Abstract
Considerable evidence, particularly from genetics, points to the aggregation-prone amyloid β-peptide as a pathogenic entity in Alzheimer’s disease. Hence, the proteases that produce this peptide from its precursor protein have been prime targets for the development of potential therapeutics. One of these proteases, γ-secretase, has been a particular focus. Many inhibitors and modulators of this membrane-embedded protease complex have been identified, with some brought into late-stage clinical trials, where they have spectacularly failed. The reasons for these failures will be discussed, along with recent findings on the mechanism of γ-secretase and of Alzheimer-causing mutations that may suggest new strategies for targeting this enzyme.
Keywords: amyloid, biochemistry, genetics, inhibitors, modulators, protease
Alzheimer’s disease (AD) is a progressive and ultimately fatal neurodegenerative disorder and major public health crisis, with over 5 million affected in the USA and some 30 million worldwide. With age being the greatest risk factor and more people expected to live well into old age, AD is projected to dramatically rise in the coming decades [1]. Despite the devastating course of AD, the numbers afflicted, and the huge social and economic costs, current treatments are only symptomatic and marginally effective. No therapeutic agents are available that slow or stop the inexorable neurodegeneration and progressive loss of learning and memory that are well-known hallmarks of the disease. Toward the discovery of such agents, the underlying molecular mechanisms must be understood and in sufficient detail to formulate appropriate strategies for effective prevention or treatment.
The deposition of extracellular amyloid plaques and neurofibrillary tangles in the brain are cardinal pathological features of AD [2]. The former are composed primarily of the amyloid β-peptide (Aβ), while the latter are comprised of filaments of the otherwise microtubule-associated protein tau. The question of whether either of these two pathological features are involved in the pathogenesis or merely coincidental with the true pathogenic entities has been much debated, but the general consensus is that Aβ and tau are both critical to the neurodegenerative process of AD, with Aβ being upstream of tau in the pathway.
However, the plaques and tangles per se may indeed be markers rather than causal agents. In the past decade or so, evidence has mounted suggesting that soluble oligomeric forms of Aβ cause synaptic dysfunction and are more detrimental than the plaques [3], and the same may also be true for tau [4]. Regardless of the exact forms responsible for eliciting synaptic and neuronal toxicity, genetic mutations associated with dominant hereditary dementia clearly point to alterations in Aβ or tau being sufficient for neurodegenerative disease [5,6]. Moreover, Aβ pathology in AD is coupled with tau pathology, while tau-containing neurofibillary tangles are a common feature of a range of other neurodegenerative diseases [7], suggesting that a variety of neuronal insults, including aberrant Aβ, can trigger pathogenic tau.
Aβ basics
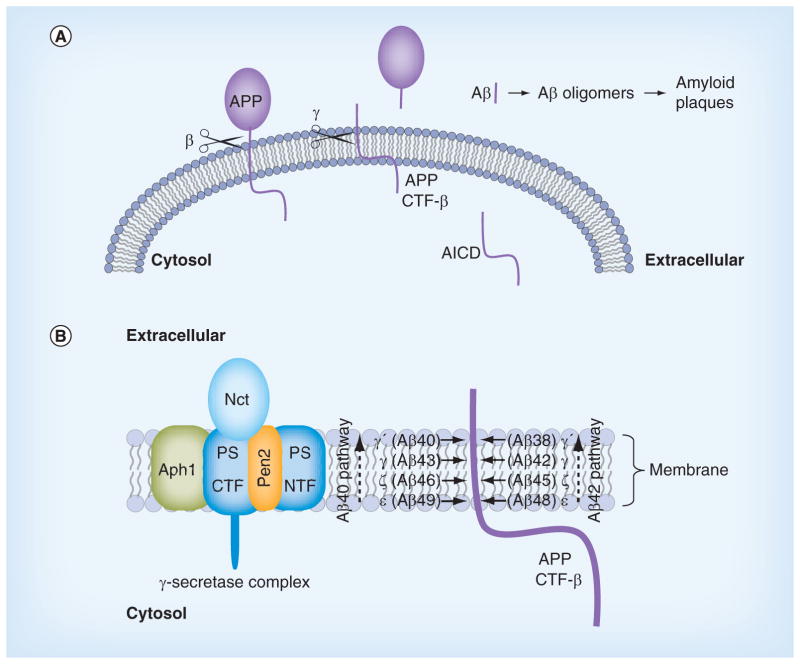
Aβ is a 38–43 amino acid secreted peptide derived from the amyloid β-protein precursor (APP), a 695–770 amino acid single-pass transmembrane protein (Figure 1A) [3]. The large extracellular/lumenal domain of APP is shed from the membrane by either α-secretase or β-secretase activity. α-secretases are membrane-anchored metalloproteases that cut APP within the Aβ region, thus precluding Aβ production, leaving an APP C-terminal fragment (APP CTF-α) in the membrane. β-secretase cuts further away from the transmembrane domain to generate the N-terminal region of Aβ as part of APP CTF-β. Both APP CTF-α and -β are subsequently cleaved by γ-secretase to produce p3 (from CTF-α) and Aβ (from CTF-β) and the APP intracellular domain (AICD).
Figure 1. Production of amyloid β-peptide from amyloid precursor protein.
(A) APP is an integral membrane protein, with the Aβ portion derived in part from the single transmembrane domain. β-secretase (β scissors) cleaves outside the membrane to release the large extracellular domain of APP. The membrane-bound remnant is then cleaved within the transmembrane domain by γ-secretase (γ-scissors) to produce Aβ and the AICD. The aggregation-prone Aβ peptide forms neurotoxic oligomers and amyloid plaques. (B)γ-secretase is composed of four different membrane proteins, with the presenilin N-terminal and C-terminal fragment heterodimer as the catalytic component that carries out proteolysis within the APP transmembrane domain. The protease complex initially cuts at the ε sites to produce long Aβ peptides Aβ48 or Aβ49. The enzyme then trims these long Aβ peptides in increments of 3–4 amino acids (at the ζ, γ and γ′ sites) along the Aβ40 or Aβ42 pathways.
