Background
Immunosuppressed patients and experimental nonhuman primates are at risk of opportunistic infection. We report a Myroides spp. infection in an immunosuppressed baboon that had received a life-supporting kidney from a genetically engineered pig.
Case Report
The baboon received a costimulation blockade-based immunosuppressive regimen as well as 2 anti-inflammatory agents (tocilizumab and etanercept). Although the pig kidney functioned well, approximately 4 months after the transplantation, the baboon became less active and ate and drank poorly. On day 136, it collapsed and died despite inotropic and fluid support. A blood culture drawn before death grew Myroides spp.
Discussion and Conclusions
To our knowledge, Myroides spp. has not been reported as a cause of opportunistic infection in either patients with organ allotransplants or experimental animals. We summarize what is known about this rare organism and suggest it should be considered in any immunocompromised patient or animal. In the present case, we suggest the baboon died of circulatory shock following infection through an indwelling intravenous catheter.
Opportunistic infection is a common complication of both clinical allotransplantation (alloTx) and experimental xenotransplantation (xenoTx).1-3 We here report a baboon that died (more than 4 months after a kidney transplant from a genetically engineered pig) in a state of circulatory shock and was found to have a blood culture positive for Myroides spp. The baboon had received an immunosuppressive regimen based on costimulation blockade, and also anti-inflammatory therapy with an IL-6R antagonist and a TNF-α antagonist.
A search of the literature has failed to identify Myroides spp. as an infectious agent in clinical alloTx or in patients receiving long-term anti-inflammatory therapy or in experimental alloTx or xenoTx in nonhuman primates (NHPs) previously.
CASE REPORT
Life-supporting pig-to-baboon kidney Tx was carried out on March 20, 2014. The baboon (B9313, Papio species, 8.3 kg, of blood type B) was from a specific pathogen-free facility at the Division of Animal Resources, Oklahoma University Health Sciences Center, Oklahoma City, OK.4 The pig (Sus scrofa, 8.5 kg, blood group O; Revivicor, Blacksburg, VA) was an α1,3-galactosyltransferase gene-knockout pig transgenic for two human complement-regulatory proteins (CD46, CD55) and human coagulation-regulatory proteins (endothelial protein C receptor).
All animal care was in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication no. 86-23, revised 1985). Protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Immunosuppressive, anti-inflammatory, and adjunctive therapies are summarized in Table 1. In summary, the baboon recovered well from the operative procedure and showed good renal function (serum creatinine, 0.6-1.6 mg/dL) with no features of consumptive coagulopathy or protein-losing nephropathy for greater than 3 months (Figure 1). The indwelling intravenous (IV) catheter that had been inserted to facilitate management during the perioperative period was withdrawn on post-Tx day 26. After day 90, urine output gradually decreased and serum creatinine increased to 1.6-2.2 mg/dL. These changes were associated with the gradual development of a stricture of the ureter at the site of implantation into the bladder. Ultrasound indicated an increase in size of the kidney and dilatation of the ureter. On day 103, an operation was carried out to excise the ureteric stricture (which showed no infiltration with leukocytes or other features of rejection) and reimplant the ureter into the bladder. A renal biopsy was taken and showed no significant abnormality (not shown).
TABLE 1.
Immunosuppressive, anti-inflammatory, and adjunctive therapy in a baboon recipient of a genetically engineered pig kidney graft
FIGURE 1.
