Abstract
Dictyostelium discoideum form groups of ~2 × 104 cells. The group size is regulated in part by a negative feedback pathway mediated by a secreted multipolypeptide complex called counting factor (CF). The CF signal transduction pathway involves CF-repressing internal glucose levels by increasing the Km of glucose-6-phosphatase. Little is known about how this enzyme is regulated. Glucose-6-phosphatase is associated with microsomes in both Dictyostelium and mammals. We find that the activity of glucose-6-phosphatase in crude microsomes from cells with high, normal, or low CF activity had a negative correlation with the amount of CF present in these cell lines. In crude cytosols (supernatants from ultracentrifugation of cell lysates), the glucose-6-phosphatase activity had a positive correlation with CF accumulation. The crude cytosols were further fractionated into a fraction containing molecules greater than 10 kDa (S>10K) and molecules less than 10 KDa (S<10K). S>10K from wild-type cells strongly repressed the activity of glucose-6-phosphatase in wild-type microsomes, whereas S>10K from countin− cells (cells with low CF activity) significantly increased the activity of glucose-6-phosphatase in wild-type microsomes by decreasing Km. The regulatory activities in the wild-type and countin− S>10Ks are heat-labile and protease-sensitive, suggesting that they are proteins. S<10K from both wild-type and countin− cells did not significantly change glucose-6-phosphatase activity. Together, the data suggest that, as a part of a pathway modulating multicellular group size, CF regulates one or more proteins greater than 10 KDa in crude cytosol that affect microsome-associated glucose-6-phosphatase activity.
Much remains to be understood about how organisms regulate the size of multicellular structures. The social amoeba Dictyostelium discoideum provides an excellent model system to study size regulation, for these cells form evenly sized groups of ~2 × 104 cells. Dictyostelium cells grow on soil surfaces as individual amoebae and feed on bacteria (for review, see Refs. 1 and 2). Upon starvation, cells secrete a cell-density sensing factor called conditioned medium factor (3–5). As more and more cells starve, the local conditioned medium factor concentration reaches a threshold level. This allows cells to aggregate using relayed pulses of cyclic AMP as a chemoattractant and form dendritic aggregation streams (for review, see Refs. 2 and 6). Each aggregate forms a fruiting body consisting of a spherical spore mass held atop by a rigid stem of stalk cells. The purpose of these structures is to facilitate spore dispersal. If a fruiting body is too small, the spore mass will be too close to the ground for optimal spore dispersal. On the other hand, if a spore mass is too big, it will slide down the stalk, or the fruiting body will fall over. Therefore, the fruiting body size is of great importance. There are several mechanisms Dictyostelium cells use to regulate the size of aggregates and the fruiting bodies, such as causing excessively large aggregation streams to fragment into groups of ~2 × 104 cells (7–9).
We previously identified a 450-kDa protein complex called counting factor (CF)2 that is involved in group size determination in Dictyostelium (10, 11). CF causes aggregate streams to fragment by increasing cell motility and decreasing cell-cell adhesion, thereby repressing group size (12, 13). Disruption of the smlA gene causes the oversecretion of CF, resulting in the formation of extensively fragmented aggregate streams and many small fruiting bodies (10). When a component of CF is disrupted, cells secrete essentially undetectable levels of CF activity (11, 14, 15). In these cells, the aggregation streams seldom break and coalesce into large fruiting bodies which tend to collapse. Starving cells in the presence of recombinant components of CF including countin, CF50, or CF45-1 mimic the effect of CF and cause streams to fragment and form small fruiting bodies (14–16).
CF regulates cell-cell adhesion and motility by repressing internal levels of glucose, and that glucose is a part of the CF signal transduction pathway (17). To understand how CF regulates glucose, we examined the effect of CF on enzymes involved in glucose metabolism (18). CF has little effect on amylase or glycogen phosphorylase, enzymes involved in glucose production from glycogen. Glucokinase activity (the first specific step of glycolysis) is repressed by high levels of CF but is not affected by an 8-min exposure to recombinant countin. The second enzyme specific for glycolysis, phosphofructokinase, is not regulated by CF. The gluconeogenic counterpart of phosphofructokinase, fructose-1,6-bisphosphatase, is not regulated by CF or recombinant countin, whereas glucose-6-phosphatase (the gluconeogenic counterpart of glucokinase) is regulated by both CF and an 8-min exposure to recombinant countin. The countin-induced changes in the Km and Vmax of glucose-6-phosphatase causes a decrease in glucose production that can account for the countin-induced decrease in glucose levels (18).
Glucose-6-phosphatase is an important enzyme in the control of glucose homeostasis (19–25). Glucose-6-phosphatase catalyzes the last biochemical process of gluconeogenesis and is a key step in glycogenolysis in which it hydrolyzes glucose 6-phosphate to glucose and Pi. It is made of multiple transmembrane components, with the active site in the lumen of the endoplasmic reticulum. Some of the components transport glucose 6-phosphate from the crude cytosol into the endoplasmic reticulum, and others transport glucose and phosphate out. However, very little is known about whether or how glucose-6-phosphatase is regulated, and the enzyme has never been purified. In humans, defects in glucose-6-phosphatase cause glycogen storage disease (26). Many efforts have been made to decrease serum glucose levels by temporarily repressing glucose-6-phosphatase as a possible therapeutic treatment for hyperglycemia and diabetes (for review, see Ref. 27).
To determine how CF regulates the activity of glucose-6-phosphatase to decrease internal glucose levels in Dictyostelium, we measured the activity of the enzyme in several different conditions. Because glucose-6-phosphatase activity is associated with microsomes, the enzyme was semipurified by fractionating cell lysates by ultracentrifugation (28). We find that CF appears to regulate at least two different components in crude cytosol to decrease the activity of the glucose-6-phosphatase in crude microsomes.