Aβ: Amyloid β-peptide; AICD: Amyloid precursor protein intracellular domain; APP: Amyloid precursor protein.
The biological roles of APP and its proteolytic fragments are largely unknown [8]. Aβ can interact with nicotinic acetylcholine receptors to modulate its activity [9], although it is unclear if this is a normal and physiologically relevant role. The AICD has been suggested to translocate to the nucleus, where it may interact with certain transcription factors and control gene expression; however, none of the candidate genes have been sufficiently validated [10]. Secreted APP ectodomain has been reported to regulate neuronal apoptosis (pruning) during development [11], although again, this has not been confirmed. A variety of roles have been suggested for full-length APP, including cell proliferation, differentiation, neurite outgrowth, cell adhesion and synaptogenesis [8].
The predominant form of Aβ is composed of 40 amino acids, while ~5–10% is 42 amino acids, with the difference at the C-terminus, which is formed by cleavage of the transmembrane domain of APP by γ-secretase [12]. Although Aβ42 is a minor variant, this peptide is much more prone to aggregation than is Aβ40 [13] and is the primary component of the amyloid plaques in the AD brain [14]. Aβ42 has been extensively studied for many years to understand the aggregation process; the structure of the monomer, oligomers, protofibrils, fibrils and plaques [15]; the neurotoxic species and possible neurotoxic pathways [16]. The current dogma is that one or more soluble oligomeric forms of Aβ42 are the neurotoxic entity, although this leading hypothesis has been met with reasonable skepticism [17].
Genetics
The strongest evidence for the pathogenicity of Aβ in AD comes from genetics [5]. Dominant missense mutations in the APP gene are associated with early-onset familial AD (FAD) with virtually 100% penetrance [18]. Although APP is a large membrane protein, all the FAD mutations in APP are found in and around the small Aβ region and alter the production or aggregation propensity of Aβ peptides. An FAD double mutation found immediately proximal to the N-terminus of Aβ is cleaved more effectively by β-secretase, leading to increased Aβ production [19,20]. Recently, a protective mutation in this region was discovered, substantially reducing the risk of developing AD, even when carriers reach their 80s or 90s [21]. This protective mutation decreases cleavage by β-secretase, leading to lower production of Aβ. Other mutations found within the Aβ sequence increase its aggregation propensity (e.g., [22]). Still others, found near the γ-secretase cleavage site, lead to increased ratios of Aβ42 to Aβ40 (e.g., [23]), thereby increasing the aggregation propensity as well.
Dominant FAD missense mutations are also found in genes encoding presenilin-1 and -2 (PS1 and PS2), multipass membrane proteins that comprise the catalytic component of γ-secretase [5]. Over 100 such mutations have been identified [18], and some can cause AD even before age 30 [24]. Like those FAD mutations in APP near the γ-secretase cleavage site, FAD-mutant PS1 and PS2 lead to increased Aβ42/Aβ40 and overall increased aggregation propensity [25–28]. Other than the strong heredity factor and early age of onset, these dominant missense mutations in APP and the presenilins result in a similar course of disease onset, progression and brain pathology (including neurofibrillary tangles containing tau filaments) that are observed in sporadic, late-onset AD.
While dominant mutations in the gene encoding tau can also cause neurodegenerative diseases, AD is not one of them; rather, these mutations typically result in frontotemporal lobar degeneration (FTLD) [7,29]. FTLD-associated tau mutations, however, lead to similar tau-based neuropathology (intraneuronal filaments and neurofibrillary tangles) that are seen in AD. FTLD-associated mutations in the tau gene (MAPT) are of two types: missense mutations and silent or intronic mutations. The former mutations change the sequence of the protein and can increase its propensity to aggregation [30–37], while the latter mutations change alternative RNA splicing to increase the proportion of a particular tau isoform [38,39]. Like Aβ, it is unclear which form of tau is pathogenic. Nevertheless, the discovery of these mutations demonstrated that changes in tau alone are sufficient for tau pathology, neurodegeneration and dementia. Mutation of MAPT is apparently one of several ways to elicit tau pathogenicity, with aberrant Aβ being the trigger in AD [40].
As for sporadic late-onset AD, the strongest genetic risk factor is apolipoprotein E (APOE) genotype [41]. Apolipoprotein E is a cholesterol-carrying protein with three allelic variants (E2, E3 and E4). One copy of the E4 allele increases the risk of late-life AD by threefold to fourfold; two copies increases the risk 12- to 15-fold. The ApoE4 allele is responsible for many more cases of AD than the FAD mutations found in APP and the presenilins: FAD is quite rare, while sporadic, late-onset AD is much more common. How ApoE4 increases the risk of AD is not clear, although evidence supports decreased Aβ clearance from the brain [42]. While the FAD cases are very rare, because the presentation, course and pathology are similar to sporadic AD, the mechanisms by which the FAD mutations result in neurodegeneration and dementia should be informative about the pathogenic mechanism of AD in general. For this reason, Aβ and Aβ production, particularly via proteolytic cleavage by γ-secretase, has been such a major focus in the field. FAD mutations alter Aβ or Aβ production, with the large majority of these mutations found in the presenilins, which alter γ-secretase activity to increase Aβ propensity to aggregation. In the next section, we discuss the details of γ-secretase biochemistry and the effects of FAD mutations on its activity.