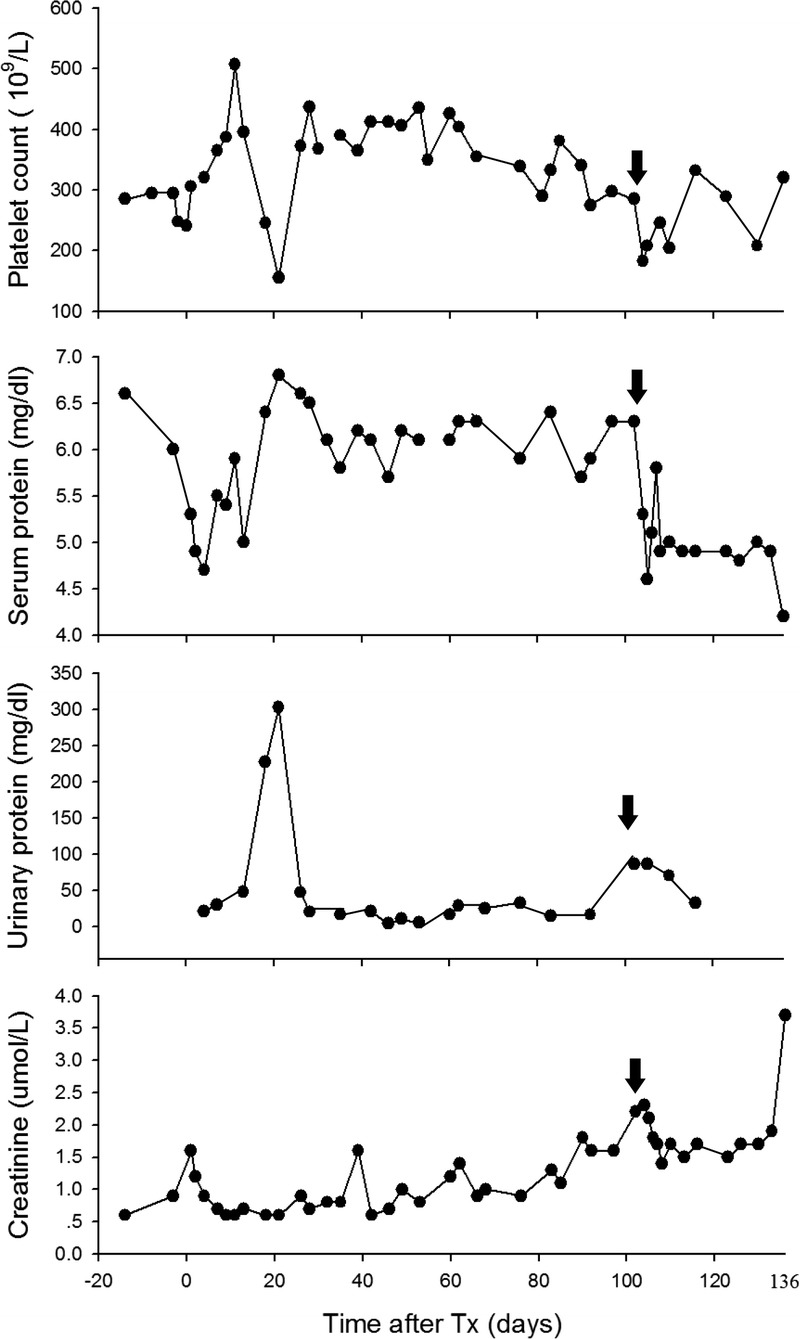
Platelet count, serum, and urinary protein, and serum creatinine in a baboon recipient of a life-supporting genetically engineered pig kidney. Graft survival (of 136 days) was longer than any previously reported case (arrows indicate the day of ureter reimplantation and renal biopsy.)
Subsequently, the serum creatinine remained slightly increased (1.5-1.9 mg/dL). The baboon was active, but was not drinking well and so on day 105 a single IV catheter was inserted into a branch of the right femoral vein using a jacket and tether system5 and remained in place for the remainder of the experiment. The presence of the catheter was likely a major factor in the subsequent bloodstream infection.
On day 135, the baboon became much less active and quiet, but otherwise unchanged. On the evening of day 136, the baboon had what appeared to be a “neurological event” (conscious with some limb rigidity, but no paralysis), the cause of which remained uncertain. Its temperature was 36.2 °C. The white blood cell count was higher than previously at 8300/mm3 (neutrophils, 6100/mm3; monocytes, 1400/mm3; lymphocytes, 800/mm3), platelet count was 320,000/mm3, hematocrit 27.4%, with basically normal electrolytes and metabolic parameters. With inotropic and IV fluid support, the baboon appeared to improve but, 2 hours later, suddenly died.