MATERIALS AND METHODS
Cell Culture
D. discoideum Ax4 wild-type, smlA− (HDB7YA), and countin− (HDB2B/4) cells were grown in HL5 media (with maltose as the carbohydrate source) and starved in PBM buffer (20 mm KH2PO4, 10 μm CaCl2, 1 mm MgCl2, pH 6.1) at 5 × 106 cells/ml as previously described (10, 11, 17, 18). At 6 h of development, cells were collected as previously described (17, 18). The pellets were lysed by freezing in dry ice/ethanol and thawing on ice. 1.5 ml of PB (3.2 mm Na2HPO4, 7.0 mm KH2PO4, pH 6.5) was added to the pellets obtained from 100 ml of shaking starvation culture, and the lysate was clarified by centrifugation at 19,000 × g for 2 min. 2 μl of clarified cell lysates were used for protein determination.
Preparation of Liver Homogenates
Crude microsomes from rat liver were prepared following Mithieux et al. (29). The liver was rinsed with ice-cold 0.15 m NaCl and homogenized in a 6× volume of PB using a tissue homogenizer (Tekmar, Cincinnati, OH). Clarified liver homogenate was obtained by centrifugation at 19,000 × g for 20 min at 4 °C twice. 2 μl of clarified liver homogenates were used for protein determination.
Glucose-6-phosphatase Assays
Because glucose-6-phosphatase activity is associated with microsomes, we fractionated clarified Dictyostelium lysates and liver homogenates by ultracentrifugation at 100,000 × g for 1 h at 4 °C (28). The resulting supernatant (crude cytosol) was removed. 2 ml of PB was gently added to the wall of the ultracentrifugation tube, the tube was swirled gently, and the PB was removed without disturbing the pellet. Where indicated, crude cytosol was further fractionated using 10-kDa cutoff Amicon spin filters (Millipore, Hercules, CA) at 2000 × g for 30 min. This generated a fraction that contained molecules retained by the 10-kDa cutoff spin filter (S>10K) and a second fraction that contained molecules that passed through the 10-kDa cutoff spin filter (S<10K). Before use, the S>10K fraction was brought up to the original volume of the crude cytosol by adding PB. For some experiments, reconstituted crude cytosol was prepared by resuspending the S>10K fraction in the associated S<10K fraction rather than in PB. Where indicated, the pellet from the ultracentrifugation (crude microsomes) was resuspended in PB, crude cytosol, fractionated crude cytosol, or reconstituted crude cytosol. To compare the enzyme activities in the crude microsomes resuspended in different fractions, we measured the glucose-6-phosphatase activity (at different glucose 6-phosphate concentrations) of the crude microsomes resuspended in PB, S>10K, or S<10K and then subtracted the glucose-6-phosphatase activity (at the same glucose 6-phosphate concentrations) of the resuspension media. In some cases the microsomal preparation was resuspended in PB with 0.1% Triton X-100 to measure the activity of the catalytic subunit of the glucose-6-phosphatase complex. For all the assays, 100 μl of sample was used for a glucose-6-phosphatase assay, and 2 μl of sample was used for a Bio-Rad protein assay with bovine serum albumin as a standard. Glucose-6-phosphatase activity was measured as previously described (18). The Vmax and Km of glucose-6-phosphatase were measured by varying the concentration of glucose 6-phosphate in the assay. For unknown reasons we were unable to reproducibly measure glucose-6-phosphatase activity at glucose 6-phosphate concentrations below 0.1 mm. Nonlinear regressions to fit the activity to the Michaelis-Menten equation used the Prism software package (Graph-Pad, San Diego, CA).
Supernatant Treatments
To determine the nature of the glucose-6-phosphatase-inhibiting activity of wild-type S>10K and the stimulating activity of countin− S>10K, we subjected wild-type or countin− S>10K to various treatments. After fractionating crude cytosol, S>10K was brought up to half of the original volume of the crude cytosol by adding PB, and the S>10K was separated into aliquots in Eppendorf tubes. Tubes were incubated at 37 and 55 °C for 30 min and 100 °C for 5 min. After incubation at 55 and 100 °C, the S>10K was clarified by centrifugation at 19,000 × g for 2 min. In addition, the S>10K was treated with DNase I (Sigma) at 0.5 μg/μl, RNase A (Sigma) at 15 ng/μl, or proteinase K agarose beads (Sigma) at 0.1 mg/μl for 30 min at 37 °C. The proteinase K-agarose beads were removed by centrifugation at 19,000 × g for 5 min, twice. Wild-type crude microsomes were resuspended in half of the original volume by adding PB. Resuspended wild-type crude microsomes were mixed with an equal volume of treated countin− S>10K, and glucose-6-phosphatase activities were measured as described above.