Biochemistry
As mentioned earlier, presenilin is the catalytic component of γ-secretase. Specifically, two completely conserved transmembrane aspartic acids are required for proteolytic activity [43], placing γ-secretase in the aspartyl protease family, even though it otherwise bears no resemblance to the many well-known soluble aspartyl proteases (e.g., pepsin, renin, HIV protease). The presence of the two transmembrane aspartates within a multipass membrane protein is consistent with the ability of γ-secretase to cleave within the transmembrane domain of APP in generating Aβ. Three other membrane proteins are components of γ-secretase along with presenilin: nicastrin, Aph-1 and Pen-2 [44–46]. After all four components assemble in the endoplasmic reticulum, presenilin undergoes autoproteolysis [43,47] into an N-terminal fragment (NTF) and C-terminal fragment (CTF) to generate the active γ-secretase complex (Figure 1B). Each of these presenilin subunits contributes one of the catalytic aspartates to the active site; that is, the active site is located at the interface between presenilin NTF and CTF [48,49]. Although several other proteins have been reported as activators or modulators of γ-secretase [50–52], none of these have been validated. The protease is trafficked through the secretory pathway to the cell surface [53–55]. APP proteolysis takes place everywhere from the Golgi apparatus to the cell membrane and endocytic vesicles [56–59].
γ-secretase has many more substrates besides APP [60]. Indeed, so many substrates have been identified that the γ-secretase complex has been called the proteasome of the membrane [61], implying that one of its major functions is to clear out membrane protein stubs that remain after ectodomain release by sheddases. While membrane protein clearance may be an important function of γ-secretase, the protease also plays essential roles in certain cell signaling pathways. The most important of these is signaling from the Notch family of receptors [62]. Notch receptors are single-pass membrane proteins such as APP, and proteolytic processing of Notch, triggered by interaction with cognate ligands on neighboring cells, leads to release of its intracellular domain. The Notch intracellular domain translocates to the nucleus and interacts with specific transcription factors that control the expression of genes involved in cell differentiation. These signaling pathways, particularly from Notch1 receptors, are essential to proper development in all multicellular animals. Knockout of presenilin genes in mice leads to phenotypes that are virtually identical with those observed upon knockout of the Notch1 gene [63,64], findings that, as explained in the next section, have major implications for the potential of γ-secretase inhibitors (GSIs) as AD therapeutics.
Inhibitors
The search for GSIs has gone on for over two decades, ever since Aβ was discovered to be a normally secreted peptide produced from a variety of cell types in culture [65]. Such inhibitors were identified even before the components of the protease were known and ultimately served as critical tools for discovery of presenilin as the catalytic component [48,49]. Initially, these inhibitors were simple peptidomimetics, but pharmaceutical companies quickly developed compounds with much better drug-like properties that allowed in vivo testing for the ability to lower Aβ in the brains of transgenic AD mice (e.g., expressing FAD mutant APP and presenilins).
Acute treatment with GSIs did show such proof of principle for these drug candidates [66,67]; however, chronic treatment revealed serious peripheral toxicities [68,69], such as gastrointestinal bleeding and immunosuppression and skin lesions, all effects that could be traced to inhibition of Notch1 proteolysis and signaling. As AD patients would be required to take GSIs for years and perhaps decades, the severe toxic consequences of γ-secretase inhibition caused great concern. While there were hopes for a therapeutic window that would allow lowering brain Aβ levels without the peripheral Notch-deficient toxicity, the failure of one GSI, semagacestat, in Phase III clinical trials, dashed these hopes [70]. The trial resulted in unacceptable peripheral toxicities, and more worrisome, but cognition that was worse than the placebo control groups.
Because all the serious toxic effects were apparently caused by inhibition of Notch1 signaling, the focus then went into finding GSIs that could selectively inhibit the proteolysis of APP by γ-secretase without affecting Notch1 proteolysis. The finding that the γ-secretase complex contained a nucleotide binding site that could selectively influence the cleavage of APP over Notch1 [71,72] led to the discovery of so-called ‘Notch-sparing’ GSIs [73,74]. The name is somewhat misleading, however, as these compounds show APP/Notch selectivity, not complete lack of effect on Notch proteolysis. Moreover, the degree of selectivity has been a matter of debate, with some reports of a lack of any selectivity for APP [75,76]. One such compound, avagacestat, went as far as Phase II clinical trials, and like semagacestat caused Notch-deficient toxicities at higher doses, equivocal CSF Aβ lowering at lower doses and worsening of cognition [77]. Evidence from mouse models suggest that the cognitive worsening may be due to increased γ-secretase substrates (APP CTF-α and CTF-β) [78], although elevation of total Aβ, seen in plasma at low inhibitor concentrations, may be responsible [79,80]. These findings have had a tremendous chilling effect on further development of GSIs for AD, and it is unlikely that another GSI will be brought into clinical trials in the foreseeable future. Interestingly though, these compounds may be repurposed for oncology, as many clinical trials are ongoing for the treatment of various cancers that involve overactive Notch signaling.
Modulators
While GSIs appear to be out of further consideration for AD therapeutics, γ-secretase modulators (GSMs) are still of keen interest. These compounds have the remarkable effect of lowering Aβ42 levels without decreasing overall Aβ levels or otherwise inhibiting general γ-secretase activity [81,82]. The decrease in Aβ42 is correlated with an increase in Aβ38, thus replacing a highly aggregation-prone form of Aβ with a much more soluble form. Thus, these compounds can prevent the formation of Aβ42-containing oligomers and plaques in the brain. GSMs, however, have no effect, even at very high concentrations, on Notch proteolysis and signaling, nor do they elevate γ-secretase substrates APP CTF-α and CTF-β. Presumably for these reasons, these compounds have shown excellent safety profiles, both in animal models and in human trials.