Blood drawn an hour before death of the baboon grew Myroides spp. Using the Bruker Biotyper MALDI-TOF instrument (Bruker Daltonics, Bilerica, MA), the isolates were identified as Myroides odoratus (blood had not been drawn for culture during the previous month.)
A necropsy was performed, and histopathology of the kidney graft showed patchy lesions in all 3 anatomic components of the transplanted kidney—glomerular, tubular, and interstitial—with thrombosis of primarily small vessels in both glomeruli and the interstitium, associated with focal infarction and tissue destruction, but little fibrosis (not shown). The pathologic features were not considered widespread or severe enough to account for the death of the animal.
DISCUSSION
This baboon represents the longest-surviving NHP supported by a pig kidney, all previous experiments having been terminated within 90 days.6,7 We attribute this relative success to the combination of (i) Tx of a kidney from a pig with multiple genetic modifications directed to prevent injury from the primate immune response and from the effects of coagulation dysfunction between pig and baboon,8 (ii) an immunosuppressive regimen that has been demonstrated to prevent a T-cell response to pig cardiac xenografts,9,10 and (iii) a regimen that reduces the inflammatory response.11
Using costimulation-based regimens, we have documented fewer infectious complications if all intravascular catheters are removed as soon as possible. In the present baboon, we felt forced to reinsert an IV catheter to ensure that the animal remained well hydrated, and we believe its presence may have been a major actor in the development of the Myroides infection. There were no features of infection in the organ-source pig or in the baboon before Tx. The clinical features in the baboon on day 136 were those of septic shock rather than acute rejection of the graft. Only 1 previous blood culture had been drawn from the baboon (day 7), which was negative, and so we have no record of the length of time the microorganism had been present in the baboon.
The positive blood culture indicated the presence of infection with Myroides spp., which is a rare cause of bloodstream infection, but which is widely distributed in nature. In recent years, novel species have been isolated from water and soil.12-16 Although they are not usually components of human microflora, they have been isolated from several different clinical specimens,17 and have been implicated in small nosocomial outbreaks.18-20 (Flavobacterium spp. have also caused fatal infections in patients with human immunodeficiency virus.21)
They are gram-negative, nonfermentative, obligately aerobic, yellow-pigmented, and nonmotile rods, with a characteristic fruity odor.22 Whether the death of the baboon was solely a result of the Myroides infection remains uncertain, but the development of circulatory collapse would correlate with a septic cause (even though the white blood count remained within the normal range, which is not unusual in immunosuppressed baboons). Nevertheless, its culture from the blood, in the absence of other microorganisms, seems worthy of reporting.
After its first isolation in 1923,23 the organism was designated as Flavobacterium odoratum and placed under genus Flavobacterium. However, it showed certain features that differentiated it from other Flavobacteriaceae, such as lack of gliding motility, good growth at 37 °C, halotolerance (tolerance to high salt concentration), and differences in the fatty acid profile. Based on genotypic and phenotypic data, the new genus Myroides was created in 1996,24 in which 2 species, Myroides odoratus (formerly F. odoratum) and Myroides odoratimimus, were included.
The diagnosis of Myroides spp. depends on culture and molecular techniques. Schröttner et al25 analyzed 22 strains using 3 identification methods. The results demonstrated that VITEK2 reliably identifies the genus Myroides, but cannot differentiate between Myroides odoratus and Myroides odoratimimus. In contrast, both MALDI_TOF MS and 16S rDNA sequencing efficiently distinguish between the species.
