Abstract
Purpose
Given the metabolic and neurologic side effects of antipsychotics and concerns about the increased risks associated with concomitant use, antipsychotic polypharmacy is a quality concern. This study assessed the operating characteristics of a Medicaid claims-based measure of antipsychotic polypharmacy.
Methods
A random sample from 10 public mental health clinics and 312 patients met criteria for this study. Medical record extractors were blind to measure status. We examined the prevalence, sensitivity, specificity, and positive predictive value (PPV) in Medicaid claims, testing nine different definitions of antipsychotic polypharmacy, including >14, >60, or >90 days concurrent use of ≥2 antipsychotic agents, each with allowable gaps of up to 0, 14, or 32 days in days’ supply of antipsychotic medications.
Results
All Medicaid claims measure definitions tested had excellent specificity and PPV (>91%). Good to excellent sensitivity was dependent upon use of a 32-day gap allowance, particularly as duration of concurrent antipsychotic use increased. The proposed claims-based measure (90-day concurrent use of ≥2 or more antipsychotics, allowing for a 32-day gap) had excellent specificity (99.1%, 95%CI: 98.2–99.6) and PPV (90.9%, 95%CI: 83.1–95.7) with good sensitivity (79.4%, 95%CI: 70.4–86.6). The overall level of concordance between claims and medical record-based categorization of antipsychotic polypharmacy was high (96.4%, n = 301/312 clients, Cohen's K = 84.7, 95%CI: 75.9–93.5). Discrepant cases were reviewed, and implications are discussed.
Conclusions
Administrative claims data can be used to construct valid measures of antipsychotic polypharmacy.
Keywords: antipsychotic polypharmacy, administrative data, sensitivity, positive predictive value, Medicaid, pharmacoepidemiology
Antipsychotic polypharmacy is common,1–5 despite sparse evidence supporting the practice.6–8 Evidence of efficacy is limited to small randomized controlled clinical trials, case reports, and individual clinician experience.8–13 At the same time, antipsychotic polypharmacy has been associated with an increased risk of metabolic syndrome14–16 and increased healthcare costs,17–21 and may carry an increased risk of mortality.22 Although some individuals may benefit from ongoing antipsychotic polypharmacy, in one clinical trial, a majority of individuals on antipsychotic polypharmacy were able to successfully transition to a single antipsychotic medication.23 Given well-established metabolic and neurologic side effects of antipsychotics, concerns about the increased risks associated with multiple antipsychotics, and high costs of these agents, antipsychotic polypharmacy has been identified as a quality concern by states and national accrediting bodies.24,25
Wide variability has been reported in the prevalence of antipsychotic polypharmacy among those treated with antipsychotics, ranging from 7% to 50%,14,26–29 although most studies report rates between 10% and 30%.6,9 This variation is likely related to differences in the general demographic and clinical characteristics of the study populations, year of the study, variations in study method, treatment setting, and the duration of the study period. For example, antipsychotic polypharmacy was found to be 8% among adolescents in Florida's Medicaid program2, 29% among adults enrolled in a California Medicaid program,28 and over 50% among psychiatric inpatients.30
Some of the variability in reported prevalence estimates likely results from differences in the methods of measuring polypharmacy. Definitions that use narrow time frames of concurrent treatment with multiple antipsychotics may overestimate true prevalence, as they may include instances when a change in medications occurs before the previous prescription has been finished (e.g., within 30 days), or temporary medication overlaps such as those for patients switching medications using a cross-taper.31 Previous research has variously defined polypharmacy to be concomitant therapy lasting over 14,28 60,4 and 90 days.29 In administrative data, observed periods without medication may represent poor adherence, short hospital admissions, use of free medication samples, eligibility gaps, or actual discontinuation of medication. In previous claims-based antipsychotic polypharmacy measures, medication gaps have been operationalized by not allowing gaps,4,31 or allowing gaps of 143 or ≤31 days.28 When measuring long-term polypharmacy, such breaks may represent changes in the antipsychotic regimen, which occur in the context of long-term polypharmacy.
