Abstract
SPECT myocardial perfusion imaging (MPI) plays a central role in coronary artery disease diagnosis; but concerns exist regarding its radiation burden. Compared to standard Anger-SPECT (A-SPECT) cameras, new high-efficiency (HE) cameras with specialized collimators and solid-state cadmium-zinc-telluride detectors offer potential to maintain image quality (IQ), while reducing administered activity and thus radiation dose to patients. No previous study has compared IQ, interpretation, total perfusion deficit (TPD), or ejection fraction (EF) in patients receiving both ultra-low-dose (ULD) imaging on a HE-SPECT camera and standard low-dose (SLD) A-SPECT imaging.
Methods
We compared ULD-HE-SPECT to SLD-A-SPECT imaging by dividing the rest dose in 101 patients at 3 sites scheduled to undergo clinical A-SPECT MPI using a same day rest/stress Tc-99m protocol. Patients received HE-SPECT imaging following an initial ~130 MBq (3.5mCi) dose, and SLD-A-SPECT imaging following the remainder of the planned dose. Images were scored visually by 2 blinded readers for IQ and summed rest score (SRS). TPD and EF were assessed quantitatively.
Results
Mean activity was 134 MBq (3.62 mCi) for ULD-HE-SPECT (effective dose 1.15 mSv) and 278 MBq (7.50 mCi, 2.39 mSv) for SLD-A-SPECT. Overall IQ was superior for ULD-HE-SPECT (p<0.0001), with twice as many studies graded excellent quality. Extracardiac activity and overall perfusion assessment were similar. Between-method correlations were high for SRS (r=0.87), TPD (r=0.91), and EF (r=0.88).
Conclusion
ULD-HE-SPECT rest imaging correlates highly with SLD-A-SPECT. It has improved image quality, comparable extracardiac activity, and achieves radiation dose reduction to 1 mSv for a single injection.
Keywords: SPECT, radiation dose reduction, high-efficiency camera
SPECT myocardial perfusion imaging (MPI) plays a central role in the diagnosis of patients with established or suspected coronary artery disease (CAD), in predicting outcomes, and in guiding management. However, concern has been raised regarding its radiation burden. In a landmark 2009 report, the National Council on Radiation Protection and Measurements noted a six-fold increase in ionizing radiation exposure to the American population from medical procedures since the early 1980s (1), in particular finding that >10% of the entire U.S. population radiation burden was related to MPI (2). The concern raised by this high radiation burden underscores the importance of efforts to keep radiation exposure as low as possible, and in particular has generated considerable interest in developing methods to reduce radiation dose to patients from MPI, while preserving its benefits.
Based on a design advanced by Berkeley electrical engineer Hal Anger (3) in 1957, conventional Anger SPECT (A-SPECT) cameras now typically have two large thallium-doped sodium-iodide (NaI(Tl)) crystal detectors coupled to arrays of photomultiplier vacuum tubes. Used with low-energy, high-resolution collimators, these traditional cameras are only able to detect less than 0.02% of photon events (4). In contrast, new high-efficiency (HE) cameras incorporate multiple solid-state cadmium-zinc-telluride (CZT) detectors arrayed surrounding the patient with a collimator geometry designed to optimize scintillation detection (5). Two such HE cameras have been introduced into clinical practice, and offer potential to maintain image quality, while reducing administered activity and thus radiation dose to patients. HE-SPECT imaging acquires up to 8 times as many scintillation counts per minute as does conventional A-SPECT (6), an advantage offering the potential to improve image quality, decrease image acquisition time, and/or decrease radiation dose. Several previous studies have evaluated the performance of MPI using a HE-SPECT camera. These include studies of protocols with reduced administered activity and radiation dose (7, 8), as well as other studies comparing HE-SPECT to A-SPECT imaging with equal doses of radiopharmaceutical (9–12). However, no previous study has validated reduced-dose MPI using a HE-SPECT camera in comparison to traditional A-SPECT performed on the same patients. In this study, the MultIcenter nucLear Low-dose Imaging at a milliSIEVERT (MILLISIEVERT) Study, we directly compare image quality (IQ), interpretation, quantitative total perfusion deficit (TPD), and ejection fraction (EF) in patients who received both 1 mSv single-injection ultra-low-dose (ULD) imaging on one of the HE-SPECT cameras and a standard protocol on an A-SPECT camera.
MATERIALS AND METHODS
Study Design
At 3 centers, in 101 patients with suspected or known CAD and scheduled to undergo rest/stress 1-day Tc-99m-based MPI, we divided the rest dose so as to perform ULD (130 MBq, 3.5 mCi) rest imaging on a CZT camera followed by standard low-dose (SLD, 260-480 MBq, 7-13 mCi Tc-99m depending on standard clinical protocol) rest imaging on a conventional A-SPECT camera. Images were scored visually by 2 blinded readers for IQ, extracardiac activity, and summed rest score (SRS), while TPD and EF were assessed quantitatively. These measures were statistically compared between ULD-HE-SPECT imaging and SLD-A-SPECT imaging.