The mechanism of action of these compounds is not entirely clear, although the correlation between Aβ42 lowering and Aβ38 elevation is apparently relevant. Strong evidence supports Aβ42 as a precursor to Aβ38, with γ-secretase cleaving the Aβ42 C-terminus to release a tetrapeptide [83]. Although reports of dissociation between Aβ42 and Aβ38 have been interpreted as evidence against a precursor–product relationship [84,85], the recent finding that isolated γ-secretase converts synthetic Aβ42 to Aβ38 with release of the tetrapeptide [86] unambiguously confirms the relationship. Moreover, presenilin mutations decrease the Aβ42-to-Aβ38 conversion while GSMs stimulate it. Thus, GSMs appear to decrease Aβ42 by enhancing the carboxypeptidase activity of γ-secretase that converts this aggregation-prone peptide to Aβ38.
A critical issue with GSMs, however, like all anti-Aβ therapeutic strategies, is the design of clinical trials [87]. So far, all reported clinical trials with candidate AD therapeutic agents, including GSIs, GSMs and anti-Aβ immunotherapy, have been with individuals who already have AD. Even in those with mild or moderate AD, substantial neurodegeneration has occurred, and there are serious concerns that targeting Aβ after the onset of symptoms is too late. Aβ pathology in the brain may appear more than 10 years before the clinical manifestation of AD. As Aβ pathology apparently precedes tau pathology [88], and tau pathology may then propagate from neuron to neuron [89,90], blocking Aβ after tau pathology is initiated may not prevent or slow the progression of AD and may not even prevent or delay disease onset. For anti-Aβ strategies, including GSMs, to succeed, clearer knowledge of the pathogenic process and timing is needed, as are convenient and reliable biomarkers and diagnostics.
Moreover, the clinical success of GSMs is completely dependent on whether Aβ42 is indeed the pathogenic entity in AD. The reasons for the focus on Aβ42 are arguably more historic than a result of an objective search starting with no preconceptions. Over 100 years ago, Alois Alzheimer described extraneuronal amyloid plaques as a signature pathological characteristic of the disease, and the discovery in the late 1980s and early 1990s that the primary protein component of the plaques is Aβ [91,92] with Aβ42 being the predominant species [14] and most aggregation prone [13], led to the assumption that Aβ42 is the likely pathogenic species. Subsequent studies ranging from effects of APP and presenilin mutations to the neurotoxicity of various Aβ assemblies would seem to confirm this hypothesis. However, selectivity in the reporting of findings (negative results are more difficult to publish) in combination with incomplete knowledge of all forms of Aβ could result in mistaking correlation for causality. This issue will be addressed in further detail in the next sections.
Processive proteolysis
In recent years, the proteolysis of APP substrates by γ-secretase has been revealed to be considerably more complicated than initially believed. As noted earlier, secreted forms of Aβ range from 38 to 43 amino acids. However, identification of the N-terminus of the other product of γ-secretase cleavage of APP, AICD, revealed that proteolysis also occurs between residues 49 and 50 (Aβ numbering) [93], close to the cytosolic end of the transmembrane domain, and this cleavage event is also γ-secretase-dependent. Mass spectrometric analysis of AICD showed that a minor degree of cleavage also occurs between residues 48 and 49 [94]. Interestingly, FAD mutations were found to increase the proportion of AICD beginning at residue 49 over that beginning at residue 50, similar to the ability of these mutations to increase Aβ42 over Aβ40 [94]. Thus, γ-secretase cleaves the APP transmembrane domain at least twice, and both of these cleavage events are altered by FAD mutations.
In the analysis of γ-secretase cleavage products, however, some residues were unaccounted for. Most Aβ peptides are Aβ40, while most AICD begins at residue 50. The discovery of longer forms of Aβ was an important step in solving this mystery. Modified gel electrophoresis methods allowed separation of Aβ peptides to one amino acid resolution, and such analysis revealed Aβ45, Aβ46, Aβ48 and Aβ49 [95–97]. Thus, Aβ peptides ranging all the way to where AICD begins suggested that initial proteolysis occurs near the C-terminal end of the transmembrane domain, producing AICD and either Aβ48 or Aβ49. These long Aβ peptides are then trimmed through a carboxypeptidase function of γ-secretase to produce the secreted forms of Aβ [96,98]. Further studies refined this idea by suggesting two pathways for Aβ production: Aβ49-Aβ46-Aβ43-Aβ40 and Aβ48-Aβ45-Aβ42-Aβ38 (Figure 1B) [99]. The expected tri- and tetra-peptides formed in this process have been detected by mass spectrometry in cell-free γ-secretase assays [83]. Interestingly, the small levels of long Aβ peptides released by the protease complex before complete trimming are found in cell membrane fractions [95], as might be expected given that these peptides contain most of the APP transmembrane domain. A new study shows differences in the proportions of Aβ products formed by different isoforms of γ-secretase, with Aph-1B and PS2 associated with skewing toward longer Aβ peptides [100]. Such a finding is consistent with antisense against PS1 expression leading to increased Aβ42/Aβ40 [101].
Reports of dissociation between Aβ42 and Aβ38 have led to doubts about the precursor–product relationship between these two peptides [84,85], and other reports of dissociation between Aβ and AICD have questioned the carboxypeptidase activity of γ-secretase and the dual-pathway model (e.g., [52]). However, as mentioned above, unambiguous evidence that γ-secretase converts Aβ42 to Aβ38 has recently been provided using in vitro assays [86]. Furthermore, a dissociation of Aβ and AICD at the γ-secretase level is a stoichiometric impossibility, as cleavage of APP CTF-β must give one Aβ molecule for every AICD, and this has been formally proven to be the case through careful quantification [96]. Moreover, in unpublished work, we have found that synthetic peptides ranging from Aβ45 to Aβ49 are converted to Aβ40 and Aβ42 by purified γ-secretase and in the same proportions as expected from the dual-pathway model [Fernandez MA, Wolfe MS, Unpublished Data]. These results were seen whether using detergent solubilized γ-secretase preparations or purified protease complex reconstituted into lipid vesicles. Thus, the carboxypeptidase activity of γ-secretase and the dual-pathway model are unambiguously established as intrinsic properties of the enzyme independent of the membrane.