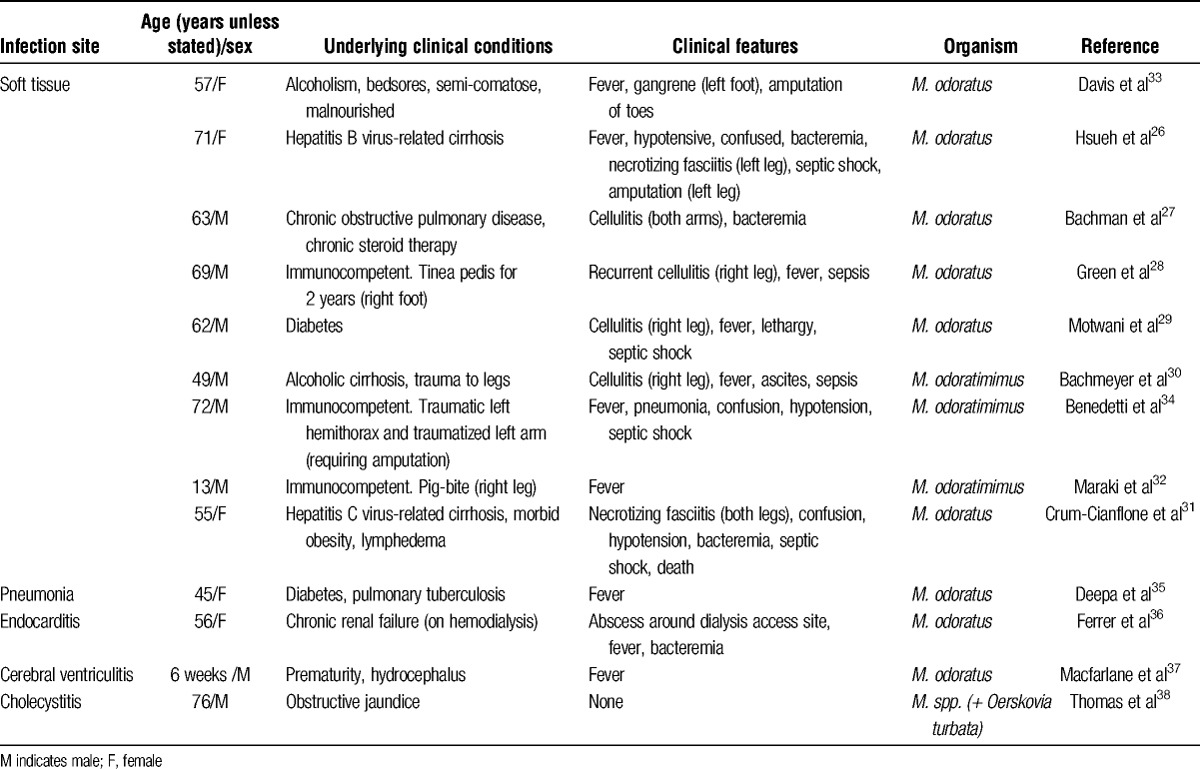
Myroides spp. microorganisms usually behave as low-grade opportunistic pathogens, though clinical infection caused by this organism is rare. A significant number of patients have been to some extent immunocompromised through, for example, liver cirrhosis, diabetes mellitus, or chronic obstructive pulmonary disease on long-term corticosteroid treatment. The organism has been responsible for soft tissue infection,26-31 including after a pig bite,32 surgical wound infection,33,34 pneumonia,35 endocarditis,36 cerebral ventriculitis,37 and cholecystitis38 (Table 2A). One patient died with a necrotizing fasciitis of both lower extremities and septic shock.31 Perhaps, surprisingly, we have not been able to identify any reports of infection caused by these microorganisms after solid organ alloTx.
TABLE 2A.
Clinical reports of infection with Myroides spp
Nosocomial outbreaks have been reported (Table 2B), including urinary tract infections, sometimes associated with urological surgery.19,20 An outbreak of central venous catheter-associated bloodstream infection was reportedly caused by commercial ampoules of sterile water contaminated by Myroides odoratum and/or Burkholderia capacia.18
TABLE 2B.