Patient Population
We prospectively enrolled patients at 3 sites (Cedars-Sinai Medical Center, Sacred Heart Medical Center, and Brigham and Women's Hospital) who were scheduled to undergo SPECT MPI for clinical indications. Patients were excluded if they had uncontrolled heart failure, uncontrolled hypertension, or obesity (body mass index [BMI] >30 kg/m2). By prespecification, to ensure sufficient patients with resting perfusion defects, we planned to enroll two patient groups, each with 50 patients. Patient Group 1 constituted patients without history of flow-limiting CAD (no known prior myocardial infarction or coronary revascularization), assessed by a treating physician as having intermediate/high pretest likelihood of CAD. Group 2 constituted patients with a history of myocardial infarction, specifically hospitalization for acute myocardial infarction or Q waves consistent with one. The study was registered (NCT01135095) and approved by the Institutional Review Boards of each institution; all subjects provided written informed consent.
Imaging Protocol
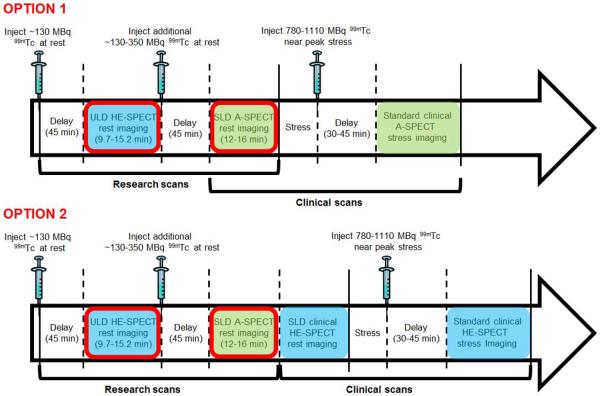
The imaging protocol is illustrated in Figure 1. For each patient, both ULD-HE-SPECT rest imaging and SLD-A-SPECT rest imaging were obtained. While site investigators had the option to choose Tc-99m-sestamibi or Tc-99m-tetrofosmin, all studies were performed using Tc-99m-sestamibi. ULD rest imaging was performed with the patient supine, approximately 45 minutes following administration of ~130 MBq (3.5 mCi) of Tc-99m-sestamibi, using a D-SPECT (Spectrum Dynamics) HE-SPECT camera. Image acquisition time was determined based on the patient's BMI, using a scheme designed by a medical physicist to ensure that a minimum of 700,000 left ventricular scintigraphic counts would be recorded (13). For patients with BMI of 20–22 m/kg2, acquisition time was 9.7 minutes, for BMI 22–24 10.7 minutes, for 24–26 13.0 minutes, for 26–28 14.1 minutes, and for 28–30 15.2 minutes. Immediately after ULD-HE-SPECT rest imaging, an additional 130–350 MBq (3.5–9.5 mCi) of Tc-99m-sestamibi were supplemented to achieve the planned rest dose of 260-480 MBq (7-13 mCi) prescribed for the patient by the local investigator, for the clinically-indicated MPI study. After another delay of ~45 minutes, SLD-A-SPECT imaging was performed using the site's standard acquisition protocol, with images acquired over 12–16 minutes. SLD-A-SPECT rest imaging was performed using at Cedars-Sinai using a Siemens ECAM or Philips Forte gamma camera, at Sacred Heart using a Philips Forte, and at Brigham and Women's using a Siemens Symbia T-6. All A-SPECT images were processed using the site's standard A-SPECT reconstruction protocol. In all three sites this employs iterative reconstruction, while at Brigham and Women's Hospital, resolution recovery and scatter correction is performed as well (Flash 3D, Siemens).
Figure 1.
Study protocol. Sites had the option to perform standard clinically-indicated imaging using either the A-SPECT camera (Option 1) or HE-SPECT camera (Option 2). Images obtained using HE-SPECT camera are denoted with blue shading, using A-SPECT camera with green shading. Comparison is made in each of the two options between images circled in red; subsequent images were obtained solely for clinical purposes and not analyzed in this study.
The remainder of imaging was performed solely for clinical purposes, and these images were not evaluated as part of the research protocol. All patients underwent stress testing with injection of a clinically-prescribed full dose (780–1110 MBq, 21–30 mCi) of Tc-99m-sestamibi near peak stress, and post-stress A-SPECT or HE-SPECT imaging as clinically warranted.
Administered Activity and Radiation Effective Dose Estimation
Administered activity was measured using a dose calibrator immediately prior to administration of the ULD injection, and residual activity in the syringe/needle was measured immediately afterwards. Received activity was determined as the difference between administered and residual activity. Similarly, administered, residual, and received activities were determined for the supplemental SLD injection. Total activities for the SLD injection were determined as the sum of ULD activities, decayed by the time between injections, and supplemental activities, using the equation
| Eq. 1 |
where 4.8 hours is Tc-99m's effective-half-life.
Effective dose of radiation was estimated from received activities using a conversion factor based on the most recent organ dosimetry for Tc-99m-sestamibi (14) and updated tissue weighting factors (15).