A valid measure of antipsychotic polypharmacy is important not only to pharmacoepidemiologists but also to state Medicaid offices, healthcare plans, and pharmacy benefit managers interested in quality of care improvement initiatives. Pharmacy programs use measures to support interventions with individual patients and physicians, including prior authorization before filling prescriptions, retrospective drug utilization review, second opinion consultation requirements, and computer-based decision support tools, like electronic health records and prescribing applications that provide messages to prescribers. As measures may be used to intervene directly in clinical care pathways, it is important to establish that they are clinically meaningful and valid. With medical records as the criterion standard, we assessed the validity of an antipsychotic polypharmacy measure that was developed for a statewide quality improvement initiative. A claims-based measure was developed in an empirically derived and clinically guided manner to enhance the utility of the measure. We examine the demographic and operating characteristics of this measure, and test the impact of alternative definitions of antipsychotic polypharmacy on the prevalence, sensitivity, specificity, and positive predictive value (PPV) of the measure, specifically 14, 60, and 90 days current use, and a gap allowance in days’ supply of individual antipsychotics of 0, 14, and 32 days.
METHODS
Validation study sample
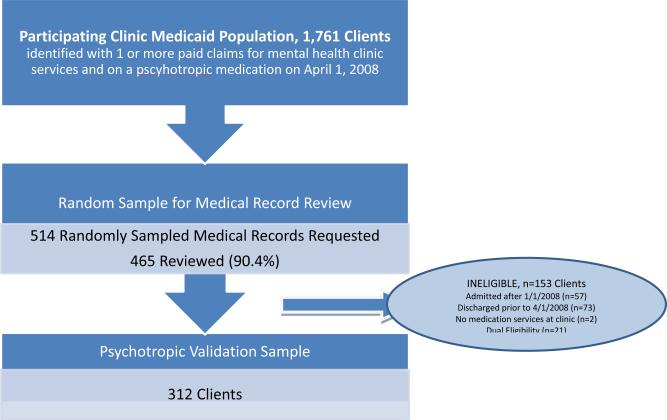
The study sample was drawn from a statewide Medicaid database that includes all Medicaid enrollees, regardless of length of Medicaid eligibility, with an active prescription for any psychotropic medication on 1 April 2008 (n = 225 439). Ten urban mental health clinics from five agencies participated in the evaluation. Medicaid enrollees with ≥1 mental health clinic claim between 1 April 2007 and 1 April 2008, and ≥1 active psychotropic medications on 1 April 2008 were included in the target population (n = 1761). A simple random sample of these clients (of up to 150 clients) were selected from the four larger agencies, and all recipients were selected from the smallest agency that served fewer than 150 Medicaid recipients (total n = 514). The New York State Office of Mental Health's Institute Review Board determined that this work was not human subjects research.
Of requested medical records, 465 (90.8%) were retrieved and reviewed. Patients who had been discharged from the clinic prior to 1 April 2008 (n = 73), were admitted to the clinic after 1 January 2008 (n = 57), were not receiving medication management services at the clinic (n = 2), or had Medicare Part D (n = 21) were excluded, resulting in a final analytic sample of 312 (Figure 1).
Figure 1.
Medical record validation sample
Record review procedures
Reviewers conducted medical record reviews on site at the 10 clinics. The six reviewers had Masters-level or higher education in social work, psychology, or medical fields. Reviewers were trained in the use of the structured data abstraction form and were kept blind to claims-based indicator status. Each review abstracted medication name, start date, and most recent date prescribed or the end date/discontinued date for all medications listed in the medication order sheet or the physician's progress notes for a 6-month period of observation, 1/10/2007–1/4/2008. When the start date preceded the period of observation, 1/10/2007 was used as the start date. If no start date was noted, 1/10/2007 or the first date written in the medical record was assumed to be the start date. If the medication was active and continuing on the last day of the period of observation, then 1/4/2008 was entered. If the end date was not noted, the trial was assumed to be ongoing as of the report date. A total of 22 medical records were abstracted both by the medical record review team leader (E. K.) and the other reviewers on the team for training and/or general review. All reviewers were able to reach a kappa of 0.90 or greater with the team leader.