Clinical reports of nosocomial outbreaks of Myroides spp. infection

Myroides spp. is characterized by resistance to a wide range of antimicrobial agents that have satisfactory activity against other gram-negative bacteria. Most strains are resistant to the β-lactams, including aztreonam and carbapenems, and exhibit variable susceptibility to aminoglycosides, quinolones, and sulfamethoxazole.39,40 Resistance to β-lactams is due to the production of chromosome-encoded metallo-β-lactamases, both in M. odoratum (TUS-1) and M. odoratimimus (MUS).41 The importance of an accurate and reliable susceptibility test has been stressed when significant infection with Myroides strains is encountered.
To our knowledge, the present report is the first of a bloodstream infection caused by Myroides spp. in an immunosuppressed NHP host. Indeed, we have been unable to identify a report of a Myroides complication in either patients with organ allografts or those receiving anti-inflammatory agents or in any NHP (whether immunosuppressed or not). Whether the nature of the immunosuppressive regimen (costimulation blockade) or the addition of a potent anti-inflammatory agent (tocilizumab) contributed to the development of this rare infectious complication remains uncertain. However, we would suggest that it should be suspected in any patient receiving immunosuppressive therapy following organ Tx or for an autoimmune disorder.
ACKNOWLEDGMENTS
The authors thank Dr. Keith Reimann for providing anti-CD40mAb from the NHP Reagent Resource (contract HHSN272200900037C).
Footnotes
Hong Liu and Hayato Iwase contributed equally.
The authors declare no conflicts of interest.
The surgical procedures were carried out by A.H., M.W., H.L., H.I., and J.S. H.L., H. I., and J.S. took responsibility for management of the baboon after transplantation, with help from M.W., R.W., and D.K.C.C. The microbiological studies were by W. P., and the kidney biopsieswere interpreted by E.K. The original article was prepared by H.L., H.I., and D.K.C.C. All authors reviewed and approved the article, which was finalized by H.L., H.I., and D.K.C.C.
Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is, or has been, supported in part by NIH grants U19 AI090959, U01 AI068642, and R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA. The baboon used in the study was from the Oklahoma University Health Sciences Center, Division of Animal Resources, which is supported by NIH P40 sponsored grant RR012317-09.
REFERENCES
- 1. Kalil AC, Florescu MC, Grant W, et al. Risk of serious opportunistic infections after solid organ transplantation: interleukin-2 receptor antagonists versus polyclonal antibodies. A meta-analysis. Expert Rev Anti Infect Ther. 2014; 12: 881. [DOI] [PubMed] [Google Scholar]
- 2. Kawecki D, Wszola M, Kwiatkowski A, et al. Bacterial and fungal infections in the early post-transplant period after kidney transplantation: etiological agents and their susceptibility. Transplant Proc. 2014; 46: 2733. [DOI] [PubMed] [Google Scholar]
- 3. Fishman JA, Scobie L, Takeuchi Y. Xenotransplantation-associated infectious risk: a WHO consultation. Xenotransplantation. 2012; 19: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou H, Iwase H, Wolf RF, et al. Are there advantages in the use of specific pathogen-free baboons in pig organ xenotransplantation models? Xenotransplantation. 2014; 21: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper DK, Ye Y, Niekrasz M. Heart transplantation in primates. In: Handbook of Animal Models in Transplantation Research. In: Cramer DV, Podesta LG, Makowka L, editors. Boca Raton: CRC Press; 1994: 173. [Google Scholar]