Assessment of Image Quality
De-identified images were transferred to the nuclear core laboratory at Cedars-Sinai. Each ULD-HE-SPECT and SLD-A-SPECT set of images was assessed for image quality and semiquantitative image analysis by two board-certified nuclear cardiologists (SWH and JDF), each having read more than 50,000 cases, blinded to the clinical and stress data. Readers were also blinded to camera type, although in principle they could have predicted camera type for a study based on visual appearance of the perfusion imaging. HE-SPECT and A-SPECT images for a given patient were read at separate times and in random order. A full data set including rotating projection image data, perfusion images, and gated images, was available to each reader for their independent assessments. Each reader assessed overall IQ on a 5 point scale (5=excellent, 4=good, 3=fair, 2=poor, 1=uninterpretable) as well as the amount of extracardiac activity (ECA) on a 5 point scale (0 = none; 1=minimal/without any interference with scan interpretability; 2 = mild/probably without any interference with interpretability; 3 = moderate/probably interfering with scan interpretability; 4 = severe/definitely interfering with scan interpretability). Where there was a discrepancy between readers, they subsequently reviewed the images jointly and assigned a consensus classification.
Semiquantitative Image Analysis
Each of the two nuclear cardiologists, as above, also visually assessed each ULDHE-SPECT and SLD-A-SPECT set of images for overall and segmental perfusion. Overall perfusion was assessed on a 5 point scale (5=normal, 4=probably normal, 3=equivocal, 2=probably abnormal, 1=abnormal, -=uninterpretable). Where there was a discrepancy between readers, the readers reviewed the images jointly and assigned a consensus classification. Segmental perfusion was assessed using a 17-segment model (16) and a 0–4 scale (0=normal uptake, 1=mildly reduced uptake, 2=moderately reduced uptake, 3= severely reduced uptake and 4= no uptake). Scoring was guided by boundaries overlaid on the SPECT slices by quantitative perfusion SPECT, and summed rest score (SRS) was calculated by summing scores of the 17 segments. Additionally, to compare agreement between ULD-HE-SPECT and SLD-A-SPECT interpretation with intra-reader reproducibility of SLD-A-SPECT interpretation, one of the readers read each study a second time, three months after completion of the initial reads, and with cases presented in random order. Subsequently, the two readers reviewed images jointly and assigned a consensus classification to resolve any discrepancies.
Quantitative Image Analysis
TPD, a metric combining defect extent and severity as a percentage of total myocardium, was obtained by a core laboratory technologist, blinded to clinical data, from ULD-HE-SPECT and SLD-A-SPECT perfusion images using previously-developed normal limits (17). The only manual step is adjustment of left ventricular contours, if required, in a minority of cases. Separate camera-specific normal limits were applied for ULD HE-SPECT and SLD A-SPECT images.. EF was determined from gated short-axis images using an optimized version of Quantitative Gated SPECT (QGS; Cedars-Sinai), with 16 frames for ASPECT and 8 for HE-SPECT. Additionally, for patients imaged at Cedars-Sinai, LV scintigraphic counts were determined from planar projections using a previously-described method (13).
Statistical Analysis
Continuous and ordinal variables are described by median and interquartile range (IQR), and/or mean±standard deviation, and were compared using Wilcoxon signed-rank tests or correlated using Pearson's correlation coefficient. Agreement is displayed graphically using scatter plots and Bland-Altman plots. Intra-reader, inter-reader, and between-method agreement of SRS were assessed by percent agreement and weighted kappa, with linear weighting. Statistical analysis was performed using Stata/SE (StataCorp, College Station, TX).
Study Approval and Role of the Funding Source
The study was conceived and initiated by the principal and senior investigators (AJE and DSB), who formulated the study design, with technical assistance, viz. acquisition time calculations, provided by a physicist employed by the funding source (Spectrum Dynamics). The decision as to the final study design remained solely that of the principal and senior investigators, with input from site investigators. At no point did the funding source have access to clinical or imaging data. The funding source played no role in the decision to publish or the content of the publication. The funding source remained blinded to the results of the study until it was accepted for publication, except for its publicly-available ACC.13 abstract.
RESULTS
Patients and Doses
Characteristics of the 101 patients enrolled are summarized in Table 1. Administered activities and radiation effective doses are summarized in Table 2. The mean received activity from ULD injections was 134 MBq (3.62 mCi), corresponding to an effective dose of 1.15 mSv.
Table 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| Total subjects | 101 |
| Group | |
| 1: Intermediate/High Likelihood of CAD | 55(54.5%) |
| 2: Prior Myocardial Infarction | 46(45.5%) |
| Site | |
| Cedars-Sinai | 49(48.5%) |
| Sacred Heart | 39(38.6%) |
| Brigham and Women's | 13(12.9%) |
| Age, years | 63.8 ± 11.3 |
| Women | 47(46.5%) |
| BMI, kg | 26.1 ± 2.8 |
| Range | 17.1–30.9 |
| Diabetes mellitus | 26(25.7%) |
| On insulin | 7 (6.9%) |
| On oral medications | 17(16.8%) |
| Hypertension | 74(73.3%) |
| Hyperlipidemia | 77(76.2%) |
| Current smoking | 13(12.9%) |
| Family history of premature heart disease | 31(30.7%) |
| No risk factors | 3(3.0%) |
| Stress type | |
| Exercise: Bruce Protocol | 46 (45.5%) |
| Exercise: Modified Bruce Protocol | 4 (4.0%) |
| Adenosine | 25(24.8%) |
| Regadenoson | 25(24.8%) |
| Dobutamine | 1(1.0%) |
Table 2.
