Abstract
Hemophores from Serratia marcescens (HasAsm) and Pseudomonas aeruginosa (HasAp) bind hemin between two loops, which harbor the axial ligands H32 and Y75. Hemin binding to the Y75 loop triggers closing of the H32 loop and enables binding of H32. Because Yersinia pestis HasA (HasAyp) presents a Gln at position 32, we determined the structures of apo-and holo-HasAyp. Surprisingly, the Q32 loop in apo-HasAyp is already in the closed conformation but no residue from the Q32 loop binds hemin in holo-HasAyp. In agreement with the minimal reorganization between the apo-and holo-structures, the hemin on-rate is too fast to detect by conventional stopped-flow measurements.
Keywords: Hemophore, Heme, Hemin, Yersinia pestis, Pseudomonas aeruginosa, HasA
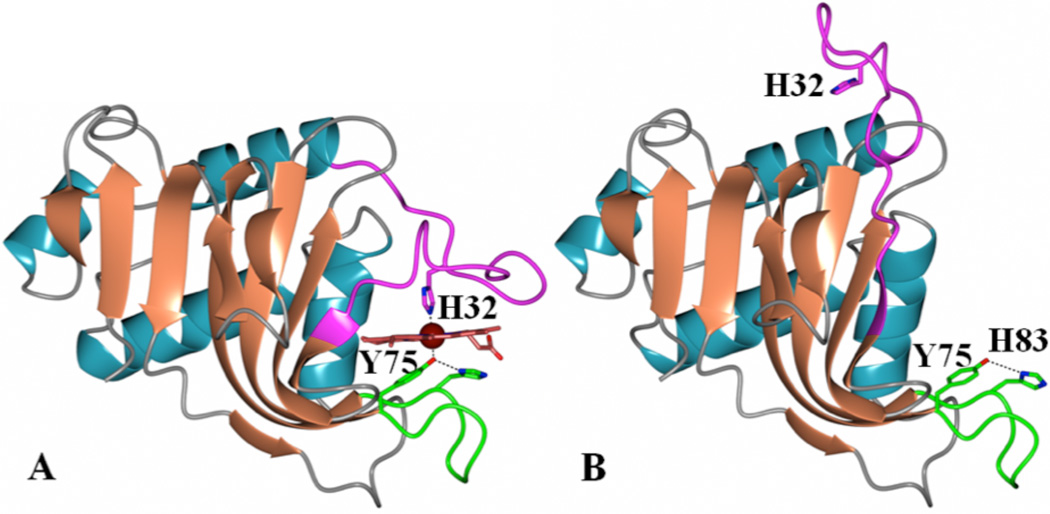
Although iron is important for many crucial biological functions, its chemical properties present unique challenges to living cells, which have to overcome the insolubility of Fe(III) and the toxicity of Fe(II) by sequestering iron in heme, iron-sulfur clusters or iron binding proteins. The very low concentrations of free iron in host cells pose significant challenges to pathogenic bacteria, which have evolved efficient strategies to scavenge iron, including secretion of hemophores, siderophores, hemolysins, proteases and cytotoxins (1, 2). Given that ~70% of total iron is bound to hemoglobin, heme is an important iron source. Thus, a strategy used by bacteria to acquire heme is the deployment of hemophores, which are outer membrane-exposed, or secreted proteins involved in the path of heme transfer from their location in the host to the bacterial cytosol (2, 3), where the macrocycle is degraded by heme-degrading enzymes to release the iron (1, 4, 5). The HasA-type hemophore was first identified in Serratia marcescens (6) and then shown to be conserved in several Gram negative pathogens including, Pseudomonas aeruginosa, Pseudomonas fluorescens, Yersinia pestis, Yersinia pseudotuberculosis, Erwinia carotovora and Pectobacterium carotovorum (7–11). Hemophores from S. marcescens (HasAsm) (12, 13) and P. aeruginosa (HasAp) (7, 14) have been structurally characterized in their apo-and heme-bound (holo) forms and found to be nearly identical. The hemin iron in holo-HasAp (and HasAsm) is coordinated by H32 and Y75. Each axial ligand is harbored in a loop, termed either the H32 or Y75 loop. The main difference between the apo-and holo-structures is a large rearrangement of the H32 loop, which relocates H32 ~30 Å (Figure 1). Structural and spectroscopic studies carried out with WT and H32A HasAp (7, 15) showed that hemin loads onto the Y75 loop within a few milliseconds. Once hemin is loaded its proximal side is likely rapidly coordinated by Y75, while coordination by H32 is significantly slower (hundreds of milliseconds to second scale). In the structure of H32A holo-HasAp in complex with imidazole, hemin-iron is coordinated by Y75 and imidazole, the H32 loop is near the distal heme face and adopts a conformation very similar to that of WT holo-HasAp; NMR studies suggest that this conformation is maintained in solution (7). These findings led to the conclusion that hemin loading onto the Y75 loop triggers closing of the H32 loop. Results from targeted molecular dynamic simulations allowed identification of motions that are likely important for transmitting the presence of heme in the Y75 loop to the H32 loop in order to initiate its closing (7).