RESULTS
Cell Lines with Different Extracellular CF Levels Have Different Glucose-6-phosphatase Activities in Crude Microsomes and Crude Cytosols
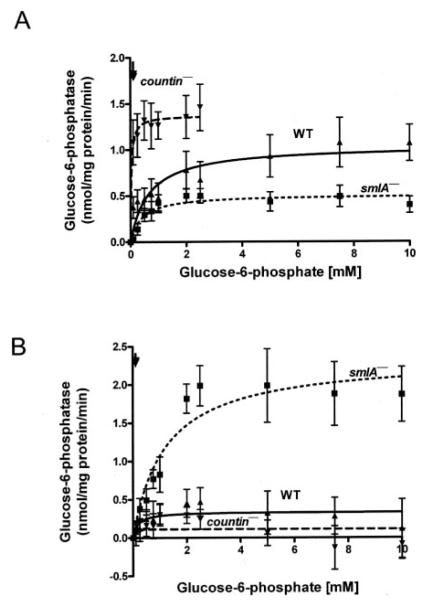
We previously found that CF decreases internal glucose levels by decreasing the activity of glucose-6-phosphatase in crude cell lysates (17, 18). Glucose-6-phosphatase is a multicomponent enzyme associated with microsomes in yeast, Dictyostelium, rats, mice, and humans (30–33). To determine how CF regulates glucose-6-phosphatase, we measured the activity of the enzyme in crude microsomes (Fig. 1A, Table 1). We have previously observed that high concentrations of glucose 6-phosphate can sometimes cause the lysates of Dictyostelium cells, especially countin− cells, to become highly turbid.3 We were, thus, unable to reproducibly measure the activity of glucose-6-phosphatase at high substrate concentrations in countin− crude microsomes. We normalized the activities of glucose-6-phosphatase in crude microsomes by the amount of protein present in both crude microsomes (Fig. 1) and cleared lysates (Table 1). In both cases, compared with the activity in wild-type crude microsomes, glucose-6-phosphatase activity in crude microsomes from smlA− cells had a low Vmax and a slightly low Km, whereas the glucose-6-phosphastase activity in crude microsomes from countin− cells had a high Vmax and a low Km. At the physiological level of glucose 6-phosphate observed in wild-type cells (~114 μm), smlA− crude microsomes had a slightly lower glucose-6-phosphatase activity, and countin− crude microsomes had a higher glucose-6-phosphatase activity compared with wild-type crude microsomes (Table 2).
FIGURE 1. Cell lines with different CF accumulation have different glucose-6-phosphatase activities in crude microsomes and crude cytosols.
smlA−, Ax4 (WT), and countin− cells were starved by shaking in buffer, harvested at 6 h of starvation, and lysed by freezing/thawing. Cell lysates were then clarified by centrifugation, and the clarified lysates were further fractionated by ultracentrifugation to yield crude microsomes and crude cytosol. The activities of glucose-6-phosphatase in the crude microsomal fraction and crude cytosol fraction were then measured. The lines show Michaelis-Menten equations fit to the data using nonlinear regression. A, glucose-6-phosphatase activities were measured in crude microsomes normalized to the protein concentrations of crude microsomes from the three cell lines. Values are the means ± S.E. from seven independent assays. B, glucose-6-phosphatase activities were measured in crude cytosol fractions and normalized to the protein concentrations of the crude cytosol fractions from the three cell lines. Values are the means ± S.E. from seven independent assays. Arrows indicate the physiological concentration of glucose-6-phosphatase in wild-type cells (114 μm).
TABLE 1. The Vmax and Km of glucose-6-phosphatase activity in crude microsomes and crude cytosol.
smlA−, Ax4 (WT), and countin− cells were starved by shaking in PBM, harvested at 6 h of starvation, and lysed. Cell lysates were then clarified by centrifugation, and the clarified lysates were further fractionated by ultracentrifugation at 100,000 × g to yield crude microsomes and crude cytosol fractions. The glucose-6-phosphatase activities were normalized against the amount of protein present in cleared lysates. The Vmax and Km of glucose-6-phosphatase in cleared lysates were from Jang and Gomer (18), conservatively recalculating the countin− Km as <0.1 mm. For smlA−, wild-type, and countin− cells, crude microsome protein was 37% of cleared lysate protein, and crude cytosol protein was 63% of cleared lysate protein.
| Parameter measured | smlA − | Wild type | countin − |
|---|---|---|---|
|
Vmax (crude microsomes) (nmol/mg protein/min) |
0.19 | 0.38 | 0.51 |
| Km (crude microsomes) (mm) | 0.40 | 0.65 | <0.1 |
|
Vmax (crude cytosol) (nmol/mg protein/min) |
1.5 | 0.21 | 0.069 |
| Km (crude cytosol) (mm) | 1.2 | 0.18 | <0.1 |
|
Vmax (cleared lysate) (nmol/mg protein/min) |
2.1 | 1.5 | 0.45 |
| Km (cleared lysate) (mm) | 2.4 | 2.1 | <0.1 |
TABLE 2. The glucose-6-phosphatase activities at the physiological glucose-6-phosphate level.
The activities of glucose-6-phosphatase were calculated at the glucose 6-phosphate concentration observed in wild-type cells (~114 μm) using the Vmax and Km in crude microsomes, crude cytosol, and cleared lysates, all normalized to the protein concentration in the cleared lysates. For countin− cells, we used Km <0.1 mm (Table I). Values for glucose-6-phosphatase activity in lysates are from Jang and Gomer (18).
| Activity |
|||
|---|---|---|---|
| smlA − | Wild-type | countin − | |
| nmol/mg protein/min | |||
| Crude microsomes | 0.03 | 0.05 | >0.26 |
| Crude cytosol | 0.12 | 0.06 | >0.035 |
| Cleared lysate | 0.10 | 0.08 | >0.23 |
The fractionation procedure we used separated the cleared lysate into crude microsomes and crude cytosol fractions. To determine how much glucose-6-phosphatase activity is in the crude cytosol, we measured the rate of glucose 6-phosphate hydrolysis in the crude cytosol (Fig. 1B and Table 1). Compared with the activity in wild-type crude cytosol, the glucose-6-phosphatase activity in the crude cytosol of smlA− cells had a higher Vmax and Km, whereas the glucose-6-phosphatase activity in the crude cytosol of countin− had a lower Vmax and Km.