Activities and Radiation Effective Doses
| Mean | Standard Deviation. | Range | |
|---|---|---|---|
| Ultra-Low Dose (ULD) injection | |||
| Administered Activity in MBq (mCi) | 198 (5.35) | 37 (1.00) | 127–275 (3.44–7.44) |
| Residual Activity in MBq (mCi) | 64 (1.74) | 26 (0.71) | 6.3–144 (0.17–3.89) |
| Received Activity in MBq (mCi) | 134 (3.62) | 28 (0.75) | 67–189 (1.80–5.10) |
| Effective Dose, Received (mSv) | 1.15 | 0.24 | 0.57–1.63 |
| Supplemental injection, prior to Standard Low Dose (SLD) imaging | |||
| Administered Activity in MBq (mCi) | 229 (6.21) | 105 (2.84) | 120–588 (3.23–15.89) |
| Residual Activity in MBq (mCi) | 65 (1.75) | 46 (1.24) | 5.9–270 (0.16–7.29) |
| Received Activity in MBq (mCi) | 165 (4.46) | 68 (1.83) | 75–383 (2.04–10.34) |
| Effective Dose, Received (mSv) | 1.42 | 0.58 | 0.65–3.30 |
| Total SLD (Supplemental injection+decayed ULD injection) | |||
| Received Activity in MBq (mCi) | 277 (7.50) | 74 (1.99) | 147–497 (3.98–13.43) |
| Effective Dose, Received (mSv) | 2.39 | 0.64 | 1.27–4.29 |
Count Statistics and Image Quality
A mean of 1.34±0.51 million LV counts were obtained for ULD-HE-SPECT images. There were over 1 million counts for 36 of 49 patients (73%), and an additional 7 patients (total 88%) had over 900,000 counts. Based on consensus reads, IQ was superior for ULD-HE-SPECT images in comparison to SLD-ASPECT images (Table 3), which was the case for patients in both patient groups. Extracardiac activity was similar between ULD-HE-SPECT and SLD-A-SPECT, with borderline significantly less extracardiac activity noted in ULD-HE-SPECT images. Illustrative images comparing sub-mSv ULD-HE-SPECT and SLD-ASPECT are shown in Figure 2.
Table 3.
Image Quality and Extracardiac Activity
| ULD-HE-SPECT | SLD-A-SPECT | |
|---|---|---|
| Image Quality | ||
| Excellent | 48 | 24 |
| Good | 41 | 48 |
| Fair | 5 | 22 |
| Poor | 7 | 7 |
| Mean score±SD | 4.29±0.85 | 3.88±0.85 |
| p value (Wilcoxon) | <0.0001 | |
| Extracardiac Activity | ||
| None | 55 | 40 |
| Minimal | 27 | 35 |
| Mild | 10 | 19 |
| Moderate | 3 | 2 |
| Severe | 6 | 5 |
| Mean score±SD | 0.79±1.12 | 0.98±1.06 |
| p value (Wilcoxon) | 0.05 | |
Values are for consensus read
Figure 2.
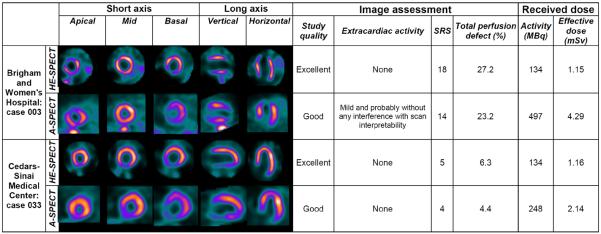
Comparison of representative images between ULD-HE-SPECT and SLD-A-SPECT imaging.
Perfusion Comparison
Abnormal or probably abnormal rest perfusion imaging on SLD-A-SPECT was noted in 22 patients, of which 18 were in Group 2, equivocal rest perfusion in 11 patients, and normal or probably normal rest perfusion in 68 patients. Overall perfusion assessment, based on consensus of the two readers on the five point scale, was similar and not significantly different (p=0.19) between ULD-HESPECT (median[IQR] 4 [3-5], mean 3.92±1.39) and SLD-A-SPECT (median[IQR] 4 [3-5], mean 3.76±1.50).
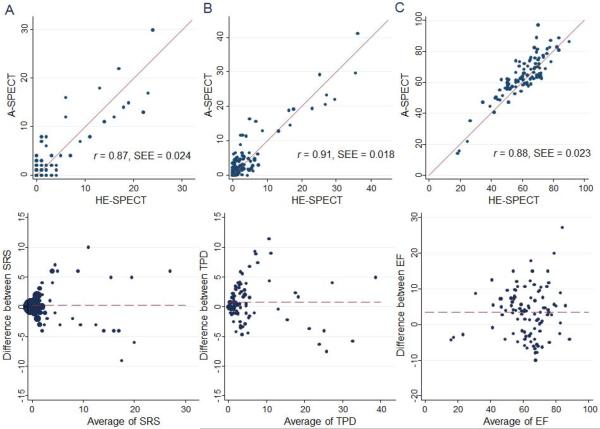
SRS was not significantly different (p=0.96) between ULD-HE-SPECT (mean 2.75±5.41) and SLD-A-SPECT (mean 2.95±5.40), and was strongly correlated between the two methods (r=0.87, p<0.0001), with few cases with notable differences in SRS (Figure 3). The strong correlation (r=0.84, p<0.0001) and lack of significant difference (p=0.63; ULD-HE-SPECT median[IQR] 2 [1–6], mean 5.19±6.69; SLD-A-SPECT median[IQR] 2 [2–8], mean 5.58±6.51) remained when considering only abnormal studies, defined here as those 52 patients with SRS>1 using either method. There was outstanding inter-reader reproducibility in SRS, which was comparable to intra-reader reproducibility. Both inter-reader and intra-reader reproducibility were slightly better for ULD-HE-SPECT imaging than for SLD-A-SPECT imaging (Table 4).
