Abstract
An asymmetric epoxidation of trisubstituted α,β-unsaturated esters is described. The oxidation utilizes a pseudo-C2-symmetric iron(II) catalyst [Fe(L*)2(CH3CN)(OTf)](OTf) and peracetic acid as oxidant, yielding the α,β-epoxyesters in high enantiomeric purity (up to 99% ee).
Keywords: iron; asymmetric epoxidation; α, β-unsaturated ester
The oxidation reaction is one of the most powerful and fundamental transformations in organic chemistry. Among oxidation reactions, epoxidation of alkenes has been extensively studied as subsequent ring opening of epoxides affords versatile building blocks for the synthesis of more complex molecules. Pioneering contributions to asymmetric epoxidation, such as Katsuki-Sharpless epoxidation[1] and Jacobsen epoxidation[2], have involved the use of chiral metal complexes.
Since then, methods for epoxidation have flourished, including those towards electron deficient olefins, which are less reactive to electrophilic oxidants. Approaches to these targets are typically nucleophilic and generally execute a Weitz-Scheffer type mechanism. Examples of such systems include chiral ligand-metal peroxides[3], phase-transfer catalysts[4] and polyamino acid catalysts[5]. However, no single method can serve as the ultimate solution for the epoxidation of electron-deficient systems.[6]
Bio-inspired iron complexes, in particular, caught our attention, due to its low-cost, abundant and environmentally benign nature. In fact, many iron-catalysts have been developed in the past, including heme and non-heme biomimetic systems.[7] Our interest in β,β-disubstituted enones and α,β-unsaturated esters prompted our development of a non-heme phenanthroline-based ligand for the epoxidation of unsaturated carbonyl compounds. This catalyst has been proven effective in the asymmetric epoxidation of β,β-disubstituted enones, which is sterically congested at the β-carbon and thus hitherto inaccessible.[8] Nevertheless, the utilization of enantioenriched epoxy ketones is relatively narrow compared to epoxy esters, which can be readily converted to other functional groups such as epoxy carboxylic acid, amides and alcohols. And given such exciting results from those of β,β-disubstituted enones, we turn our attention to α,β-unsaturated esters, from which derivations are expected to be fruitful.
Examples of other systems that target α,β-unsaturated esters are yttrium-chiral biphenyldiol[9], chiral dioxirane[10] and chiral Mn-salen complexes[11]. More recently, Cussó et al. reported a chiral Fe-bipyrrolidine catalyst[12], which is used to access a wide range of carbonyl adjacent olefins, including α,β-unsaturated esters.
Unlike the majority of epoxidation on α,β-unsaturated esters, which generally employed disubstituted trans-alkenes, we started with the less reported trisubstituted (E)-alkene. An initial trial with -C(CH3)2(iPr) ester (Table 1) using similar conditions reported in preceding work[7] gave valuable result. Upon a brief optimization of the reaction conditions, we found performing the reaction at −20 °C significantly deteriorates the yield and enantioselectivity (entry 4), while raising the temperature to 20 °C produces a lower yield but similar selectivity. Two equivalents of peracetic acid are also observed to be the most desirable condition (entry 2). In addition, stirring the complex formation and epoxidation reactions at 1200 rpm is important in providing ideal results in terms of yields and enantioselectivities. Prompt addition of oxidant is also desirable presumably due to the short lifetime of the iron-oxo species.
Table 1.
Optimizing reaction conditions for the epoxidation of α,β-unsaturated esters. [a]
| ||||
|---|---|---|---|---|
| Entry | AcOOH (equiv.) | Temperature (° C) | Yield (%)[e] | ee (%)[f] |
| 1 | 1.8 | 0 | 42 | 92 |
| 2 | 2 | 0 | 58 | 94 |
| 3 | 2.2 | 0 | 51 | 86 |
| 4 | 2 | −20 | 32 | 66 |
| 5 | 2 | 20 | 38 | 92 |
| 6[b] | 2 | 0 | 43 | 78 |
| 7[c] | 2 | 0 | 57 | 86 |
| 8[d] | 2 | 0 | 54 | 95 |
Unless otherwise stated, reactions are performed in 0.6 ml CH3CN and stirred at 1200 revolutions per minute (rpm).
Reaction performed in 0.3 mL CH3CN.
Reaction performed in 1.2 mL CH3CN.
Fe(OTf)2 (10 mol %) and L* (20 mol %) were used.
Isolated yields.
Determined by HPLC on a chiral stationary phase.