Figure 1.
Structure of (A) holo-HasAp (PDB: 3ELL) and (B) apo-HasAp (PDB: 3MOK) showing the proximal (Y75) and distal (H32) ligands. The Y75 loop is shown in green, the H32 loop in magenta and the heme in red. Heme loads onto the Y75 loop, triggers closure of the H32 loop and enables H32 to coordinate the heme iron.
Given the unusual His/Tyr coordination of the hemin-iron and the induced fit closing of the H32 loop upon hemin loading onto the Y75 loop of HasAp or HasAsm, it is intriguing that H32 is not conserved amongst HasA proteins. Hemophore sequences from Yersinia species (Figure S1) contain a Gln at position 32 and do not have a His residue close in the sequence that could coordinate the hemin iron. These observations suggest that if the hemophore structures of Yersinia species are similar to HasAp and HasAsm, the hemin iron would be axially coordinated by an unprecedented set of ligands, Q32 and Y75. In order to understand how the structures of hemophores compensate for the absence of H32, we carried out the structural characterization of the hemophore from Yersinia pestis KIM10+ (HasAyp) in its heme-free (apo-) and heme-bound (holo-) forms. As will be shown below, unlike HasAp and HasAsm, the structure of apo-HasAyp is in a closed conformation, and undergoes minimal rearrangement upon binding heme.
A synthetic gene coding for HasAyp was expressed in E. coli BL21-GOLD (DE3) cells. Purification yields a mixture of apo-and holo-proteins truncated at the C-terminus (Supporting Information). C-terminal cleavage of hemophores is common (11, 17) and may have functional relevance: The most abundant form of HasAp secreted by P. aeruginosa is the truncated protein missing 15–21 C-terminal residues (18). In contrast, the most abundant form of HasAp secreted by quorum sensing-impaired mutants of P. aeruginosa is full-length HasAp (18). Consequently, the structural characterization of HasAp was carried out with truncated protein (14). Herein we report the structural characterization of C-terminus truncated HasAyp.