Figure 3.
Comparison of Summed Rest Score from consensus reading, Total Perfusion Deficit, and resting Ejection Fraction between SLD-A-SPECT and ULD-HE-SPECT imaging. A. Summed Rest Score. B. Total Perfusion Deficit (%). C. Ejection Fraction (%). Top: Scatter plots. Bottom: Bland-Altman plots. SEE denotes standard error of the estimate.
Table 4.
Agreement in Summed Rest Scores (SRS)*
| All Studies | Abnormal Studies (SRS>1) | |||
|---|---|---|---|---|
|
|
||||
| SLD A-SPECT | ULD HE-SPECT | SLD A-SPECT | ULD HE-SPECT | |
|
| ||||
| Intra-reader agreement: | ||||
| Reader 1 two reads | 0.79 (95.7%) | 0.83 (96.7%) | 0.74 (92.9%) | 0.78 (93.7%) |
|
| ||||
| Inter-reader agreement: | ||||
| Reader 1 first read vs. Reader 2 | 0.82 (96.5%) | 0.84 (97.1%) | 0.74 (92.2%) | 0.79 (93.4%) |
| Reader 1 second read vs. Reader 2 | 0.78 (95.5%) | 0.85 (96.8%) | 0.73 (92.5%) | 0.80 (93.7%) |
| Between-method agreement: | ||
| Reader 1 first read SLD-A-SPECT vs. Reader 1 first read ULD-HE-SPECT | 0.69 (94.4%) | 0.58 (87.8%) |
| Reader 1 second read SLD-A-SPECT vs. Reader 1 second read ULD-HE-SPECT | 0.63 (92.0%) | 0.54 (87.8%) |
| Reader 1 first read SLD-A-SPECT vs. Reader 1 second read ULD-HE-SPECT | 0.65 (93.2%) | 0.57 (87.4%) |
| Reader 1 second read SLD-A-SPECT vs. Reader 1 first read ULD-HE-SPECT | 0.62 (92.4%) | 0.54 (87.6%) |
| Reader 2 SLD-A-SPECT vs. Reader 2 ULD-HE-SPECT | 0.61 (92.8%) | 0.46 (83.3%) |
| Consensus Read SLD-A-SPECT vs. Consensus Read ULD-HE-SPECT | 0.62 (93.0%) | 0.52 (87.4%) |
Entries denote linearly weighted kappa, with percent agreement in parentheses.
Median (IQR; mean) TPD was 1.8% (0.5%–4.6%; 4.5%) for ULD-HE-SPECT and 2.6% (1.0%–5.3%; 5.3%) for SLD-A-SPECT, a difference which was statistically significant (p=0.04) but whose small magnitude is not clinically significant. There were few cases with notable differences in TPD between ULD-HE-SPECT and SLD-A-SPECT (Figure 3). For the 34 patients with TPD>5%, this difference was not statistically significant (ULD-HE-SPECT median 6.1%, IQR 3.3%–16.6%, mean 10.8%; SLD-A-SPECT median 10.1%, IQR 5.3%–18.8%, mean 12.2%; p = 0.15). There was strong correlation in TPD between ULD-HE-SPECT and SLD-A-SPECT (Figure 3; r=0.91, p<0.0001 for all patients; r=0.88, p<0.0001 for patients with TPD>5%).
Left Ventricular Function Comparison
In one subject, no gated imaging was performed and in another subject, there was a gating artifact which caused an erroneously calculated EF. For the remaining 99 cases, EF was statistically but not clinically significantly different between ULDHE-SPECT (median 62.9%, IQR 51.2%–69.9%, mean 60.5%) and SLD-ASPECT (median 63.6%, IQR 57.9%–73.1%, mean 64.0%), with strong correlation (Figure 3c; r =0.88, p<0.0001).