Realizing that the -C(CH3)2(iPr) ester generates better results than t-butyl ester (Table 2, entries 1 and 2), we further screened a variety of alkoxy moieties on the ester, which could serve as an auxiliary group for improving stereochemical induction. Subsequent screening of different esters revealed the importance of the alkoxy group on the enantioselectivity of the reaction. As a general trend, tertiary alcohol based esters (entries 1, 2, 3 and 5) perform better than secondary alcohol based esters (entries 4, 6 and 7), due to higher steric hindrance. Among them, –C(CH2)2(tBu) ester (entry 3) provided optimum result with respect to both yield and ee.
Table 2.
Asymmetric epoxidation of α,β-unsaturated esters catalyzed by chiral Fe-phenanthroline catalyst.[a]
| |||
|---|---|---|---|
| Entry[a] | R1 | Yield (%) [b] | ee (%) [c] |
| 1 | tBu | 49 | 90 |
| 2 | C(CH3)2(iPr) | 58 | 94 |
| 3 | C(CH3)2(tBu) | 69 | 95 |
| 4 | iPr | 30 | 70 |
| 5 | C(Et)3 | 43 | 90 |
| 6 | CH(tBu)2 | 26 | 76 |
| 7 | cyclododecanyl | 18 | 63 |
All reactions were carried out on a 0.15 mmol scale of using Fe(OTf)2 (5 mol %), ligand (10 mol %), peracetic acid (32 wt. % in dilute acetic acid, 2 equiv) in CH3CN (0.6 mL) at 0 °C and quenched after 2 hours.
Isolated yields.
Determined by HPLC on a chiral stationary phase.
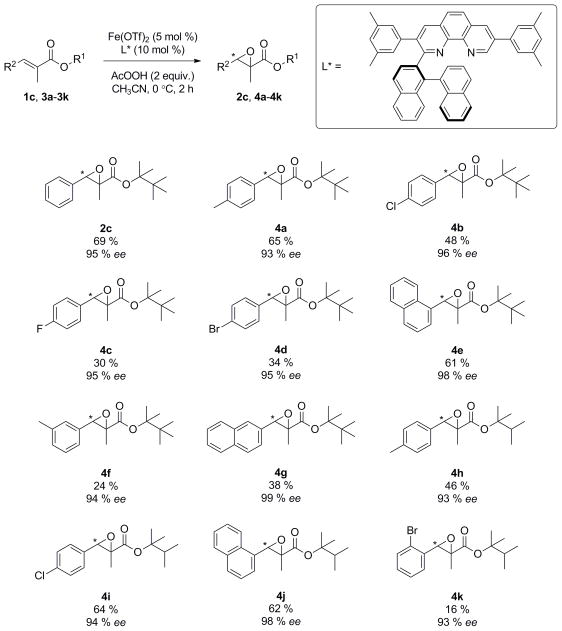
Our exploration of the substrate scope revealed that either -C(CH2)2(tBu) or –C(CH2)2(iPr) esters could be used to induce high enantioselectivity. While in some cases –C(CH2)2(tBu) ester gave higher yield and ee (Scheme 1, 4a and 2c), it would generate lower yield than its –C(CH2)2(iPr) analogue (4b and 4e) if the starting ester had lower solubility in acetonitrile. Nevertheless, high ee’s were still maintained even in such cases. Enantioselectivities are remarkably high for substrates with a large naphthyl group at the β-position (4e, 4g and 4j). In terms of reactivity, the epoxidation of para-substituted phenyl olefins gave a higher yield of the epoxide product than meta- and ortho-substituted ones. Although this catalytic system works well for phenyl and naphthyl system, it is not applicable to substrates bearing an alkyl, furyl, thienyl group at the β position of the ester.
Scheme 1.
Substrate scope of epoxidation. [a] [b] [c] [a] All reactions were carried out on a 0.15 mmol scale using Fe(OTf)2 (5 mol %), ligand (10 mol %), substrate (0.15 mmol), peracetic acid (32 wt. % in dilute acetic acid, 2 equiv) in CH3CN (0.6 mL) at 0 °C and quenched after 2 hours. [b] Isolated yields. [c] Determined by HPLC on a chiral stationary phase.
In summary, we report a highly enantioselective epoxidation of trisubstituted α,β-unsaturated esters catalyzed by a chiral iron-phenanthroline complex using peracetic acid as oxidant. This oxidation can enantioselectively target trans-α-methylcinnamic acid esters, of which –C(CH2)2(tBu) and –C(CH2)2(iPr) esters gave ideal results. The enantioselectivity was remarkably high for substrates bearing a large group at the β-position and was maintained even in cases of lower yields.