The Q-32-bearing loop in apo-HasAyp is in the closed conformation
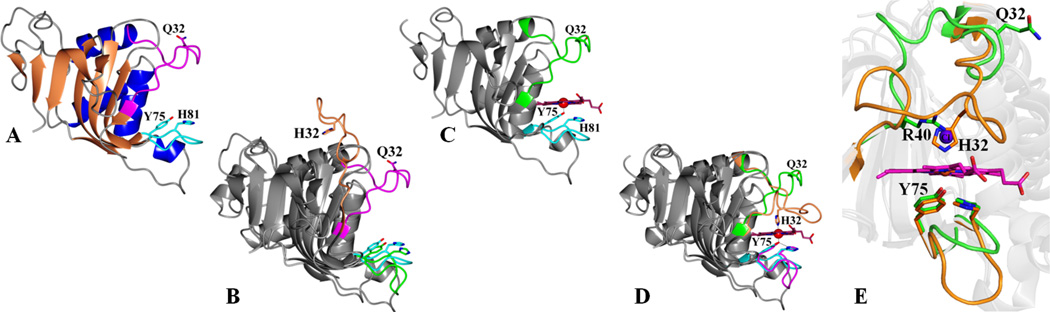
Tetragonal and hexagonal crystal forms were obtained from apo-HasAyp. The tetragonal crystal form (apo-HasAyp tet), which has one molecule in the asymmetric unit, was refined to a resolution of 1.1 Å (Table S1 and Figure 2-A). Electron density was observed from S2 to M184, except for T48 and L49, which were not modeled. Apo-HasAyp exhibits the α + β fold characteristic of HasAp and HasAsm (12, 14), which is composed of a “β-sheet wall” of nine anti-parallel β-strands connected by hairpins and an “α-helix wall” composed of three α-helices and a 310-helix. The structure of the Y75 loop, extending from Y75 to F83, is identical to the Y75 loops in HasAp and HasAsm. Interestingly, the loop bearing Q32, extending from N26 to S42, adopts a different conformation from that seen in apo-HasAp and apo-HasAsm (Figure 2-B). Its conformation is very similar to the “closed” conformation seen in the H32 loop of holo-HasAp and holo-HasAsm. The closed loop conformation of the Q32 loop in apo-HasAyp tet is also observed in the structure obtained from the hexagonal crystal form (HasAyp hex), which exhibits two molecules in the asymmetric unit and was refined to 1.6 Å resolution (Table S1, Figure S2-A). Both molecules in the asymmetric unit are nearly identical (Cα-RMSD = 0.76 Å), exhibit well-defined electron density for residues S2 to D180 and contain a PEG molecule between the Q32 and Y75 loops. Superposing the HasAyp tet structure with the structures of molecules A and B of HasAyp hex reveals near identical architectures (see Figure S2-B), except for the apparent absence of PEG or other exogenous molecules between the Q32 and Y75 loops of apo- HasAyp tet.
Figure 2.
(A) Structure of apo-HasAyp tet with the Q32 loop shown in magenta and the Y75 loop in cyan; Q32, Y75 and H81 are shown in sticks. (B) Superimposed structures of apo-HasAp (PDB ID: 3MOK) and apo-HasAyp tet where the H32 loop in apo-HasAp is shown in coral and the Q32 loop in apo-HasAyp tet in magenta. (C) The structure of holo-HasAyp is very similar to that of apo-HasAyp; the Q32 loop is in green and the Y75 loop in cyan. (D) Superposition of holo-HasAyp and holo-HasAp (PDB ID: 3ELL) structures. The hemin iron in HasAp is coordinated by Y75 and H32, whereas in holo-HasAyp it is coordinated by Y75 (also see Figure 3). (E) Zoomed-in view comparing the heme-binding pockets of holo-HasAyp (green) and holo-HasAp (orange); a chloride ion (purple sphere) in the distal pocket of HasAyp is in the position occupied by the side chain of H32 in holo-HasAp and holo-HasAsm. Structures were superimposed using the program Superpose (16).