Measures and definitions
Demographic measures
A number of variables were extracted, including demographic information (sex, ethnicity, and date of birth), mental health diagnosis, mental health clinic service utilization, and Medicaid eligibility. Mental health clinic clients were defined as all Medicaid enrollees with one or more mental health clinic service in the year before the report date (1/4/2007–1/4/2008).
Definition of antipsychotic polypharmacy in claims data. Process of developing a claims-based measure
The measure development process has been previously described.32 In brief, we convened a Scientific Advisory Committee of national experts in psycho-pharmacology that endorsed antipsychotic polypharmacy as a quality concern and recommended a duration of concurrent antipsychotic use of >90 days. A workgroup with expertise in quality indicator development and assessment of prescribing practices in Medicaid data (coauthors) was established to develop technical specifications for the measure. We conducted a series of tests to support measure definition, including frequency of the days’ supply of antipsychotics, frequency of gaps in medication trials, survival analyses of time to discontinue multiple medications, frequency of gaps in Medicaid eligibility, frequency of average hospitalization length of stay, and impact of varying gap in concurrent use of antipsychotics on prevalence (Appendix A). We examine the operating characteristics of the proposed definition, based on expert recommendation and empirical review (>90 days concurrent antipsychotics, 32-day gap allowance in days’ supply, and 15-day gap in polypharmacy; technical specification and SAS syntax available upon request), compared with nine alternative definitions varying the gap allowance in days’ supply and the duration of antipsychotic polypharmacy.
Defining medication trials in claims data
Medication information was extracted from the Medicaid database (NDC code, quantity dispensed, days’ supply, and date medication was picked up at the pharmacy). The medication trial start date was the first date the antipsychotic was filled and picked up by the client between 1/10/2007 and 1/4/2008. The end date was the last date the prescription was picked plus the days’ supply, allowing for a gap (of 0, 14, or 32 days) in days’ supply between consecutive start and end dates of the same medication. A standard days’ supply was used for long-acting injectable antipsychotic medications.33 Duration of trial was calculated as the difference in days between the trial start date and the calculated end date or 1 April, whichever is earlier, allowing for a gap in antipsychotic medication days’ supply of up to 0, 14, or 32 days.
Defining antipsychotic polypharmacy using claims data
The monthly prevalence of antipsychotic polypharmacy was defined as the concurrent use of ≥2 antipsychotic medications for >14, > 60, and >90 days, among individuals on any antipsychotics for >14, >60, and >90 days, respectively, at any time during the 35 days preceding the index date (1 April). Gaps in polypharmacy of ≤15 days were allowed.
Defining medication trials from medical record extraction
The duration of antipsychotic medication trial was defined for each antipsychotic noted in the medical record between 1/10/2007 and 1/4/2008. No gap allowance was used to calculate the length of individual antipsychotic medication trials in medical records in order to define duration as prescribed.
Defining antipsychotic polypharmacy using medical record
Antipsychotic polypharmacy was defined by the presence of ≥2 concurrent antipsychotics recorded in the medical record for >14, >60, or >90 days, among individuals on antipsychotics for >14, >60, and >90 days, respectively, at any time during the 35 days preceding the index date (1 April). No gaps in polypharmacy were allowed in order to define duration of polypharmacy as prescribed.
DATA ANALYSIS
All analyses were calculated using SAS 9.2 (Carey, NC, USA). The demographic characteristics of Medicaid enrollees receiving psychotropic medication for >90 days as of 1 April 2008 were calculated from Medicaid data for enrollees: (i) statewide all treatment settings; (ii) statewide, mental health clinic; and (iii) validation samples.