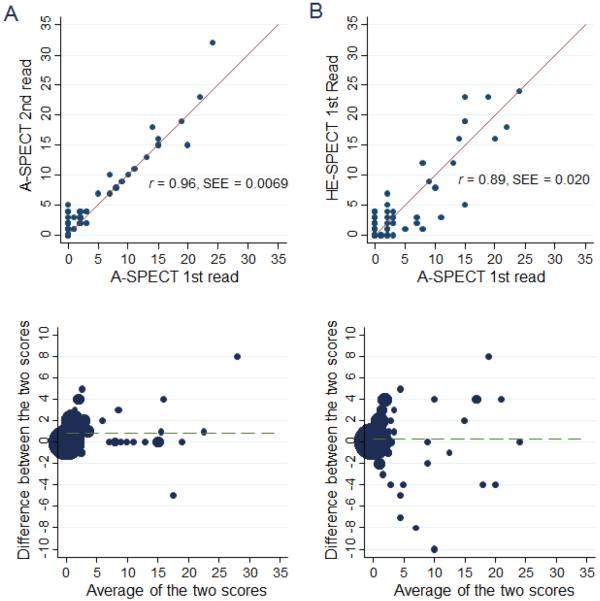
Agreement between ULD-HE-SPECT and SLD-A-SPECT
Agreement in SRS for SLD-A-SPECT between two remote reads of a single reader was outstanding, with 95.7% agreement and weighted kappa of 0.79. Agreement in SRS between the reader's first SLD-A-SPECT reading and ULDHE-SPECT reading showed nearly as good agreement, with 94.4% agreement and weighted kappa of 0.69 (Figure 4). Limiting analysis to abnormal studies with SRS>1, findings were similar, with agreements 92.9% vs. 87.8% and weighted kappas of 0.74 vs. 0.58, respectively (Table 4).
Figure 4.
Comparison of intra-reader agreement in SRS of SLD-A-SPECT, to agreement between SLD-A-SPECT and ULD-HE-SPECT imaging. A. Intra-reader agreement in SRS comparing two SLD-A-SPECT reads, spaced 3 months apart, for first reader. B. Between-method agreement in SRS comparing SLD-ASPECT to ULD-HE-SPECT, for first reader. Top: Scatter plots. Bottom: Bland-Altman plots. SEE denotes standard error of the estimate.
DISCUSSION
Introduction of high-sensitivity cameras has opened the possibility of reducing radiation dose associated with SPECT-MPI. While previous studies have evaluated HE-SPECT protocols with reduced administered activity (7, 8), or compared HE-SPECT to A-SPECT imaging using the same injected activities of radiopharmaceuticals (9–12), no previous study evaluated reduced-dose MPI using a HE-SPECT camera in comparison to traditional A-SPECT performed on the same patients. Our multicenter study found that a single-injection SPECT MPI study can be performed using a HE-SPECT camera at a mean radiation dose of 1 mSv, while attaining high correlation with conventional Anger SPECT in terms of perfusion and function, with improved image quality and comparable extracardiac activity.
These results suggest that a stress-only procedure can be performed using a HE-SPECT camera with effective dose of 1 mSv. Single-injection rest imaging was chosen as the method with which to explore the low-radiation-dose procedure as it made it possible to perform ULD-HE-SPECT imaging under identical conditions to SLD-A-SPECT imaging, using a divided rest dose. A stress dose cannot be analogously administered in a divided fashion, but rather would require patients to undergo stress testing twice; differences between the two stress tests, for example in patient hemodynamics or duration of exercise, might well result in differences in stress perfusion imaging that do not reflect any innate difference between a ULD injection imaged on a HE-SPECT camera and a standard injection imaged on an A-SPECT camera. The pharmacokinetics of Tc-99m-sestamibi are similar at rest and stress, in fact with the proportion of the injected dose that is retained in the myocardium slightly higher at stress (14, 18). Thus we expect that agreement between ULD-HE-SPECT and SLD-A-SPECT imaging should be similar for rest imaging and stress imaging. Furthermore, extracardiac activity interfering with image interpretation is higher with rest than with stress studies (19), suggesting that ULD stress studies performed with the HE-SPECT camera might be of higher quality than we observed on rest studies.
Stress-only imaging has lower radiation dose and faster laboratory throughput than traditional protocols incorporating both rest and stress imaging. It has become especially important in light of recent publications finding that an increasing percentage (>90% in one report) of rest-stress studies are normal (20), and that outcomes of normal rest-stress and stress-only studies are identical (21, 22). Reflecting these observations, a stress-only procedure is preferred for many patients in current guidelines (23, 24). Thus, we believe our findings suggesting the feasibility of 1 mSv stress-only MPI will be of interest to a broad community of physicians performing and ordering nuclear imaging studies.
The radiation dose reduction of over 50% observed here for ULD-HE-SPECT is in reference to a baseline where SLD-A-SPECT imaging was performed with an average received activity of 277 MBq (7.50 mCi). This activity, which is lower than the lower end of the recommend range of activity for low-dose rest imaging in current guidelines (25), reflects practice in 3 expert laboratories, all of which use iterative reconstruction, and one of which uses software reconstruction incorporating resolution recovery and noise reduction. Such reconstruction techniques, which are also incorporated in the HE-SPECT camera, can improve image quality and diagnostic ability, as well as contribute to reducing radiation dose to patients. The low doses observed here in both SLD-A-SPECT and ULDHE-SPECT imaging underscore the message that both software and hardware advances can be used to lower doses from SPECT MPI. In particular, laboratories without access to HE-SPECT cameras can still consider use of advanced reconstruction software to lower doses to their patients (5).