The structures of apo- and holo-HasAyp are nearly identical
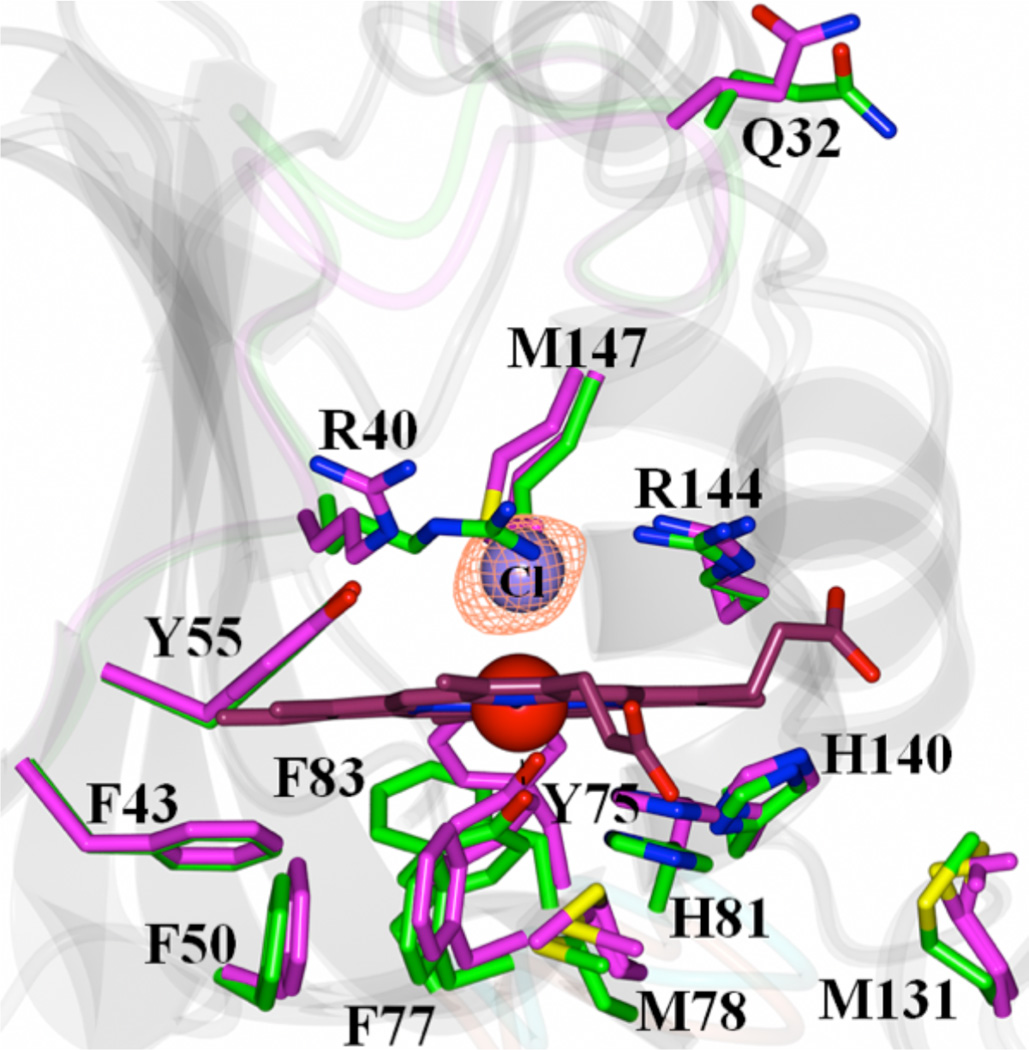
The crystal structure of holo-HasAyp was solved to a resolution of 2.2 Å (Table S1 and Figure 2-C). Well-defined electron density was traced from S2 to D180 for each of the 10 molecules in the asymmetric unit; superposing all ten chains results in a Cα-RMSD = 0.50 Å. Figure 2 illustrates the surprising observation that unlike HasAp or HasAsm, the Q32-loop in HasAyp does not change conformation upon binding hemin (Cα-RMSD = 0.56 Å). As in the previously characterized hemophores, the hemin iron in HasAyp is coordinated by Y75, which also forms a hydrogen bond (2.7 Å) with the Nδ of H81 (H83 in HasAsm and HasAp). Despite the similar coordinating environment of the proximal binding site, the environment of the distal site in HasAyp is distinct (Figure 2-D and E) in that the 6th coordination site is not occupied by a protein-provided ligand. Note that Q32, which we hypothesized may coordinate the hemin iron, is located approximately 14 Å away (Cα) from the heme iron, in a position very similar to the one it occupies in the apo-form. Q32 is clearly not part of the heme-binding pocket. The most significant change in the Q32 loop upon hemin binding is the relocation of the R40 side chain (Figure 3). This small reorganization protects the hemin distal face immediately after it binds to HasAyp, a situation that contrasts with apo-HasAp or apo-HasAsm, where the hemin distal face is exposed to the aqueous environment prior to closing of the H32 loop. The Q32-loop of HasAyp is 3-residues shorter than the H32 loop of HasAp and HasAsm (Figure S1 and Figure S3) which may decrease the conformational flexibility of the Q32 loop. This issue is currently under investigation in our laboratories.