For the validation sample, PROC SURVEYSELECT was used to create the simple random samples of patients from each agency that served over 150 clients on psychotropics (the number of potential medical records to be reviewed at each agency was capped at 150 as to not be too onerous on the participating clinics). For the smallest agency that served less than 150 Medicaid clients on a psychotropic, all patients were sampled. Data extracted from the medical records were considered the criterion standard for the evaluation of operating characteristics (sensitivity, specificity, and PPV). The sampling probability and corresponding weights were used to calculate weighted proportions of the full agency Medicaid population on psychotropics for the proposed antipsychotic measure (>90 days concurrent use of ≥2 antipsychotics, with >32 days gap allowance). The exact binomial 95% confidence intervals were calculated using the SAS %BNMLCI macro.34 Cohen's kappa was calculated to measure overall agreement between Medicaid data and claims data. Discrepancies between the Medicaid claims data and medical record data (false positives and false negatives) were examined and reasons for differences explored.
RESULTS
Demographic characteristics
The validation sample was generally similar to the other populations with the exception that the New York City-based sample had a lower proportion of White enrollees and fewer youth (Table 1). As expected, the proportion of Medicaid enrollees with serious mental illness was higher among populations served in mental health clinics than in any treatment setting statewide (Table 1).
Table 1.
Characteristics of the New York State Medicaid population with an active psychotropic (as of 1 April 2008): statewide and in the validation sample
| Statewide: all treatment settings n = 225 439 (100%) | Statewide: MH clinic clients n = 75 306 (33.4%) | Validation sample N = 312 (0.1%) | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 40.16 (18.5) | 40.4 (17.6) | 44.0 (14.4) |
| ≤6 | 1880 (0.8%) | 320 (0.4%) | 0 (0%) |
| 6-12 | 23 162 (10.4%) | 7923 (10.6%) | 22 (7.1%) |
| 13-21 | 21 539 (9.6%) | 7237 (9.6%) | 10 (3.2%) |
| 22-55 | 127 203 (56.8%) | 42 605 (56.9%) | 211 (67.6%) |
| 55+ | 49 867 (22.3%) | 16 753 (22.4%) | 68 (21.8%) |
| Gender | |||
| % Male | 100 161 (44.4%) | 32 104 (42.6%) | 141 (45.2%) |
| Race/ethnicity | |||
| White, non-Hispanic | 110 060 (48.8%) | 34720 (46.1%) | 83 (26.6%) |
| African American, non-Hispanic | 47 243 (20.9%) | 15 392 (20.4%) | 132 (42.3%) |
| Hispanic | 32 976 (14.6%) | 13 490 (17.9%) | 60 (19.2%) |
| Other | 22 272 (9.8%) | 7256 (9.6%) | 31 (9.9%) |
| Unknown | 12 888 (5.7%) | 4448 (5.9%) | 6 (1.9%) |
| Primary diagnosis | |||
| Schizophrenia spectrum | 18 702 (8.3%) | 13 775 (18.3%) | 108 (34.7%) |
| Bipolar | 10 623 (7.0%) | 8313 (11.0%) | 37 (11.9%) |
| Major depression | 26 554 (17.5%) | 21 961 (29.2%) | 101 (32.4%) |
Notes:
All comparison populations had one or more active psychotropic prescriptions as of 1/4/2008. All characteristics were extracted from Medicaid data.
MH clinic clients = Medicaid enrollee with one or more services in a mental health clinic in the 12 months prior to the report date (1/4/2007-1/4/2008).
Because of missing data, not all proportions will add to 100%.
Validation
Impact of alternative definitions of polypharmacy on prevalence, sensitivity, specificity, and positive predictive value
The operating characteristics of nine alternative claims-based definitions of antipsychotic polypharmacy are presented in Table 2. All Medicaid claims definitions examined yielded a lower prevalence than charts, with definitions using a 32-day gap best approximating the prevalence found in medical records. The prevalence dropped to less than half of that observed in medical records, when no gap allowance was used for the >60 and >90 days’ concurrent use measures. For each duration of concurrent anti-psychotic use tested (>90, >60, and >14 days), the sensitivity and specificity were enhanced by using the 32-day gap allowance for individual antipsychotic trials. For polypharmacy >90 days, the sensitivity of the measure increased from 40.9% when no allowable gap was applied to 81.8% when a 32-day allowable gap in the Medicaid records was used. A similar pattern was observed for definitions of polypharmacy of >60 and >14 days, but was less marked for short durations of concurrent use. Regardless of the definition, the PPV and the specificity remained greater than 90%. Specificity was further improved with the addition of a gap allowance. For polypharmacy of >90 days, specificity increased from 91.2% to 99.3% by incorporating a 32-day gap allowance.