At the physiological level of glucose 6-phosphate in wild-type cells, the glucose-6-phopshatase activity in smlA− crude cytosol was higher than that of wild-type and countin− crude cytosols (Table 2). Comparing the activities at this glucose 6-phosphate concentration in crude microsomes and crude cytosol, smlA− cells had a high percentage of activity in cytosol, wild-type cells appeared to have roughly equal amounts in crude microsomes and crude cytosol, and countin− cells had most of the glucose-6-phosphatase activity in crude microsomes (Table 2). A comparison of the sum of the activities in crude microsomes and the activity in crude cytosol with the previously observed activity in cleared lysates (at the time the glucose-6-phosphatase activities were measured in the cell fractions, the activities in cleared lysates were also measured, and this latter activity matched previously published cleared lysate activity) suggests that separating crude cytosol from crude microsomes causes activity in one of the fractions to increase slightly (Table 2). This could be due to a component in the crude microsomal fraction inhibiting the activity of a phosphatase in the crude cytosol fraction or vice versa. However, if we consider the error at 114 μm glucose 6-phosphate to be roughly equal to the S.E. of the mean in our assays at 100 μm glucose 6-phosphate (Fig. 1 and Ref. 18), the difference is not statistically significant.
Detergent Increases the Vmax of Glucose-6-phosphatase in countin− Crude Microsomes
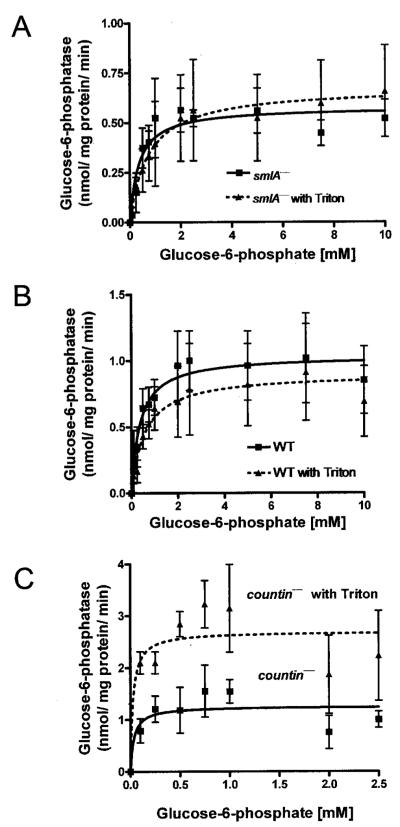
Because glucose-6-phosphatase is a multicomponent enzyme with catalytic and transporter subunits and with the catalytic subunit inside the lumen of the endoplasmic reticulum, one way to measure the activity of the catalytic subunit itself is to measure the glucose-6-phosphatase activity in detergent-disrupted microsomes (34). We treated crude microsomes with detergent (0.1% Triton X-100) to determine whether the differences in the enzyme activity in crude microsomes of the three cell lines with different levels of CF accumulation are due to regulation of the transporter subunits. In smlA− cells, the discernable activities of the enzyme were quite similar with or without Triton X-100 treatment (Fig. 2A). Wild-type cells also had no discernable differences in the activity of the enzyme with or without Triton X-100 treatment (Fig. 2B). In countin− cells, treatment with Triton X-100 increased the Vmax of the glucose-6-phosphatase (Fig. 2C). Together, the data suggest that in smlA− and wild-type cells, detergent treatment to allow direct access of glucose 6-phosphate to the catalytic subunit does not increase enzymatic activity, suggesting that in these cell lysates the glucose 6-phosphate transporter does not limit enzyme activity. In countin− crude microsomes, the detergent treatment did increase glucose-6-phosphatase activity, suggesting that in this cell line the glucose-6-phosphatase activity is limited by the transporter.
FIGURE 2. Treatment with 0.1% Triton X-100 affects glucose-6-phosphatase activity in countin− cells.
Crude microsomes were prepared from smlA−, Ax4 (WT), and countin− cells The crude microsomes were resuspended in buffer with or without 0.1% Triton X-100, and the activities of glucose-6-phosphatase were measured as in Fig. 1. The lines show Michaelis-Menten equations fit to the data using nonlinear regression. A, glucose-6-phosphatase activities were measured in crude microsomes from smlA− cells with or without Triton X-100. The presence of Triton X-100 did not significantly alter Vmax and Km. Values are the means ± S.E. from four independent assays. B, glucose-6-phosphatase activities were measured in crude microsomes from wild-type cells with or without Triton X-100. The treatment of Triton X-100 did not significantly alter the activity of the enzyme. Values are the means ± S.E. from four independent assays. C, glucose-6-phosphatase activities were measured in crude microsomes from countin− cells with or without Triton X-100. The presence of Triton X-100 significantly increased the glucose-6-phosphatase activity at 0.1, 0.25, 0.5, and 0.75 mm substrate concentrations (paired t test, p < 0.05). Treatment of countin− crude microsomes with Triton X-100 did not affect the Km of glucose-6-phosphatase. Treatment of countin− cells with Triton X-100 increased the Vmax, but did not affect the Km of glucose-6-phosphatase. Values are the means ± S.E. from five independent assays.