Experimental Section
General procedure for the asymmetric epoxidation of unsaturated esters
To a flame-dried test tube charged with ligand L* (10.3 mg, 0.016 mmol) and stir-bar under nitrogen was added a solution of Fe(OTf)2 (0.32 mL, 0.008 mmol, 0.025 M in CH3CN) followed by CH3CN (0.32 mL), resulting in a light yellow solution. The complex was stirred vigorously (1200 rpm) for 2–3 hours at room temperature. Another dry test tube was charged with the substrate (0.15 mmol) and flushed with nitrogen. The iron complex (0.6 mL, 0.0075 mmol) in CH3CN from the first test tube was added via syringe and the vessel was cooled to 0 °C for 10 min with vigorous stirring (1200 rpm). Peracetic acid (63 μL, 32 wt. % in dilute acetic acid, 0.3 mmol) was added to the reaction via microsyringe at once, yielding a near black solution. The reaction was stirred at this temperature for 2 hours and quenched with 10% Na2S2O3 in 1:1 saturated NaHCO3 : H2O (3 mL). The mixture was extracted with CH2Cl2, dried over Na2SO4, and concentrated in vacuo. The residue was purified by flash chromatography (hexane/CH2Cl2 = 1:1) to furnish the epoxide. The column was flushed with ethyl acetate (100%) to elute the ligand.
Supplementary Material
Acknowledgments
This work was supported by the NIH (2R01GM068433). We would like to thank Dr. Yasuhiro Nishikawa for his preliminary experimental results. We would like to thank Dr. Antoni Jurkiewicz, and Dr. Jin Qin for their assistance in NMR spectroscopy and mass spectrometry, respectively.
References
- 1.Katsuki T, Sharpless KB. J Am Chem Soc. 1980;102:5974–5976. [Google Scholar]
- 2.Zhang W, Loebach JL, Wilson SR, Jacobsen EN. J Am Chem Soc. 1990;112:2801–2803. [Google Scholar]
- 3.Enders D, Zhu J, Raabe G. Angew Chem, Int Ed. 1996;35:1725–1728. [Google Scholar]; Bougauchi M, Watanabe S, Arai T, Sasai H, Shibasaki M. J Am Chem Soc. 1997;119:2329–2330. [Google Scholar]; Elston CL, Jackson RFW, MacDonald SJF, Murray PJ. Angew Chem, Int Ed. 1997;36:410–412. [Google Scholar]
- 4.Lygo B, Wainwright PG. Tetrahedron Lett. 1998;39:1599–1602. [Google Scholar]; Corey EJ, Zhang FY. Org Lett. 1999;1:1287–1290. doi: 10.1021/ol990964z. [DOI] [PubMed] [Google Scholar]; Arai S, Tsuge H, Shioiri T. Tetrahedron Lett. 1998;39:7563–7566. [Google Scholar]
- 5.Juliá S, Masana J, Vega JC. Angew Chem, Int Ed. 1980;19:929–931. [Google Scholar]
- 6.For reviews on the epoxidation of electron deficient olefins: Porter MJ, Skidmore J. Chem Commun. 2000:1215–1225.Weiß KM, Tsogoeva SB. Chem Rec. 2011;11:18–39. doi: 10.1002/tcr.201000006.Diez D, Nunez MG, Anton AB, Garcia P, Moro RF, Garrido NM, Marcos IS, Basabe P, Urones JG. Curr Org Synth. 2008;5:186–216.
- 7.For review on biomimetic iron-catalyzed asymmetric epoxidation: Adv Synth Catal. 2014;356:261–299.
- 8.Nishikawa Y, Yamamoto H. J Am Chem Soc. 2011;133:8432–8435. doi: 10.1021/ja201873d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakei H, Tsuji R, Ohshima T, Shibasaki M. J Am Chem Soc. 2005;127:8962–8963. doi: 10.1021/ja052466t. [DOI] [PubMed] [Google Scholar]
- 10.Wu XY, She X, Shi Y. J Am Chem Soc. 2002;124:8792–8793. doi: 10.1021/ja020478y. [DOI] [PubMed] [Google Scholar]
- 11.Chang S, Galvin JM, Jacobsen EN. J Am Chem Soc. 1994;116:6937–6938. [Google Scholar]
- 12.Cussó O, Garcia-Bosch I, Ribas X, Lloret-Fillol J, Costas M. J Am Chem Soc. 2013;135:14871–14878. doi: 10.1021/ja4078446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.