Table 2.
Comparing the prevalence, sensitivity, positive predictive value (PPV), and specificity of nine alternative claims-based definitions of antipsychotic polypharmacy
| Source | Parameters | Identified with polypharmacy | Prevalence | Sensitivity | PPV | Specificity |
|---|---|---|---|---|---|---|
| >14 days of polypharmacy | N | (%) | ||||
| Chart | 51 | (30.0%) | ||||
| Medicaid | Allowable gap: 32 days | 50 | (29.4%) | 92.16% | 94.0% | 98.47% |
| Allowable gap: 14 days | 46 | (27.1%) | 82.35% | 91.3% | 96.63% | |
| Allowable gap: none | 45 | (26.5%) | 82.35% | 93.3% | 96.63% | |
| >60 days of polypharmacy | ||||||
| Chart | 48 | (28.9%) | ||||
| Medicaid | Allowable gap: 32 days | 43 | (25.9%) | 83.33% | 93.0% | 97.03% |
| Allowable gap: 14 days | 36 | (21.7%) | 68.75% | 91.7% | 94.57% | |
| Allowable gap: none | 24 | (14.5%) | 50.00% | 96.0% | 91.64% | |
| >90 days of polypharmacy | ||||||
| Chart | 44 | (27.5%) | ||||
| Medicaid | Allowable gap: 32 days | 39 | (24.4%) | 81.82% | 92.3% | 99.25% |
| Allowable gap: 14 days | 32 | (20.0%) | 68.18% | 93.8% | 95.00% | |
| Allowable gap: none | 18 | (11.3%) | 40.91% | 100.0% | 91.16% |
Note: The Medical chart was the criterion standard (n = 312 medical records). The chart-based assessment did not allow for medication gaps in order to capture the duration of antipsychotic medications and polypharmacy as prescribed. Prevalence of the measures is calculated based on nine alternative definitions, using the medical chart-based measure to establish the denominator for individuals on antipsychotics for >14 (n = 170), >60 (n = 166), and >90 days (n = 160).
The proposed measure of polypharmacy (>90 days concurrent antipsychotics, with an allowable gap of 32 days) had a weighted sensitivity of 79.4% (95%CI: 70.4–86.6) and specificity of 99.1% (95%CI: 98.2–99.6). The PPV was 90.9% (95%CI: 83.1–95.9) (Table 3).
Table 3.
Weighted sensitivity, specificity, and positive predictive value (PPV) of the antipsychotic polypharmacy claims-based measure in a random sample of Medicaid mental health clinic clients (n = 312)
| Validation criterion: medical record-based measure |
||||||||
|---|---|---|---|---|---|---|---|---|
| Antipsychotic polypharmacy |
No antipsychotic polypharmacy |
Total | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | |||
| Medicaid claims-based measure | Antipsychotic polypharmacy | 36 | 3 | 39 | 81.8% (67.7-91.8) | 98.8% (96.7-99.7) | 92.3% (79.1-98.4) | Raw |
| No antipsychotic polypharmacy | 8 | 265 | 273 | 79.4% (70.4-86.6) | 99.1% (98.2-99.6) | 90.9% (83.1-95.9) | Weighted | |
| Total | 44 | 267 | 312 | |||||
Notes:
95% exact binomial confidence intervals were calculated for each proportion. Weighted results reflect the underlying sampling probability.
Antipsychotic polypharmacy: two or more antipsychotics for longer than 90 days, as of 1/4/2008.
No antipsychotic polypharmacy: did not meet criteria for antipsychotic polypharmacy, as of 1/4/2008.