Fractionated Crude Cytosols from Wild-type and countin− Cells Affect the Glucose-6-phosphatase Activity of Wild-type Crude Microsomes
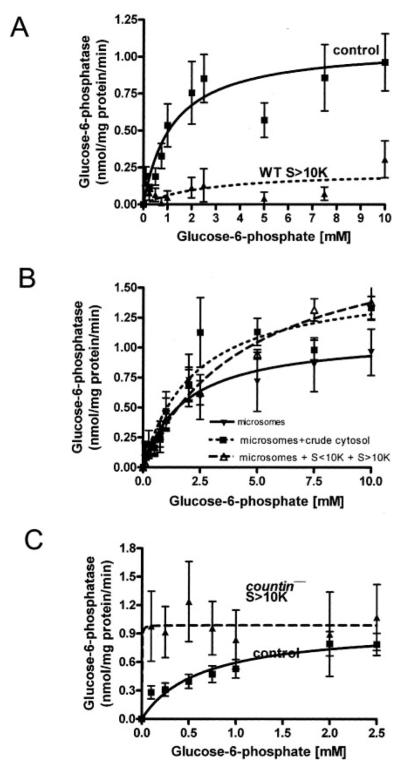
Having established that the glucose-6-phosphatase activities of smlA−, wild-type, and countin− cells are different when measured in crude microsomes, we then asked whether there exists any factors in the crude cytosol that regulate the glucose-6-phosphatase activity in crude microsomes. To identify CF-regulated components that affect the activity of glucose-6-phosphatase in the crude cytosol, we fractionated the crude cytosol by using 10-kDa cutoff spin filters to generate S<10K and S>10K fractions and then measured the activities of glucose-6-phosphatase in crude microsomes with or without fractionated crude cytosol. Crude cytosol from smlA− cells was not used, since it had a very high glucose-6-phosphatase activity (Fig. 1B). The S<10K and the S>10K fractions from both wild-type and countin− cells had essentially undetectable glucose-6-phosphatase activities (data not shown). The glucose-6-phosphatase activity of wild-type crude microsomes resuspended in S<10K was not significantly different from the activity of wild-type crude microsomes resuspended in PB (data not shown). However, when wild-type crude microsomes were resuspended in S>10K from wild-type cells, the Km increased, and the Vmax of the enzyme decreased (Fig. 3A, Table 3). When unfractionated crude cytosol or reconstituted crude cytosol (a mixture of S>10K and S<10K) from wild-type cells was added to wild-type crude microsomes, glucose-6-phosphatase activities were restored to roughly the cleared lysate level (Fig. 3B). The data suggest that there is one or more factors in wild-type S>10K that decrease glucose-6-phosphatase activity and another factor(s) in wild-type S<10K that suppresses the activity of the factors in wild-type S>10K. The presence of S<10K from countin− cells did not alter the glucose-6-phosphatase activity of wild-type crude microsomes (data not shown), whereas S>10K from countin− cells decreased the Km of glucose-6-phosphatase in wild-type crude microsomes but had little effect on Vmax (Fig. 3C and Table 3). This effect was also seen using crude cytosol from countin− cells (data not shown). At the physiological level of glucose 6-phosphate in wild-type cells (~114 μm), S>10K from countin− cells increased the activity of wild-type crude microsomes by ~6-fold compared with the activity of wild-type crude microsomes resuspended in PB.
FIGURE 3. The effect of different cell fractions on the glucose-6-phosphatase activity of wild-type crude microsomes.
WT and countin− cells were starved by shaking in PBM, harvested at 6 h of starvation, and lysed by freezing/thawing. Cell lysates were then clarified by centrifugation, and the clarified lysates were fractionated by ultracentrifugation. The supernatants from the ultracentrifugation were further fractionated by using 10-kDa cutoff spin filters. Wild-type crude microsomes were resuspended in S>10K, crude cytosol, or reconstituted crude cytosol from wild-type and countin− cells, and the activities of glucose-6-phosphatase were measured. The lines show Michaelis-Menten equations fit to the data using nonlinear regression. A, glucose-6-phosphatase activities were measured in crude microsomes resuspended in PB (control) or wild-type S>10K. Values are the means ± S.E. from six independent assays. B, glucose-6-phosphatase activities were measured in crude microsomes from wild-type cells resuspended in PB (microsomes), WT crude cytosol (microsomes + crude cytosol), or reconstituted WT crude cytosol (microsomes + S<10K + S>10K). Values are the means ± S.E. from seven independent assays. C, glucose-6-phosphatase activities were measured in WT crude microsomes resuspended in PB (control) or S>10K from countin− cells. The presence of S>10K from countin− cells significantly increased the glucose-6-phosphatase activity at the physiological level of glucose 6-phosphate in wild-type cells (~114 μm) (paired t test, p < 0.05). Values are means ± S.E. from 10 independent assays.
TABLE 3. One or more factors in S>10K regulates the Vmax and Km of glucose-6-phosphatase in wild-type crude microsomes.
The activities of wild-type crude microsomes resuspended in PB (control), wild-type S>10K, or countin− S>10K were measured (Figure 3). The Vmax and Km values were calculated using nonlinear regressions to fit the activity (as a function of the substrate concentration) to the Michaelis-Menten equation.
| Control | WT S>10K | countin− S>10K | |
|---|---|---|---|
|
Vmax (nmol/mg protein/min) |
0.96 | 0.22 | 0.90 |
| Km (mm) | 0.63 | 2.7 | <0.1 |
The Factors in Wild-type and countin− S>10K That Affect Microsomal Glucose-6-phosphatase Activity Are Destroyed by Heat or Protease Treatments
To determine the nature of the factors in the wild-type and countin− S>10Ks, we subjected the S>10K from wild-type or countin− cells to a variety of treatments. The treated wild-type or countin− S>10K was then added to wild-type microsomes. There was no decrease of the wild-type S>10K activity (the ability of the wild-type S>10K to decrease wild-type microsomal glucose-6-phosphatase activity at 10 mm glucose 6-phosphate) after 30-min treatments at 37 °C or 37 °C with DNase. There was an ~70% decrease of the wild-type S>10K activity upon treatment of wild-type S>10K at 55 °C. When wild-type S>10K was heated to 100 °C for 5 min or treated with proteinase K agarose beads at 37 °C for 30 min, the wild-type S>10K activity was abolished. We were unable to measure the effect of RNase on wild-type S>10K, as this treatment caused severe turbidity when mixed with wild-type microsomes.