Presenilin mutations: new findings
Recent findings on the more detailed effects of presenilin FAD mutations on Aβ production suggest how these mutations may cause disease. In a study from our laboratory [102], five different FAD PS1-mutant γ-secretase complexes were compared with wild-type using in vitro assays and PAGE analysis of the range of Aβ peptides as well as AICD. Four of the five FAD mutations caused a reduction in AICD and total Aβ production compared with the wild-type enzyme, consistent with previous findings that the majority of PS1 mutations cause a ‘loss of function’ in proteolysis. One of these mutations, however, produced AICD and Aβ to the same degree as the wild-type enzyme, demonstrating that such general reduction of proteolytic function is not essential for pathogenicity. However, all five mutations skewed Aβ production in favor of the longer forms (>Aβ42), suggesting that the mutations all caused a deficiency in the carboxypeptidase function of γ-secretase. Another report from the laboratory of Bart De Strooper [76] confirmed these findings, that presenilin FAD mutations do not necessarily inhibit general proteolysis by γ-secretase but do cause qualitative changes in the types of Aβ peptides produced, in favor of longer forms. Most recently, in unpublished work we have further found that PS1 FAD-mutant γ-secretase complexes are dramatically deficient in their ability to trim Aβ48 and Aβ49 to Aβ40 and Aβ42, providing important confirmation of reduced carboxypeptidase function as the common feature of disease-causing γ-secretase, independent of initial cleavage of APP CTF-β to form AICD and Aβ48/49 [Fernandez MA, Wolfe MS, Unpublished Data].
Collectively, these recent findings show that the increase in Aβ42-to-Aβ40 observed by presenilin FAD mutations may be a consequence of reduced trimming function. However, the relationship between these two Aβ peptides is not the only change. In general, the proportion of longer forms of Aβ, including membrane-associated forms Aβ45-Aβ49, are substantially increased, begging the question of what pathogenic role, if any, these Aβ peptides may play. In any event, the apparent reduction in carboxypeptidase function caused by presenilin FAD mutations suggests that therapeutic agents targeting γ-secretase should correct this biochemical defect; that is, rather than searching for inhibitors of γ-secretase, stimulators of the carboxypeptidase function should be sought. The GSMs identified to date do this, but only for the last of the trimming step (e.g., Aβ42 to Aβ38), making their therapeutic potential entirely dependent on Aβ42 being the primary pathogenic species. A more general stimulator of γ-secretase carboxypeptidase activity might have a better chance of working, as it would not depend on knowing exactly which form of Aβ is responsible for the cascade of events that result in neurotoxicity, neurodegeneration and dementia.
Conclusion
Presenilin and the γ-secretase complex are central to the pathogenesis of AD, as dominant mutations in the presenilins alter γ-secretase activity and Aβ production and cause hereditary AD in midlife that is otherwise indistinguishable from sporadic, late-life AD. The search for potential AD therapeutics that target γ-secretase has gone on for many years, even before the discovery of the components of this membrane-embedded protease complex and the plethora of other substrates besides APP. Potent brain-penetrant inhibitors have been identified and brought into clinical trials; however, these compounds show serious toxic effects due to inhibition of proteolysis and signaling from the Notch1 receptor. More worrisome, cognitive worsening was observed, which may be due to increased levels of APP γ-secretase substrates. Modulators of γ-secretase have been discovered that selectively lower Aβ42 and are currently in clinical trials. Although these compounds are apparently safe, their potential to prevent or treat AD is dependent on Aβ42 being the primary pathogenic entity. New findings on the effects of AD-causing presenilin mutations reveal a common defect in carboxypeptidase function, resulting in increased proportions of other Aβ peptides, even longer than Aβ42. The pathogenicity of these longer Aβ peptides is unknown.
Future perspective
Given the clear failure of GSIs in the clinic for the treatment of AD, such agents will likely not be further pursued any time in the foreseeable future, although they may have potential for the treatment of cancers involving overstimulated Notch signaling [103]. GSMs remain viable candidates, but clinical evidence that these agents slow or stop AD progression is so far lacking. Independent evidence that Aβ42 is indeed a worthwhile target (e.g., via immunotherapy) would increase interest in GSMs for AD. New evidence shows that presenilin mutations reduce the carboxypeptidase function of γ-secretase to increase proportions of longer forms of Aβ, suggesting that more general stimulators of this trimming function might be more appropriate. However, all anti-Aβ strategies would be expected to fail as treatments for AD patients if pathogenic Aβ triggers neurotoxic events that then become Aβ-independent (e.g., propagation of pathological tau from neuron to neuron). The potential of these agents for prevention will require identification of appropriate biomarkers that enable the formulation of convenient diagnostic tests capable of discerning those pre-symptomatic individuals who are at high risk of developing AD.
EXECUTIVE SUMMARY.
Background
Current treatments for Alzheimer’s disease (AD) are symptomatic and largely ineffective.
Evidence strongly supports the amyloid β-peptide (Aβ) as the pathogenic initiator of AD. In particular, dominant mutations in genes encoding the Aβ precursor protein (APP) and the presenilins all alter Aβ properties or production.
Presenilin is the catalytic component of γ-secretase, a protease that determines the length of Aβ peptides.
Current approaches
Inhibitors of γ-secretase have failed in clinical trials, causing toxicities resulting from blocking the proteolysis of another substrate and cognitive worsening that may be due to elevation of APP substrates.
Modulators of γ-secretase can selectively lower Aβ42 and appear to be safe, but their potential as AD therapeutics is unclear.
Presenilin mutations specifically reduce the trimming function of γ-secretase, which converts long Aβ peptides to shorter secreted forms.
Future prospects
Stimulation of the trimming function of γ-secretase would take an unbiased view of which particular Aβ peptide is pathogenic.
All anti-Aβ strategies may require diagnostics that identify at-risk individuals years before the onset of symptoms.