Overall level of agreement and review of discrepancies between Medicaid and chart-based measure
The overall level of agreement between the proposed claims-based measure (>90 days concurrent antipsychotics, 32-day allowable gap in days’ supply) and medical record-based categorization of clients on antipsychotic polypharmacy was 96.4% (n = 301/312 clients, Cohen's K = 84.7, 95%CI: 75.9–93.5). Results from the in-depth review of discrepancies of this definition between medical records and Medicaid claims are available upon request. There were three cases identified as antipsychotic polypharmacy by the claims-based measure but not by the medical record-based measure. In one case, an antipsychotic was observed 53 days earlier in the claims than medical record. In the second case, the medical record indicated the physician's intent to discontinue an antipsychotic, although the patient continued to fill the prescription. In the third case, an antipsychotic was not listed in the medical record, although the patient filled a series of prescriptions for it.
There were nine cases where the medical record indicated polypharmacy >90 days, but the Medicaid claims measure did not. In eight cases, the medical records suggested continuous use of two antipsychotics throughout the period of observation, whereas pharmacy claims revealed gaps in medication fills for one of the antipsychotic agents ranging from 50 to 93 days. In the ninth case, one medication that was listed in the chart was not observed in the Medicaid records.
DISCUSSION
The claims-based measure of long-term antipsychotic polypharmacy demonstrated high specificity and PPV, and acceptable sensitivity. Inclusion of a gap allowance, in particular, emerged as an important variable to define. It was required for an acceptable sensitivity and for accurate prevalence estimates for >60 and >90 days polypharmacy.
Changing the required duration of concurrent antipsychotic use from >14 to >60 to >90 days (assuming a 32-day allowable medication gap) did not have a marked impact on the sensitivity, specificity, or PPV. Therefore, duration of polypharmacy should be based on the clinical relevance and intended purpose of the measure. Brief episodes of polypharmacy, while accurately detected in claims data, may represent a planned cross-taper that occurs while changing medications and is consistent with current best practice.35–37 Measures designed for clinical decision support or quality improvement need to minimize false positives to promote clinical utility and focus limited quality improvement resources by selecting a longer duration of polypharmacy, like the recommended >90 days criteria.32
The study also sheds light on the strengths and limitations of using medical records as the criterion standard in claims-based measurement development. Claims-based measures rely on dates that prescriptions are picked up from the pharmacy and the days’ supply to determine discontinuation dates, and may overestimate the duration of a medication trial and polypharmacy. Our study suggests that claims-based measures do not overestimate prevalence of polypharmacy. Review of “false positives” suggests that Medicaid claims data were able to identify prescriptions being filled that were not recorded in the medical record. Physicians may not always update medication sheets or systematically record medication changes in their notes. In addition, physicians may not be aware of prescriptions received by their patients in other treatment settings, whereas claims data can identify prescriptions from any prescriber paid by Medicaid anywhere within the state (via prescriber identifiers on the record).
In some cases, the medical record noted ongoing prescribing of two antipsychotic medications, whereas claims data had gaps in medication prescriptions or the absence of a medication. The medical record may be accurately reflecting ongoing use; yet, these absences may represent periods of non-adherence. This study suggests that Medicaid data may, in some cases, be more inclusive than medical records data, with implications for the clinical value of Medicaid claims data for physicians. New York State is an example of one state that has given physicians secure, web-based access to Medicaid data to support clinical decision making and quality of care.38
The study has several limitations. First, the study was limited to Medicaid patients from a small number of urban specialty mental health clinics, and different results might be obtained from different settings, selection criteria, or payment groups. Second, the migration to electronic health records may improve access and reliability of electronic data. Third, the sample size was not sufficiently large to examine patient groups of particular clinical and policy interest such as patients with treatment-resistant disorders or those with a known history of antipsychotic non-adherence. Fourth, the patients’ actual medication taking was not observed. Fifth, because physicians were not questioned, the clinical intent of the use of polypharmacy was not ascertained. Finally, a small number of medical records were not available for review and may have biased estimation of the operating characteristics.