There was no decrease of the countin− S>10K activity (the ability of the countin− S>10K to stimulate wild-type microsomal glucose-6-phosphatase activity at 0.1 mm glucose 6-phosphate) after treatment at 37 °C or at 37 °C with DNase or RNase. There was an ~50% decrease of the countin− S>10K activity upon treatment at 55 °C for 30 min. The stimulating activity in the countin− S>10K was completely abolished with treatment at 100 °C for 5 min or incubation with proteinase K agarose beads at either 55 or 37 °C for 30 min. Together, the data suggest that the glucose-6-phosphatase-modulating activities in the wild-type and countin− S>10K are heat-labile proteins.
Fractionated Crude Cytosols from Wild-type and countin− Cells Affect the Glucose-6-phosphatase Activity of Crude Microsomes from Rat Liver
To find out whether Dictyostelium S>10K has similar effects on mammalian glucose-6-phosphatase, crude microsomes from rats were resuspended in PB, wild-type S>10K, or countin− S>10K. The addition of wild-type (WT) and countin− S>10 to rat liver crude microsomes significantly decreased the Vmax and Km of the rat liver glucose-6-phosphatase (Fig. 4 and Table 4). However, the countin− S>10K decreased the Km more than the WT S>10K decreased the Km, suggesting that CF regulates a component in Dictyostelium S>10K that affects the Km of mammalian glucose-6-phosphatase.
FIGURE 4. The effect of WT and countin− S>10K on the glucose-6-phosphatase activities of rat liver crude microsomes.
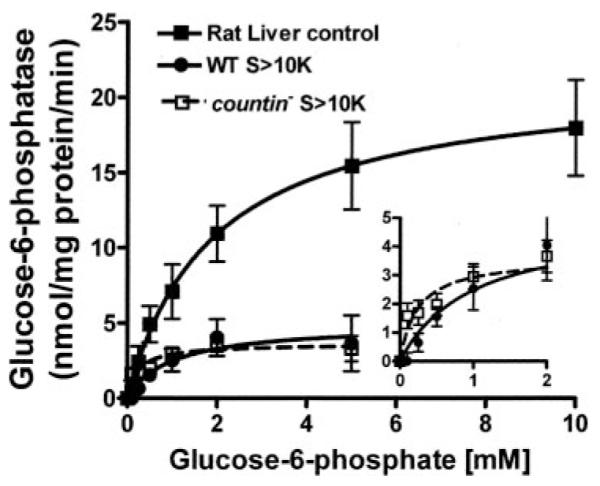
Rat liver crude microsomes were resuspended in PB (control), WT S>10K, or countin− S>10K, and the activities of glucose-6-phosphatase were measured by varying the concentration of glucose 6-phosphate in the assay buffer. The lines show Michaelis-Menten equations fit to the data using nonlinear regression. The inset shows the curves for the effect of WT and countin− S>10K on the glucose-6-phosphatase activities of rat liver crude microsomes at low substrate concentrations. Values are the means ± S.E. from seven independent assays.
TABLE 4. One or more factors in S>10K regulate the glucose-6-phosphatase in rat liver crude microsomes.
The activities of rat liver crude microsomes resuspended in PB (control), WT S>10K, or countin− S>10K were measured (Fig. 4). The Vmax and Km values were calculated using nonlinear regressions to fit the activity (as a function of the substrate concentration) to the Michaelis-Menten equation.
| Control | WT S>10K | countin− S>10K | |
|---|---|---|---|
|
Vmax (nmol/mg protein/min) |
21.4 | 4.9 | 3.6 |
| Km (mm) | 1.9 | 1.0 | 0.24 |
DISCUSSION
Glucose-6-phosphatase is a crucial enzyme involved in glucose homeostasis. It catalyzes the final step of both glycogenolysis and gluconeogenesis, where its substrate, glucose 6-phosphate, serves as a branching point for many biochemical reactions (for review, see Refs. 19 and 20–25). The activity of glucose-6-phosphatase can be regulated through gene expression and/or biochemical inhibition (35–39). Due to the importance and implication in diseases, considerable effort has been made to identify the regulation mechanism of this enzyme. However, the exact nature of glucose-6-phosphatase regulation is still largely unknown.
The observation that glucose-6-phosphatase activities in crude microsomes from smlA−, wild-type, and countin− cells are different from the previously observed glucose-6-phosphatase activities in cleared lysates of the three cell lines suggested that there may exist factors or other nonspecific glucose-6-phosphatase activities in the crude cytosol and that interactions between factors in the crude cytosol and crude microsomes may regulate glucose-6-phosphatase activity in these cell lines.
Numerous cytosolic phosphatases other than glucose-6-phosphatase such as alkaline phosphatase hydrolyze glucose 6-phosphate (for review, see Ref. 25). The high glucose-6-phosphatase activity observed in the clarified lysates of smlA− cells and the glucose-6-phosphatase activity observed in the crude cytosol from wild-type and countin− cells may, thus, be due to cytosolic enzymes such as alkaline phosphatase or may be due to ineffective separation of the microsomes from the crude cytosol or solubilization of glucose-6-phosphatase.
The finding that the presence of detergent (Triton X-100) increased glucose-6-phosphatase activity in countin− cells by increasing Vmax suggests that glucose-6-phosphatase, glucose, or phosphate transporter subunits limit the activity of glucose-6-phosphatase in countin− cells and that the amount or Vmax of the catalytic subunit in countin− cells is higher than in wild-type or smlA− cells. This then suggests that CF regulates the amount or Vmax of the glucose-6-phosphatase catalytic subunit as well as the Km.