For patients requiring both stress and rest imaging on the same day, guidelines suggest that the second dose of radiopharmaceutical should generally be 3–4 times the activity of the first dose (25), to minimize the problem of shine-through, wherein residual activity from the initial dose interferes with the interpretation of the second dose. Thus the findings of our manuscript suggest that a complete rest-stress or stress-rest HE-SPECT study could be completed with an effective dose of less than 5 mSv (1 + 3 or 4 mSv). Even further, initial data from Nkoulou et al (26) suggest that using a HE-SPECT camera, accurate assessment of ischemic myocardial disease can be performed with the second dose of radiopharmaceutical having the same activity as the first dose. This suggests the possibility of 1 mSv stress-first imaging performed with a HE-SPECT camera, followed only if abnormal by same-day 1 mSv rest imaging. This approach would reduce the radiation burden from a complete, one-day stress and rest MPI study to less than a year's background radiation. Thus, the findings here may be foundational for future efforts to lower radiation dose to patients using a variety of protocols on the new generation of HE-SPECT cameras.
An important issue raised by our study is the challenge posed in low-dose MPI in accurately delivering a desired activity (mCi). In contrast to Tl-201, Tc-99m-based radiopharmaceuticals are “sticky,” adhering to their syringes. Residual activities of Tc-99m-sestamibi in the syringe averaged around 70 (2 mCi), but ranged from less than 7 MBq (0.2 mCi) up to nearly 150 MBq (4 mCi) for an ULD injection. While for high-dose injections, typically around 1110 MBq (30 mCi), and even for SLD injections, typically 300–440 MBq (8–12 mCi) (25), this variability in residual activity represents a modest fraction of the actual received activity, for ULD imaging this residual dose can represent the majority of the administered activity. Better understanding of determinants of Tc-99m residual activity, and development of methods to ensure reproducibility of received activity, will be important to ensure the quality of ULD MPI.
Our study is not without limitations. We excluded obese patients with BMI≥30kg/m2, for whom data about the suitability of MPI with a HE-SPECT camera is mixed. For example, Fiechter et al (27) found non-diagnostic IQ in the majority of patients with BMI≥40 due to image truncation, and IQ less than “good” in a third of patients with BMI of 35–39.5 undergoing multipinhole HE-SPECT imaging. Our calculations performed prior to the study to determine acquisition times suggested that for a 1 mSv injection, an acquisition of more than 15 minutes would be required for obese patients to ensure adequate count statistics. Since 15 minutes is often used as a maximum acquisition time, beyond which many patients are unable to lie still and thus the frequency of motion artifacts increases, obese patients were not included in this study aiming for a radiation dose of 1 mSv. Thus, the generalizability of our ULD HE-SPECT protocol to obese patients remains to be demonstrated. Moreover, few patients were determined to have EF<30% and thus further validation is required in this population. Another limitation is that the study was confined to examination of only one of the two HE-SPECT cameras currently available. One recent comparative study suggests that while both HE-SPECT cameras have better performance characteristics than do Anger cameras, they differ in that the D-SPECT camera studied here has greater count sensitivity, whereas the other camera (Discovery NM 530c, GE Healthcare) has better spatial resolution and modestly higher contrast-to-noise ratio (4). A final limitation is that our images were limited to rest MPI. As discussed above, because we wanted to compare ULD-HE-SPECT to SLD-A-SPECT MPI, evaluation of stress images would have required all patients to undergo stress testing on two separate occasions, since stress imaging cannot be performed with a divided dose as can rest imaging. We desired that both ULD-HE-SPECT and SLD-A-SPECT would be performed under identical conditions and thus chose to study the rest examination. However, as discussed above, the increased cardiac uptake and decreased extracardiac activity observed in stress images as compared to rest images suggest that our positive findings from ULD rest imaging are likely to be applicable to stress-only imaging as well.
CONCLUSION
Our study demonstrates that in non-obese individuals with a high-efficiency scintillation camera, MPI can be performed at high quality with an effective dose of 1 mSv for a single injection.
Acknowledgments
The authors thank Dr. Lynne Johnson, Ming Feng, Nathaniel Roth, and Jim Gerlach for their assistance.
Supported by a grant from Spectrum Dynamics.