Figure 3.
View of the heme-binding pocket in the superimposed structures of apo-HasAyp tet and holo-HasAyp illustrate the minor structural differences between apo-(magenta) and holo-(green) HasAyp. The distal site in the holo protein is coordinated by a chloride ion; the Fo-Fc omit map contoured at 3 σ is shown in mesh representation.
Electron density immediately above the hemin iron was best modeled as a chloride ion (Figure 3). The modeled Cl− is not within binding distance of Fe(III) (Fe-Cl = 2.8 Å). In contrast, the resonance Raman and EPR spectra show that in solution the heme iron(III) in holo-HasAyp adopts a 6-coordinate high-spin configuration (Figure S4). Although attempts to detect Fe(III)-Cl and Fe(III)-OH stretching frequencies were unsuccessful we presume that the sixth ligand might be a solvent molecule and/or a loosely bound chloride ion. Because HasA sequences with Q32 contain R40 but those with H32 do not (Figure S1), we hypothesize that R40 may stabilize the chloride ion observed in the holo-HasAyp structure. In contrast to the large differences observed in the H32 loop, the structure of the Y75 loop is conserved amongst the three hemophores (Figure S5). As pointed out previously (7) the proximal loop forms a conserved hydrophobic surface for hemin to interact with the hemophore. Accordingly, hemin binding to HasAyp causes minimal changes to the Y75 loop; the largest change is a rearrangement of the F83 side chain to accommodate the incoming hemin (Figure 3).
HasAyp loads hemin from solution faster than HasAp
Stopped-flow experiments reveal that hemin capture by apo-HasAyp is complete within the millisecond deadtime of the apparatus and is thus much faster than in apo-HasAp (Figure S6). These observations support the idea that apo-HasAyp adopts a closed configuration in solution and that minimal reorganization occurs upon binding hemin. This distinctive behavior of HasAyp is likely to also affect hemin transfer to the receptor protein, which in S. marcescens is thought to modulate hemin binding affinity to HasAsm via interaction with the H32 loop (19).
Supplementary Material
ACKNOWLEDGMENTS
Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Sciences, under Contact No. DE-AC02-06CH11357. Use of the IMCA-CAT beamline 17-ID at the Advanced Photon Source was supported by the companies of the Industrial Macromolecular Crystallography Association through a contract with Hauptman-Woodward Medical Research Institute. Use of the KU COBRE-PSF Protein Structure Laboratory was supported by grants from the National Center for Research Resources (5P20RR017708-10) and the National Institute of General Medical Sciences (8 P20 GM103420-10) from the National Institutes of Health. M.R. thanks David R. Benson for insightful discussion.