The high level of agreement between pharmacy claims and medical records suggests that the two sources of data are closely correlated, and either can be used to support pharmacy measure development. It is hoped that the results will spur claims-based evaluations of the safety and effectiveness of antipsychotic polypharmacy as well as quality of care initiatives aimed at reducing potentially inappropriate reliance on this prescribing practice. These findings support the clinically guided empirical approach that was used to develop these indicators, and sufficient numbers of individuals on ≥2 antipsychotics were observed to support a quality improvement focus in this area.
Supplementary Material
KEY POINTS.
• A claims-based measure of antipsychotic polypharmacy demonstrated high specificity and positive predictive value, and acceptable sensitivity against a medical record criterion standard.
• The definition of antipsychotic polypharmacy used has an impact on the prevalence and the validity of the measure. Inclusion of an allowance for gaps in days’ supply of individual medications was particularly important to support specificity and accurate estimates of prevalence for claims-based measures.
ACKNOWLEDGEMENT
This project was funded by the New York State Office of Mental Health.
Footnotes
An earlier version of this work was presented at the Mental Health Services Research Conference in Washington D.C. on 21 July 2009.
CONFLICT OF INTEREST
This project was funded by the New York State Office of Mental Health. The New York State Office of Mental Health reserves the right to review and approve work conducted under its auspices. Dr. Finnerty has been the PI on research grants/contracts from Bristol Myers Squibb and Sunovion; her time spent on these projects is fully supported by the New York State Office of Mental Health.
ETHICS STATEMENT
The New York State Office of Mental Health's Institute Review Board determined that this work was not human subjects research and no approval was needed.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher's web site.
REFERENCES
- 1.Constantine RJ, Andel R, Tandon R. Trends in adult antipsychotic polypharmacy: progress and challenges in Florida's Medicaid program. Community Ment Health J. 2010;46(6):523–530. doi: 10.1007/s10597-009-9288-2. DOI: 10.1007/s10597-009-9288-2. [DOI] [PubMed] [Google Scholar]
- 2.Constantine RJ, Boaz T, Tandon R. Antipsychotic polypharmacy in the treatment of children and adolescents in the fee-for-service component of a large state Medicaid program. Clin Ther. 2010;32(5):949–959. doi: 10.1016/j.clinthera.2010.04.021. DOI: 10.1016/j.clinthera.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Gilmer TP, Dolder CR, Folsom DP, et al. Antipsychotic polypharmacy trends among Medicaid beneficiaries with schizophrenia in San Diego County, 1999–2004. Psychiatr Serv. 2007;58(7):1007–1010. doi: 10.1176/ps.2007.58.7.1007. DOI: 10.1176/appi.ps.58.7.1007. [DOI] [PubMed] [Google Scholar]
- 4.Morrato EH, Dodd S, Oderda G, et al. Prevalence, utilization patterns, and predictors of antipsychotic polypharmacy: experience in a multistate Medicaid population, 1998–2003. Clin Ther. 2007;29(1):183–195. doi: 10.1016/j.clinthera.2007.01.002. DOI: 10.1016/j.clinthera.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18–28. doi: 10.1016/j.schres.2012.03.018. DOI: 10.1016/j.schres.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443–457. doi: 10.1093/schbul/sbn018. DOI: 10.1093/schbul/sbn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goff DC, Freudenreich O. Focus on polypharmacy in schizophrenia: does anyone truly benefit? Int J Neuropsychopharmacol. 2004;7(2):109–111. doi: 10.1017/S1461145704004183. DOI: 10.1017/S1461145704004183. [DOI] [PubMed] [Google Scholar]