References
- 1.National Council on Radiation Protection and Measurements . Report No. 160, Ionizing Radiation Exposure of the Population of the United States. Bethesda, MD: 2009. [Google Scholar]
- 2.Einstein AJ. Effects of radiation exposure from cardiac imaging how good are the data? J Am Coll Cardiol. 2012;59:553–565. doi: 10.1016/j.jacc.2011.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anger HO. Scintillation camera. Rev Sci Instrum. 1958;29:27–33. [Google Scholar]
- 4.Imbert L, Poussier S, Franken PR, et al. Compared performance of high-sensitivity cameras dedicated to myocardial perfusion SPECT: a comprehensive analysis of phantom and human images. J Nucl Med. 2012;53:1897–1903. doi: 10.2967/jnumed.112.107417. [DOI] [PubMed] [Google Scholar]
- 5.Slomka PJ, Dey D, Duvall WL, Henzlova MJ, Berman DS, Germano G. Advances in nuclear cardiac instrumentation with a view towards reduced radiation exposure. Curr Cardiol Rep. 2012;14:208–216. doi: 10.1007/s11886-012-0248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharir T, Ben-Haim S, Merzon K, Prochorov V, Dickman D, Berman DS. High-speed myocardial perfusion imaging initial clinical comparison with conventional dual detector anger camera imaging. JACC Cardiovasc Imaging. 2008;1:156–163. doi: 10.1016/j.jcmg.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Oddstig J, Hedeer F, Jogi J, Carlsson M, Hindorf C, Engblom H. Reduced administered activity, reduced acquisition time, and preserved image quality for the new CZT camera. J Nucl Cardiol. 2012;20:38–44. doi: 10.1007/s12350-012-9634-6. [DOI] [PubMed] [Google Scholar]
- 8.Berman DS, Kang X, Tamarappoo B, et al. Stress thallium-201/rest technetium-99m sequential dual isotope high-speed myocardial perfusion imaging. JACC Cardiovasc Imaging. 2009;2:273–282. doi: 10.1016/j.jcmg.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Esteves FP, Raggi P, Folks RD, et al. Novel solid-state-detector dedicated cardiac camera for fast myocardial perfusion imaging: multicenter comparison with standard dual detector cameras. J Nucl Cardiol. 2009;16:927–934. doi: 10.1007/s12350-009-9137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharir T, Slomka PJ, Hayes SW, et al. Multicenter trial of high-speed versus conventional single-photon emission computed tomography imaging: quantitative results of myocardial perfusion and left ventricular function. J Am Coll Cardiol. 2010;55:1965–1974. doi: 10.1016/j.jacc.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Songy B, Lussato D, Guernou M, Queneau M, Geronazzo R. Comparison of myocardial perfusion imaging using thallium-201 between a new cadmium-zinc-telluride cardiac camera and a conventional SPECT camera. Clin Nucl Med. 2011;36:776–780. doi: 10.1097/RLU.0b013e31821a294e. [DOI] [PubMed] [Google Scholar]
- 12.Duvall WL, Croft LB, Ginsberg ES, et al. Reduced isotope dose and imaging time with a high-efficiency CZT SPECT camera. J Nucl Cardiol. 2011;18:847–857. doi: 10.1007/s12350-011-9379-7. [DOI] [PubMed] [Google Scholar]
- 13.Nakazato R, Berman DS, Hayes SW, et al. Myocardial perfusion imaging with a solid-state camera: simulation of a very low dose imaging protocol. J Nucl Med. 2013;54:1–7. doi: 10.2967/jnumed.112.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53). ICRP Publication 80. Ann ICRP. 1998;28:1–126. doi: 10.1016/s0146-6453(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 15.The 2007 recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 17.Slomka PJ, Nishina H, Berman DS, et al. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol. 2005;12:66–77. doi: 10.1016/j.nuclcard.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Wackers FJ, Berman DS, Maddahi J, et al. Technetium-99m hexakis 2-methoxyisobutyl isonitrile: human biodistribution, dosimetry, safety, and preliminary comparison to thallium-201 for myocardial perfusion imaging. J Nucl Med. 1989;30:301–311. [PubMed] [Google Scholar]
- 19.Rehm PK, Atkins FB, Ziessman HA, et al. Frequency of extra-cardiac activity and its effect on 99Tcm-MIBI cardiac SPET interpretation. Nucl Med Commun. 1996;17:851–856. doi: 10.1097/00006231-199610000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Rozanski A, Gransar H, Hayes SW, et al. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol. 2013;61:1054–1065. doi: 10.1016/j.jacc.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 21.Chang SM, Nabi F, Xu J, Raza U, Mahmarian JJ. Normal stress-only versus standard stress/rest myocardial perfusion imaging: similar patient mortality with reduced radiation exposure. J Am Coll Cardiol. 2010;55:221–230. doi: 10.1016/j.jacc.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Duvall WL, Wijetunga MN, Klein TM, et al. The prognosis of a normal stress-only Tc-99m myocardial perfusion imaging study. J Nucl Cardiol. 2010;17:370–377. doi: 10.1007/s12350-010-9210-x. [DOI] [PubMed] [Google Scholar]
- 23.Depuey EG, Mahmarian JJ, Miller TD, et al. Patient-centered imaging. J Nucl Cardiol. 2012;19:185–215. doi: 10.1007/s12350-012-9523-z. [DOI] [PubMed] [Google Scholar]
- 24.Cousins C, Miller DL, Bernardi G, et al. Radiological protection in cardiology. ICRP Publication 120. Ann ICRP. 2013;42:1–125. doi: 10.1016/j.icrp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS. Stress protocols and tracers. J Nucl Cardiol. 2006;13:e80–90. doi: 10.1016/j.nuclcard.2006.08.011. Updated at: http://www.asnc.org/imageuploads/ImagingGuidelinesStressProtocols021109.pdf. [DOI] [PubMed] [Google Scholar]
- 26.Nkoulou R, Pazhenkottil AP, Kuest SM, et al. Semiconductor detectors allow low-dose-low-dose 1-day SPECT myocardial perfusion imaging. J Nucl Med. 2011;52:1204–1209. doi: 10.2967/jnumed.110.085415. [DOI] [PubMed] [Google Scholar]
- 27.Fiechter M, Gebhard C, Fuchs TA, et al. Cadmium-zinc-telluride myocardial perfusion imaging in obese patients. J Nucl Med. 2011;53:1401–1406. doi: 10.2967/jnumed.111.102434. [DOI] [PubMed] [Google Scholar]