Funding
This study was supported by grants from the National Science Foundation (M.R., MCB1158469 and MCB 0818488; PML MCB0811888) and by a fellowship from the Japan Society for the Promotion of Science (H.M.).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Detailed experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
Accession codes
PDB entries 4JER (apo-HasAyp tet), 4JES (apo-HasAyp hex), 4JET (holo-HasAyp)
REFERENCES
- 1.Benson DR, Rivera M. Met. Ions Life Sci. 2013;12:279–332. doi: 10.1007/978-94-007-5561-1_9. [DOI] [PubMed] [Google Scholar]
- 2.Wandersman C, Delepelaire P. Mol Microbiol. 2012;85:618–631. doi: 10.1111/j.1365-2958.2012.08136.x. [DOI] [PubMed] [Google Scholar]
- 3.Tullius MV, Harmston CA, Owens CP, Chim N, Morse RP, McMath LM, Iniguez A, Kimmey JM, Sawaya MR, Whitelegge JP, Horwitz MA, Goulding CW. P Natl Acad Sci USA. 2011;108:5051–5056. doi: 10.1073/pnas.1009516108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith AD, Wilks A. Current topics in membranes. 2012;69:359–392. doi: 10.1016/B978-0-12-394390-3.00013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skaar EP, Gaspar AH, Schneewind O. J. Biol. Chem. 2004;279:436–443. doi: 10.1074/jbc.M307952200. [DOI] [PubMed] [Google Scholar]
- 6.Létoffé S, Ghigo JM, Wandersman C. Proc. Natl. Acad. SciU.SA. 1994;91:9876–9880. doi: 10.1073/pnas.91.21.9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jepkorir G, Rodriguez JC, Rui H, Im W, Lovell S, Battaile KP, Alontaga AY, Yukl ET, Moenne-Loccoz P, Rivera M. J Am Chem Soc. 2010;132:9857–9872. doi: 10.1021/ja103498z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cescau S, Cwerman H, Letoffe S, Delepelaire P, Wandersman C, Biville F. Biometals. 2007;20:603–613. doi: 10.1007/s10534-006-9050-y. [DOI] [PubMed] [Google Scholar]
- 9.Rossi MS, Fetherston JD, Letoffe S, Carniel E, Perry RD, Ghigo JM. Infect Immun. 2001;69:6707–6717. doi: 10.1128/IAI.69.11.6707-6717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochsner UA, Johnson Z, Vasil ML. Microbiology. 2000;146(Pt 1):185–198. doi: 10.1099/00221287-146-1-185. [DOI] [PubMed] [Google Scholar]
- 11.Letoffe S, Redeker V, Wandersman C. Mol Microbiol. 1998;28:1223–1234. doi: 10.1046/j.1365-2958.1998.00885.x. [DOI] [PubMed] [Google Scholar]
- 12.Arnoux P, Haser R, Izadi N, Lecroisey A, Delepierre M, Wandersman C, Czjzek M. Nat Struct Biol. 1999;6:516–520. doi: 10.1038/9281. [DOI] [PubMed] [Google Scholar]
- 13.Wolff N, Izadi-Pruneyre N, Couprie J, Habeck M, Linge J, Rieping W, Wandersman C, Nilges M, Delepelairre P, Lecroisey A. J. Mol. Biol. 2008;376:517–525. doi: 10.1016/j.jmb.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 14.Alontaga AY, Rodriguez JC, Schonbrunn E, Becker A, Funke T, Yukl ET, Hayashi T, Stobaugh J, Moenne-Loccoz P, Rivera M. Biochemistry. 2009;48:96–109. doi: 10.1021/bi801860g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yukl ET, Jepkorir G, Alontaga AY, Pautsch L, Rodriguez JC, Rivera M, Moenne-Loccoz P. Biochemistry. 2010;49:6646–6654. doi: 10.1021/bi100692f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krissinel E, Henrick K. Acta Crystallogr D Biol Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 17.Letoffe S, Ghigo JM, Wandersman C. J Bacteriol. 1994;176:5372–5377. doi: 10.1128/jb.176.17.5372-5377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arevalo-Ferro C, Hentzer M, Reil G, Gorg A, Kjelleberg S, Givskov M, Riedel K, Eberl L. Environ Microbiol. 2003;5:1350–1369. doi: 10.1046/j.1462-2920.2003.00532.x. [DOI] [PubMed] [Google Scholar]
- 19.Krieg S, Huche F, Diederichs K, Izadi-Pruneyre N, Lecroisey A, Wandersman C, Delepelaire P, Welte W. P Natl Acad Sci USA. 2009;106:1045–1050. doi: 10.1073/pnas.0809406106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.