Footnotes
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
For reprint orders, please contact: reprints@futuremedicine.com
References
- 1.Dorsey ER, George BP, Leff B, Willis AW. The coming crisis: obtaining care for the growing burden of neurodegenerative conditions. Neurology. 2013;80(21):1989–1996. doi: 10.1212/WNL.0b013e318293e2ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314(5800):777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 3.Masters CL, Selkoe DJ. Biochemistry of amyloid beta-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(6):a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger Z, Roder H, Hanna A, et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27(14):3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8(8):423–434. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8(9):663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 8.Muller UC, Zheng H. Physiological functions of APP family proteins. Cold Spring Harb Perspect Med. 2012;2(2):a006288. doi: 10.1101/cshperspect.a006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckingham SD, Jones AK, Brown LA, Sattelle DB. Nicotinic acetylcholine receptor signalling: roles in Alzheimer’s disease and amyloid neuroprotection. Pharmacol Rev. 2009;61(1):39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebert SS, Serneels L, Tolia A, et al. Regulated intramembrane proteolysis of amyloid precursor protein and regulation of expression of putative target genes. EMBO Rep. 2006;7(7):739–745. doi: 10.1038/sj.embor.7400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457(7232):981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.De Strooper B, Iwatsubo T, Wolfe MS. Presenilins and γ-secretase: structure, function, and role in alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(1):a006304. doi: 10.1101/cshperspect.a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 14.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 15.Roychaudhuri R, Yang M, Hoshi MM, Teplow DB. Amyloid β-protein assembly and Alzheimer disease. J Biol Chem. 2009;284(8):4749–4753. doi: 10.1074/jbc.R800036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2(7):a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci. 2012;15(3):349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 18.ALZFORUM: Networking for a cure. www.alzforum.org/mutations.
- 19.Citron M, Oltersdorf T, Haass C, et al. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature. 1992;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 20.Cai XD, Golde TE, Younkin SG. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson T, Atwal JK, Steinberg S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488(7409):96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 22.Massi F, Klimov D, Thirumalai D, Straub JE. Charge states rather than propensity for beta-structure determine enhanced fibrillogenesis in wild-type Alzheimer’s beta-amyloid peptide compared with E22Q Dutch mutant. Protein Sci. 2002;11(7):1639–1647. doi: 10.1110/ps.3150102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki N, Cheung TT, Cai XD, et al. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 24.Moehlmann T, Winkler E, Xia X, et al. Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Abeta 42 production. Proc Natl Acad Sci USA. 2002;99(12):8025–8030. doi: 10.1073/pnas.112686799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borchelt DR, Thinakaran G, Eckman CB, et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17(5):1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 26.Citron M, Westaway D, Xia W, et al. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3(1):67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- 27.Tomita T, Maruyama K, Saido TC, et al. The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue. Proc Natl Acad Sci USA. 1997;94(5):2025–2030. doi: 10.1073/pnas.94.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia W, Zhang J, Kholodenko D, et al. Enhanced production and oligomerization of the 42-residue amyloid beta-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J Biol Chem. 1997;272(12):7977–7982. doi: 10.1074/jbc.272.12.7977. [DOI] [PubMed] [Google Scholar]
- 29.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 30.Pickering-Brown SM, Baker M, Nonaka T, et al. Frontotemporal dementia with Pick-type histology associated with Q336R mutation in the tau gene. Brain. 2004;127(Pt 6):1415–1426. doi: 10.1093/brain/awh147. [DOI] [PubMed] [Google Scholar]
- 31.Nacharaju P, Lewis J, Easson C, et al. Accelerated filament formation from tau protein with specific FTDP-17 missense mutations. FEBS Lett. 1999;447(2–3):195–199. doi: 10.1016/s0014-5793(99)00294-x. [DOI] [PubMed] [Google Scholar]
- 32.Goedert M, Jakes R, Crowther RA. Effects of frontotemporal dementia FTDP-17 mutations on heparin-induced assembly of tau filaments. FEBS Lett. 1999;450(3):306–311. doi: 10.1016/s0014-5793(99)00508-6. [DOI] [PubMed] [Google Scholar]
- 33.Gamblin TC, King ME, Dawson H, et al. In vitro polymerization of tau protein monitored by laser light scattering: method and application to the study of FTDP-17 mutants. Biochemistry. 2000;39(20):6136–6144. doi: 10.1021/bi000201f. [DOI] [PubMed] [Google Scholar]
- 34.Barghorn S, Zheng-Fischhofer Q, Ackmann M, et al. Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry. 2000;39(38):11714–11721. doi: 10.1021/bi000850r. [DOI] [PubMed] [Google Scholar]
- 35.von Bergen M, Barghorn S, Li L, et al. Mutations of tau protein in frontotemporal dementia promote aggregation of paired helical filaments by enhancing local beta-structure. J Biol Chem. 2001;276(51):48165–48174. doi: 10.1074/jbc.M105196200. [DOI] [PubMed] [Google Scholar]
- 36.Grover A, England E, Baker M, et al. A novel tau mutation in exon 9 (1260 V) causes a four-repeat tauopathy. Exp Neurol. 2003;184(1):131–140. doi: 10.1016/s0014-4886(03)00393-5. [DOI] [PubMed] [Google Scholar]
- 37.Neumann M, Diekmann S, Bertsch U, Vanmassenhove B, Bogerts B, Kretzschmar HA. Novel G335V mutation in the tau gene associated with early onset familial frontotemporal dementia. Neurogenetics. 2005;6(2):91–95. doi: 10.1007/s10048-005-0210-y. [DOI] [PubMed] [Google Scholar]
- 38.Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta. 2005;1739(2–3):240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe MS. Tau mutations in neurodegenerative diseases. J Biol Chem. 2009;284(10):6021–6025. doi: 10.1074/jbc.R800013200. [DOI] [PubMed] [Google Scholar]