We previously showed that internal glucose levels are elevated in countin− cells (17). Because glucose increases the expression levels of glucose-6-phosphatase mRNA in mammals (for review, see Ref. 24), it is possible that the increased activity of glucose-6-phosphatase in countin− cells is due to the elevated levels of internal glucose up-regulating glucose-6-phosphtase expression. In addition, metabolites of the glycolytic/gluconeogenic pathway can play an important role in the expression and inhibition of glucose-6-phosphatase (34), and we observed that cell lines with different levels of CF accumulation had different levels of metabolites (18). Finally, the activity of glucose-6-phosphatase is inhibited by the presence of phosphatidylinositol kinase and its metabolites (33), and the levels of one such metabolite, inositol 3,4,5-trisphosphate, are affected by CF.4 Therefore, it is possible that some of the differences in the activities of glucose-6-phosphatase in smlA−, wild-type, and countin− cells are due to differences in the amount of the enzyme present in cells or due to metabolites regulating the activity of the enzyme.
We observed that S>10K from wild-type cells decreased the Vmax and increased the Km of glucose-6-phosphatase in wild-type crude microsomes, whereas crude cytosol or reconstituted cytosol from wild-type cells did not have such effects. In addition, wild-type S<10K did not have any significant effect on wild-type crude microsomes. Together, the data suggest that there is a factor in S>10K from wild-type cells that decreases Vmax and increases Km of glucose-6-phosphatase in wild-type crude microsomes, and the effect of this factor is suppressed by another factor in S<10K from wild-type cells. S>10K from countin− cells strongly decreased the Km of wild-type crude micro-somes with little effect on the Vmax. Heat and protease treatment of the S>10Ks indicated that the activities are heat-labile proteins. There is, thus, a protein in the S>10K fraction of countin− cells that increases the activity rather than the level of glucose-6-phosphatase. Rat liver crude microsomes have a higher glucose-6-phosphatase activity than Dictyostelium crude microsomes. The addition of Dictyostelium wild-type and countin− S>10K greatly decreased the Vmax and Km of the rat liver glucose-6-phosphatase. However, the presence of countin− S>10K decreased the Km of mammalian glucose-6-phosphatase more than wild-type S>10K, suggesting that the CF-regulated protein in Dictyostelium S>10K that decreased the Km of Dictyostelium glucose-6-phosphatase is also effective on a mammalian glucose-6-phosphatase. Our working hypothesis is, thus, that extracellular CF inhibits the presence or activity of at least one protein in crude cytosol (Fig. 5). When extracellular CF levels are low, this factor increases glucose-6-phosphatase activity by decreasing the Km of the enzyme. Thus, at low CF levels, the high glucose-6-phosphatase activity increases intracellular glucose levels, which in turn increases cell-cell adhesion and decreases cell motility. An increase in CF levels would then cause glucose-6-phosphatase activity to decrease, and the resulting decreased cell-cell activity and increased motility would lead to stream breakup and allow a regulation of group and fruiting body size.
FIGURE 5. Model showing how CF may regulate glucose-6-phosphatase, glucose, and group size.
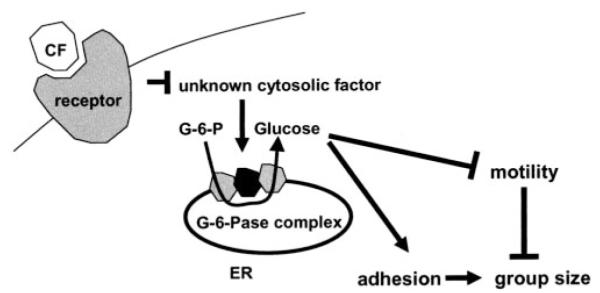
Our working hypothesis is that when CF binds to its surface receptor, this signal inhibits an unknown cytosolic protein. In the absence of CF this protein increases glucose-6-phosphatase activity by decreasing the Km to produce more glucose. A protein found in wild-type cytosol that affects glucose-6-phosphatase may be the same cytosolic protein or may be a different protein. Glucose inhibits cell motility and increases cell-cell adhesion to increase group size. ER, endoplasmic reticulum.
Acknowledgments
We thank Drs. Jennifer West and Darrell Pilling for their kind gift of rat liver.
Footnotes
The abbreviations used are: CF, counting factor; WT, wild type.
T. Gao and R. H. Gomer, unpublished result.
Y. Tang and R. H. Gomer, unpublished data.
This work was supported by Robert A. Welch Foundation Grant C-1555.