- 6. Lambrigts D, Sachs DH, Cooper DK. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation. 1998; 66: 547. [DOI] [PubMed] [Google Scholar]
- 7. Cooper DK, Satyananda V, Ekser B, et al. Progress in pig-to-nonhuman primate transplantation models (1998-2013): a comprehensive review of the literature. Xenotransplantation. 2014; 21: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ayares D, Phelps C, Vaught TD, et al. Multi-transgenic pigs for vascularized pig organ xenografts. Xenotransplantation. 2011; 18: 269 (abstract 119).21923866 [Google Scholar]
- 9. Mohiuddin MM, Corcoran PC, Singh AK, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012; 12: 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohiuddin MM, Singh AK, Corcoran PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014; 14: 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwase H, Ekser B, Zhou H, et al. Further evidence for a sustained systemic inflammatory response in xenograft recipients (SIXR). 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang XY, Zhang YJ, Chen XL, et al. Myroides profundi sp. nov., isolated from deep-sea sediment of the southern Okinawa Trough. FEMS Microbiol Lett. 2008; 287: 108. [DOI] [PubMed] [Google Scholar]
- 13. Cho SH, Chae SH, Im WT, et al. Myroides marinus sp. nov., a member of the family Flavobacteriaceae, isolated from seawater. Int J Syst Evol Microbiol. 2011; 61: 938. [DOI] [PubMed] [Google Scholar]
- 14. Yan S, Zhao N, Zhang XH. Myroides phaeus sp. nov., isolated from human saliva, and emended descriptions of the genus Myroides and the species Myroides profundi Zhang et al. 2009 and Myroides marinus Cho et al. 2011. Int J Syst Evol Microbiol. 2012; 62: 770. [DOI] [PubMed] [Google Scholar]
- 15. Hairul Islam VI, Saravanan S, Preetam Raj JP, et al. Myroides pelagicus from the gut of Drosophila melanogaster attenuates inflammation on dextran sodium sulfate-induced colitis. Dig Dis Sci. 2014; 59: 1121. [DOI] [PubMed] [Google Scholar]
- 16. Yoon JW, Maneerat S, Kawai F, et al. Myroides pelagicus sp. nov., isolated from seawater in Thailand. Int J Syst Evol. 2006; 56: 1917. [DOI] [PubMed] [Google Scholar]
- 17. Kallman O, Lundberg C, Wretlind B, et al. Gram-negative bacteria from patients seeking medical advice in Stockholm after the tsunami catastrophe. Scand J Infect Dis. 2006; 38: 448. [DOI] [PubMed] [Google Scholar]
- 18. Douce RW, Zurita J, Sanchez O, et al. Investigation of an outbreak of central venous catheter-associated bloodstream infection due to contaminated water. Infect Control Hosp Epidemiol. 2008; 29: 364. [DOI] [PubMed] [Google Scholar]
- 19. Ktari S, Mnif B, Koubaa M, et al. Nosocomial outbreak of Myroides odoratimimus urinary tract infection in a Tunisian hospital. J Hosp Infect. 2012; 80: 77. [DOI] [PubMed] [Google Scholar]
- 20. Yagci A, Cerikcioglu N, Kaufmann ME, et al. Molecular typing of Myroides odoratimimus (Flavobacterium odoratum) urinary tract infections in a Turkish hospital. Eur J Clin Microbiol Infect Dis. 2000; 19: 731. [DOI] [PubMed] [Google Scholar]
- 21. Manfredi R, Nanetti A, Ferri M, et al. Flavobacterium spp. organisms as opportunistic bacterial pathogens during advanced HIV disease. J Infect. 1999; 39: 146. [DOI] [PubMed] [Google Scholar]
- 22. Bernardet J-F, Segers P, Vancanneyt M, et al. Cutting a gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom nov (basonym, Cytophaga aquatilis Strohl and Tait 1978). Int J Syst Bacteriol. 1996; 46: 128. [Google Scholar]
- 23. Stutzer M. Zur Frage uber die Faulnisbakterien im Darm. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg I Abt Orig. 1923; 91: 87. [Google Scholar]