- 40.Roberson ED, Scearce-Levie K, Palop JJ, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 41.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 42.Castellano JM, Kim J, Stewart FR, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 44.Takasugi N, Tomita T, Hayashi I, et al. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422(6930):438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 45.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. γ-secretase is a membrane protein complex comprised of presenilin, nicastrin, aph-1, and pen-2. Proc Natl Acad Sci USA. 2003;100(11):6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5(5):486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 47.Fukumori A, Fluhrer R, Steiner H, Haass C. Three-amino acid spacing of presenilin endoproteolysis suggests a general stepwise cleavage of gamma-secretase-mediated intramembrane proteolysis. J Neurosci. 2010;30(23):7853–7862. doi: 10.1523/JNEUROSCI.1443-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li YM, Xu M, Lai MT, et al. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405(6787):689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 49.Esler WP, Kimberly WT, Ostaszewski BL, et al. Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nat Cell Biol. 2000;2(7):428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- 50.Chen F, Hasegawa H, Schmitt-Ulms G, et al. TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature. 2006;440(7088):1208–1212. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- 51.Zhou S, Zhou H, Walian PJ, Jap BK. CD147 is a regulatory subunit of the gamma-secretase complex in Alzheimer’s disease amyloid beta-peptide production. Proc Natl Acad Sci USA. 2005;102(21):7499–7504. doi: 10.1073/pnas.0502768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He G, Luo W, Li P, et al. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature. 2010;467(7311):95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ray WJ, Yao M, Mumm J, et al. Cell surface presenilin-1 participates in the γ-secretase-like proteolysis of Notch. J Biol Chem. 1999;274(51):36801–36807. doi: 10.1074/jbc.274.51.36801. [DOI] [PubMed] [Google Scholar]
- 54.Kaether C, Capell A, Edbauer D, et al. The presenilin C-terminus is required for ER-retention, nicastrin-binding and gamma-secretase activity. EMBO J. 2004;23(24):4738–4748. doi: 10.1038/sj.emboj.7600478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaether C, Lammich S, Edbauer D, et al. Presenilin-1 affects trafficking and processing of betaAPP and is targeted in a complex with nicastrin to the plasma membrane. J Cell Biol. 2002;158(3):551–561. doi: 10.1083/jcb.200201123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia W, Zhang J, Ostaszewski BL, et al. Presenilin 1 regulates the processing of beta-amyloid precursor protein C-terminal fragments and the generation of amyloid beta-protein in endoplasmic reticulum and Golgi. Biochemistry. 1998;37(47):16465–16471. doi: 10.1021/bi9816195. [DOI] [PubMed] [Google Scholar]
- 57.Annaert WG, Levesque L, Craessaerts K, et al. Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. J Cell Biol. 1999;147(2):277–294. doi: 10.1083/jcb.147.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Runz H, Rietdorf J, Tomic I, et al. Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J Neurosci. 2002;22(5):1679–1689. doi: 10.1523/JNEUROSCI.22-05-01679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartmann T, Bieger SC, Bruhl B, et al. Distinct sites of intracellular production for Alzheimer’s disease A beta40/42 amyloid peptides. Nat Med. 1997;3(9):1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- 60.Haapasalo A, Kovacs DM. The many substrates of presenilin/gamma-secretase. J Alzheimers Dis. 2011;25(1):3–28. doi: 10.3233/JAD-2011-101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kopan R, Ilagan MX. Gamma-secretase: proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5(6):499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- 62.Selkoe D, Kopan R. Notch and presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 63.Wong PC, Zheng H, Chen H, et al. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387(6630):288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 64.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89(4):629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 65.Haass C, Schlossmacher MG, Hung AY, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 66.Dovey HF, John V, Anderson JP, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76(1):173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 67.Barten DM, Guss VL, Corsa JA, et al. Dynamics of {beta}-amyloid reductions in brain cerebrospinal fluid and plasma of {beta}-amyloid precursor protein transgenic mice treated with a {gamma}-secretase inhibitor. J Pharmacol Exp Ther. 2004;312(2):635–643. doi: 10.1124/jpet.104.075408. [DOI] [PubMed] [Google Scholar]
- 68.Searfoss GH, Jordan WH, Calligaro DO, et al. Adipsin: a biomarker of gastrointestinal toxicity mediated by a functional gamma secretase inhibitor. J Biol Chem. 2003;278:46107–46116. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- 69.Wong GT, Manfra D, Poulet FM, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279(13):12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 70.Doody RS, Raman R, Farlow M, et al. A Phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med. 2013;369(4):341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 71.Netzer WJ, Dou F, Cai D, et al. Gleevec inhibits beta-amyloid production but not Notch cleavage. Proc Natl Acad Sci USA. 2003;100(21):12444–12449. doi: 10.1073/pnas.1534745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fraering PC, Ye W, Lavoie MJ, Ostaszewski BL, Selkoe DJ, Wolfe MS. Gamma-secretase substrate selectivity can be modulated directly via interaction with a nucleotide binding site. J Biol Chem. 2005;280:41987–41996. doi: 10.1074/jbc.M501368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mayer SC, Kreft AF, Harrison B, et al. Discovery of begacestat, a Notch-1-sparing gamma-secretase inhibitor for the treatment of Alzheimer’s disease. J Med Chem. 2008;51(23):7348–7351. doi: 10.1021/jm801252w. [DOI] [PubMed] [Google Scholar]
