dynamics in the spotted hyaena. Anim. Behav. 2008;76:619–636. [Google Scholar]
- Snijders TAB, van de Bunt GG, Steglich CEG. Introduction to stochastic actor-based models for network dynamics. Soc. Net. 2010;32:44–60. [Google Scholar]
- Sundaresan SR, Fischhoff IR, Dushoff J, Rubenstein DI. Network metrics reveal differences in social organization between two fission-fusion species, Grevy’s zebra and onager. Oecologia. 2007;151:140–149. doi: 10.1007/s00442-006-0553-6. [DOI] [PubMed] [Google Scholar]
- Szykman M, Engh AL, Van Horn RC, Funk SM, Scribner KT, Holekamp KE. Association patterns among male and female spotted hyenas (Crocuta crocuta) reflect male mate choice. Behav. Ecol. Sociobiol. 2001;50:231–238. [Google Scholar]
- Van Horn RC, Engh AL, Scribner KT, Funk SM, Holekamp KE. Behavioural structuring of relatedness in the spotted hyena (Crocuta crocuta) suggests direct fitness benefits of clan-level cooperation. Mol. Ecol. 2004;13:449–458. doi: 10.1046/j.1365-294x.2003.02071.x. [DOI] [PubMed] [Google Scholar]
- Wahaj SA, Van Horn RC, Van Horn TL, Dreyer R, Hilgris R, Schwarz J, et al. Kin discrimination in the spotted hyena (Crocuta crocuta): nepotism among siblings. Behav. Ecol. Sociobiol. 2004;56:237–247. [Google Scholar]
- Wey TW, Blumstein DT. Social cohesion in yellow-bellied marmots is established through age and kin structuring. Anim. Behav. 2010;79:1343–1352. [Google Scholar]
- Wolf JBW, Mawdsley D, Trillmich F, James R. Social structure in a colonial mammal: unravelling hidden structural layers and their foundations by network analysis. Anim. Behav. 2007;74:1293–1302. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.