- 24. Vancanneyt M, Segers P, Torck U, et al. Reclassification of Flavobacterium odoratum (Stutzer 1929) strains to a new genus, Myroides, as Myroides odoratus comb. nov. and Myroides odoratimimus sp nov. Int J Syst Bacteriol. 1996; 46: 926. [Google Scholar]
- 25. Schrottner P, Rudolph WW, Eing BR, et al. Comparison of VITEK2, MALDI-TOF MS, and 16S rDNA sequencing for identification of Myroides odoratus and Myroides odoratimimus. Diagn Microbiol Infect Dis. 2014; 79: 155. [DOI] [PubMed] [Google Scholar]
- 26. Hsueh PR, Wu JJ, Hsiue TR, et al. Bacteremic necrotizing fasciitis due to Flavobacterium odoratum. Clin Infect Dis. 1995; 21: 1337. [DOI] [PubMed] [Google Scholar]
- 27. Bachman KH, Sewell DL, Strausbaugh LJ. Recurrent cellulitis and bacteremia caused by Flavobacterium odoratum. Clin Infect Dis. 1996; 22: 1112. [DOI] [PubMed] [Google Scholar]
- 28. Green BT, Green K, Nolan PE. Myroides odoratus cellulitis and bacteremia: case report and review. Scand J Infect Dis. 2001; 33: 932. [DOI] [PubMed] [Google Scholar]
- 29. Motwani B, Krezolek D, Symeonides S, et al. Myroides odoratum cellulitis and bacteremia: a case report. Infect Dis Clin Pract. 2004; 12: 343. [Google Scholar]
- 30. Bachmeyer C, Entressengle H, Khosrotehrani K, et al. Cellulitis due to Myroides odoratimimus in a patient with alcoholic cirrhosis. Clin Exp Dermatol. 2008; 33: 97. [DOI] [PubMed] [Google Scholar]
- 31. Crum-Cianflone NF, Matson RW, Ballon-Landa G. Fatal case of necrotizing fasciitis due to Myroides odoratus. Infection. 2014; 42: 931. [DOI] [PubMed] [Google Scholar]
- 32. Maraki S, Sarchianaki E, Barbagadakis S. Myroides odoratimimus soft tissue infection in an immunocompetent child following a pig bite: case report and literature review. Braz J Infect Dis. 2012; 16: 390. [DOI] [PubMed] [Google Scholar]
- 33. Davis JM, Peel MM, Gillians JA. Colonization of an amputation site by Flavobacterium odoratum after gentamicin therapy. Med J Aust. 1979; 2: 703. [DOI] [PubMed] [Google Scholar]
- 34. Benedetti P, Rassu M, Pavan G, et al. Septic shock, pneumonia, and soft tissue infection due to Myroides odoratimimus: report of a case and review of Myroides infections. Infection. 2011; 39: 161. [DOI] [PubMed] [Google Scholar]
- 35. Deepa R, Venkatesh KG, Parveen JD, et al. Myroides odoratus and Chryseobacterium indologenes: two rare isolates in the immunocompromised. Indian J Med Microbiol. 2014; 32: 327. [DOI] [PubMed] [Google Scholar]
- 36. Ferrer C, Jakob E, Pastorino G, et al. Right-sided bacterial endocarditis due to Flavobacterium odoratum in a patient on chronic hemodialysis. Am J Nephrol. 1995; 15: 82. [DOI] [PubMed] [Google Scholar]
- 37. Macfarlane DE, Baum-Thureen P, Crandon I. Flavobacterium odoratum ventriculitis treated with intraventricular cefotaxime. J Infect. 1985; 11: 233. [DOI] [PubMed] [Google Scholar]
- 38. Thomas M, Padmini SB, Govindan VK, et al. Oerskovia turbata and Myroides species: rare isolates from a case of acalculus cholecystitis. Indian J Med Microbiol. 2007; 25: 297. [DOI] [PubMed] [Google Scholar]
- 39. Holmes B, Snell JJ, Lapage SP. Flavobacterium odoratum: a species resistant to a wide range of antimicrobial agents. J Clin Pathol. 1979; 32: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang JC, Hsueh PR, Wu JJ, et al. Antimicrobial susceptibility of flavobacteria as determined by agar dilution and disk diffusion methods. Antimicrob Agents Chemother. 1997; 41: 1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mammeri H, Bellais S, Nordmann P. Chromosome-encoded beta-lactamases TUS-1 and MUS-1 from Myroides odoratus and Myroides odoratimimus (formerly Flavobacterium odoratum), new members of the lineage of molecular subclass B1 metalloenzymes. Antimicrob Agents Chemother. 2002; 46: 3561. [DOI] [PMC free article] [PubMed] [Google Scholar]