- 74.Gillman KW, Starrett JE, Parker MF, et al. Discovery and evaluation of BMS-708163, a potent, selective and orally bioavailable gamma-secretase inhibitor. ACS Med Chem Lett. 2010;1(3):120–124. doi: 10.1021/ml1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Crump CJ, Castro SV, Wang F, et al. BMS-708, 163 targets presenilin and lacks notch-sparing activity. Biochemistry. 2012;51(37):7209–7211. doi: 10.1021/bi301137h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chavez-Gutierrez L, Bammens L, Benilova I, et al. The mechanism of γ-secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31(10):2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coric V, van Dyck CH, Salloway S, et al. Safety and tolerability of the gamma-secretase inhibitor avagacestat in a Phase 2 study of mild to moderate Alzheimer disease. Arch Neurol. 2012;69(11):1430–1440. doi: 10.1001/archneurol.2012.2194. [DOI] [PubMed] [Google Scholar]
- 78.Mitani Y, Yarimizu J, Saita K, et al. Differential effects between gamma-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice. J Neurosci. 2012;32(6):2037–2050. doi: 10.1523/JNEUROSCI.4264-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lanz TA, Karmilowicz MJ, Wood KM, et al. Concentration-dependent modulation of amyloid-beta in vivo and in vitro using the gamma-secretase inhibitor, LY-450139. J Pharmacol Exp Ther. 2006;319(2):924–933. doi: 10.1124/jpet.106.110700. [DOI] [PubMed] [Google Scholar]
- 80.Barnwell E, Padmaraju V, Baranello R, et al. Evidence of a novel mechanism for partial gamma-secretase inhibition induced paradoxical increase in secreted amyloid beta protein. PLoS ONE. 2014;9(3):e91531. doi: 10.1371/journal.pone.0091531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 82.Golde TE, Koo EH, Felsenstein KM, Osborne BA, Miele L. γ-secretase inhibitors and modulators. Biochim Biophys Acta. 2013;1828(12):2898–2907. doi: 10.1016/j.bbamem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takami M, Nagashima Y, Sano Y, et al. Gamma-secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29(41):13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Page RM, Baumann K, Tomioka M, et al. Generation of Abeta38 and Abeta42 is independently and differentially affected by familial Alzheimer disease-associated presenilin mutations and gamma-secretase modulation. J Biol Chem. 2008;283(2):677–683. doi: 10.1074/jbc.M708754200. [DOI] [PubMed] [Google Scholar]
- 85.Czirr E, Cottrell BA, Leuchtenberger S, et al. Independent generation of Abeta42 and Abeta38 peptide species by gamma-secretase. J Biol Chem. 2008;283(25):17049–17054. doi: 10.1074/jbc.M802912200. [DOI] [PubMed] [Google Scholar]
- 86.Okochi M, Tagami S, Yanagida K, et al. gamma-secretase modulators and presenilin 1 mutants act differently on presenilin/ gamma-secretase function to cleave Abeta42 and Abeta43. Cell Rep. 2013;3(1):42–51. doi: 10.1016/j.celrep.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 87.Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron. 2011;69(2):203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12(10):383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 89.de Calignon A, Polydoro M, Suarez-Calvet M, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73(4):685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu L, Drouet V, Wu JW, et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE. 2012;7(2):e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Comm. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 92.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weidemann A, Eggert S, Reinhard FB, et al. A novel var epsilon-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with notch processing. Biochemistry. 2002;41(8):2825–2835. doi: 10.1021/bi015794o. [DOI] [PubMed] [Google Scholar]
- 94.Sato T, Dohmae N, Qi Y, et al. Potential link between amyloid beta-protein 42 and C-terminal fragment gamma 49–99 of beta-amyloid precursor protein. J Biol Chem. 2003;278(27):24294–24301. doi: 10.1074/jbc.M211161200. [DOI] [PubMed] [Google Scholar]
- 95.Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, et al. Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci. 2005;25(2):436–445. doi: 10.1523/JNEUROSCI.1575-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kakuda N, Funamoto S, Yagishita S, et al. Equimolar production of amyloid beta-protein and amyloid precursor protein intracellular domain from beta-carboxyl-terminal fragment by gamma-secretase. J Biol Chem. 2006;281(21):14776–14786. doi: 10.1074/jbc.M513453200. [DOI] [PubMed] [Google Scholar]
- 97.Zhao G, Cui MZ, Mao G, et al. Gamma-cleavage is dependent on zeta-cleavage during the proteolytic processing of amyloid precursor protein within its transmembrane domain. J Biol Chem. 2005;280(45):37689–37697. doi: 10.1074/jbc.M507993200. [DOI] [PubMed] [Google Scholar]
- 98.Funamoto S, Morishima-Kawashima M, Tanimura Y, Hirotani N, Saido TC, Ihara Y. Truncated carboxyl-terminal fragments of beta-amyloid precursor protein are processed to amyloid beta-proteins 40 and 42. Biochemistry. 2004;43(42):13532–13540. doi: 10.1021/bi049399k. [DOI] [PubMed] [Google Scholar]
- 99.Yagishita S, Morishima-Kawashima M, Ishiura S, Ihara Y. Abeta46 is processed to Abeta40 and Abeta43, but not to Abeta42, in the low density membrane domains. J Biol Chem. 2008;283(2):733–738. doi: 10.1074/jbc.M707103200. [DOI] [PubMed] [Google Scholar]
- 100.Acx H, Chavez-Gutierrez L, Serneels L, et al. Signature amyloid beta profiles are produced by different gamma-secretase complexes. J Biol Chem. 2014;289(7):4346–4355. doi: 10.1074/jbc.M113.530907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Refolo LM, Eckman C, Prada CM, et al. Antisense-induced reduction of presenilin 1 expression selectively increases the production of amyloid beta42 in transfected cells. J Neurochem. 1999;73(6):2383–2388. doi: 10.1046/j.1471-4159.1999.0732383.x. [DOI] [PubMed] [Google Scholar]
- 102.Quintero-Monzon O, Martin MM, Fernandez MA, et al. Dissociation between the processivity and total activity of gamma-secretase: implications for the mechanism of Alzheimer’s disease-causing presenilin mutations. Biochemistry. 2011;50(42):9023–9035. doi: 10.1021/bi2007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141(2):140–149. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]