REFERENCES
- 1.Loomis WF. Dictyostelium discoideum: A Developmental System. Academic Press, Inc.; New York: 1975. [Google Scholar]
- 2.Kessin RH. In: Dictyostelium Evolution, Cell Biology, and the Development of Multicellularity, Developmental and Cell Biology Series. Bard JBL, Barlow PW, Kirk DL, editors. Cambridge University Press; New York, NY: 2001. [Google Scholar]
- 3.Mehdy MC, Firtel RA. Mol. Cell. Biol. 1985;5:705–713. doi: 10.1128/mcb.5.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuen IS, Jain R, Bishop JD, Lindsey DF, Deery WJ, Van Haastert PJM, Gomer RH. J. Cell Biol. 1995;129:1251–1262. doi: 10.1083/jcb.129.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain R, Yuen IS, Taphouse CR, Gomer RH. Genes Dev. 1992;6:390–400. doi: 10.1101/gad.6.3.390. [DOI] [PubMed] [Google Scholar]
- 6.Varnum-Finney B, Edwards KB, Voss E, Soll DR. Cell Motil. Cytoskeleton. 1987;8:7–17. doi: 10.1002/cm.970080103. [DOI] [PubMed] [Google Scholar]
- 7.Shaffer BM. Q. J. Microsc. Sci. 1957;98:393–405. [Google Scholar]
- 8.Hohl HR, Raper KB. Dev. Biol. 1964;9:137–153. [Google Scholar]
- 9.Kopachik WJ. J. Embryol. Exp. Morphol. 1982;68:23–35. [PubMed] [Google Scholar]
- 10.Brock DA, Buczynski F, Spann TP, Wood SA, Cardelli J, Gomer RH. Development. 1996;122:2569–2578. doi: 10.1242/dev.122.9.2569. [DOI] [PubMed] [Google Scholar]
- 11.Brock DA, Gomer RH. Genes Dev. 1999;13:1960–1969. doi: 10.1101/gad.13.15.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roisin-Bouffay C, Jang W, Gomer RH. Mol. Cell. 2000;6:953–959. [PubMed] [Google Scholar]
- 13.Tang L, Gao T, McCollum C, Jang W, Vickers MG, Ammann R, Gomer RH. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1371–1376. doi: 10.1073/pnas.022516099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brock DA, Hatton RD, Giurgiutiu D-V, Scott B, Ammann R, Gomer RH. Development. 2002;129:3657–3668. doi: 10.1242/dev.129.15.3657. [DOI] [PubMed] [Google Scholar]
- 15.Brock DA, Ehrenman K, Ammann R, Tang Y, Gomer RH. J. Biol. Chem. 2003;278:52262–52272. doi: 10.1074/jbc.M309101200. [DOI] [PubMed] [Google Scholar]
- 16.Gao T, Ehrenman K, Tang L, Leippe M, Brock DA, Gomer RH. J. Biol. Chem. 2002;277:32596–32605. doi: 10.1074/jbc.M203075200. [DOI] [PubMed] [Google Scholar]
- 17.Jang W, Chiem B, Gomer RH. J. Biol. Chem. 2002;277:31972–31979. [Google Scholar]
- 18.Jang W, Gomer RH. Eukaryot. Cell. 2005;4:72–81. doi: 10.1128/EC.4.1.72-81.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burchell A, Allan BB, Hume R. Mol. Memb. Biol. 1994;11:217–227. doi: 10.3109/09687689409160431. [DOI] [PubMed] [Google Scholar]
- 20.Nordlie RC, Bode AM, Foster JD. Proc. Soc. Exp. Biol. Med. 1993;203:274–285. doi: 10.3181/00379727-203-43600. [DOI] [PubMed] [Google Scholar]
- 21.Foster JD, Pederson BA, Nordlie RC. Proc. Soc. Exp. Biol. Med. 1997;215:314–332. doi: 10.3181/00379727-215-44142. [DOI] [PubMed] [Google Scholar]
- 22.Nordlie RC, Foster JD, Lange AJ. Annu. Rev. Nutr. 1999;19:379–406. doi: 10.1146/annurev.nutr.19.1.379. [DOI] [PubMed] [Google Scholar]
- 23.Foster JD, Nordlie RC. Exp. Biol. Med. 2002;227:601–608. doi: 10.1177/153537020222700807. [DOI] [PubMed] [Google Scholar]
- 24.van Schaftingen E, Gerin I. Biochem. J. 2002;362:513–532. doi: 10.1042/0264-6021:3620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mithieux G, Rajas F, Gautier-Stein A. J. Biol. Chem. 2004;279:44231–44234. doi: 10.1074/jbc.R400011200. [DOI] [PubMed] [Google Scholar]
- 26.Cori GT, Cori CF. J. Biol. Chem. 1952;199:661–667. [PubMed] [Google Scholar]
- 27.Barthel A, Schmoll D. Am. J. Physiol. Endocrinol. Metab. 2003;285:685–692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- 28.van de Werve G, Lange A, Newgard C, Mechin MC, Li Y, Berteloot A. Eur. J. Biochem. 2000;267:1533–1549. doi: 10.1046/j.1432-1327.2000.01160.x. [DOI] [PubMed] [Google Scholar]
- 29.Mithieux G, Bordeto JC, Minassian C, Ajzannay A, Mercier I, Riou JP. Eur. J. Biochem. 1993;213:461–466. doi: 10.1111/j.1432-1033.1993.tb17782.x. [DOI] [PubMed] [Google Scholar]
- 30.Hers HG, Berthet J, Berthet L, De Duve C. Bull. Soc. Chim. Biol. 1951;33:21–41. [PubMed] [Google Scholar]
- 31.Bjorntorp P, Bjorkerud S, Schersten T. Biochim. Biophys. Acta. 1965;111:375–383. doi: 10.1016/0304-4165(65)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Gilkes NR, Weeks G. Biochim. Biophys. Acta. 1977;464:142–156. doi: 10.1016/0005-2736(77)90377-7. [DOI] [PubMed] [Google Scholar]
- 33.Mithieux G, Daniele N, Payrastre B, Zitoun C. J. Biol. Chem. 1998;273:17–19. doi: 10.1074/jbc.273.1.17. [DOI] [PubMed] [Google Scholar]
- 34.Robbins BL, Foster JD, Nordlie RC. Life Sci. 1991;48:1075–1081. doi: 10.1016/0024-3205(91)90509-a. [DOI] [PubMed] [Google Scholar]
- 35.Haber BA, Chin S, Chuang E, Buikhuisen W, Naji A, Taub R. J. Clin. Investig. 1995;95:832–841. doi: 10.1172/JCI117733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minassian C, Zitoun C, Mithieux G. Mol. Cell. Biochem. 1996;155:37–41. doi: 10.1007/BF00714331. [DOI] [PubMed] [Google Scholar]
- 37.Mithieux G, Vidal H, Zitoun C, Bruni N, Daniele N, Minassian C. Diabetes. 1996;45:891–896. doi: 10.2337/diab.45.7.891. [DOI] [PubMed] [Google Scholar]
- 38.Gardner LB, Liu Z, Barrett EJ. Diabetes. 1993;42:1614–1620. doi: 10.2337/diab.42.11.1614. [DOI] [PubMed] [Google Scholar]
- 39.Minassian C, Mithieux G. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 1994;109:99–104. doi: 10.1016/0305-0491(94)90146-5. [DOI] [PubMed] [Google Scholar]