- 8.Tranulis C, Skalli L, Lalonde P, et al. Benefits and risks of antipsychotic polypharmacy: an evidence-based review of the literature. Drug Saf. 2008;31(1):7–20. doi: 10.2165/00002018-200831010-00002. http://www.ncbi.nlm.nih.gov/pubmed/18095743. [DOI] [PubMed] [Google Scholar]
- 9.Correll CU, Gallego JA. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am. 2012;35(3):661–681. doi: 10.1016/j.psc.2012.06.007. DOI: 10.1016/j.psc.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Megna JL, Kunwar AR, Mahlotra K, et al. A study of polypharmacy with second generation antipsychotics in patients with severe and persistent mental illness. J Psychiatr Pract. 2007;13(2):129–137. doi: 10.1097/01.pra.0000265773.03756.3e. DOI: 10.1097/01.pra.0000265773.03756.3e. [DOI] [PubMed] [Google Scholar]
- 11.Stahl SM, Grady MM. A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation. Curr Med Chem. 2004;11(3):313–327. doi: 10.2174/0929867043456070. http://www.ncbi.nlm.nih.gov/pubmed/14965234. [DOI] [PubMed] [Google Scholar]
- 12.Zink M, Englisch S, Meyer-Lindenberg A. Polypharmacy in schizophrenia. Curr Opin Psychiatry. 2010;23(2):103–111. doi: 10.1097/YCO.0b013e3283366427. DOI: 10.1097/YCO.0b013e3283366427. [DOI] [PubMed] [Google Scholar]
- 13.Centorrino F, Ventriglio A, Vincenti A, et al. Changes in medication practices for hospitalized psychiatric patients: 2009 versus 2004. Hum Psychopharmacol. 2010;25(2):179–186. doi: 10.1002/hup.1095. DOI: 10.1002/hup.1095. [DOI] [PubMed] [Google Scholar]
- 14.Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1–3):91–100. doi: 10.1016/j.schres.2006.08.017. DOI: 10.1016/j.schres.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misawa F, Shimizu K, Fujii Y, et al. Is antipsychotic polypharmacy associated with metabolic syndrome even after adjustment for lifestyle effects?: a cross-sectional study. BMC Psychiatry. 2011;11:118. doi: 10.1186/1471-244X-11-118. DOI: 10.1186/1471-244X-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centorrino F, Masters GA, Talamo A, et al. Metabolic syndrome in psychiatrically hospitalized patients treated with antipsychotics and other psychotropics. Hum Psychopharmacol. 2012;27(5):521–526. doi: 10.1002/hup.2257. DOI: 10.1002/hup.2257. [DOI] [PubMed] [Google Scholar]
- 17.Baandrup L, Sorensen J, Lublin H, et al. Association of antipsychotic polypharmacy with health service cost: a register-based cost analysis. Eur J Health Econ. 2012;13(3):355–363. doi: 10.1007/s10198-011-0308-0. DOI: 10.1007/s10198-011-0308-0. [DOI] [PubMed] [Google Scholar]
- 18.Rupnow MF, Greenspan A, Gharabawi GM, et al. Incidence and costs of polypharmacy: data from a randomized, double-blind, placebo-controlled study of risperidone and quetiapine in patients with schizophrenia or schizoaffective disorder. Curr Med Res Opin. 2007;23(11):2815–2822. doi: 10.1185/030079907x233359. DOI: 10.1185/030079907X233359. [DOI] [PubMed] [Google Scholar]
- 19.Stahl SM, Grady MM. High-cost use of second-generation antipsychotics under California's Medicaid program. Psychiatr Serv. 2006;57(1):127–129. doi: 10.1176/appi.ps.57.1.127. DOI: 10.1176/appi.ps.57.1.127. [DOI] [PubMed] [Google Scholar]
- 20.Valuck RJ, Morrato EH, Dodd S, et al. How expensive is antipsychotic polypharmacy? Experience from five US state Medicaid programs. Curr Med Res Opin. 2007;23(10):2567–2576. doi: 10.1185/030079907X233214. DOI: 10.1185/030079907X233214. [DOI] [PubMed] [Google Scholar]
- 21.Zhu B, Ascher-Svanum H, Faries DE, et al. Cost of antipsychotic polypharmacy in the treatment of schizophrenia. BMC Psychiatry. 2008;8:19. doi: 10.1186/1471-244X-8-19. DOI: 10.1186/1471-244X-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Windfuhr K, Turnbull P, While D, et al. The incidence and associated risk factors for sudden unexplained death in psychiatric in-patients in England and Wales. J Psychopharmacol. 2011;25(11):1533–1542. doi: 10.1177/0269881110379288. DOI: 10.1177/0269881110379288. [DOI] [PubMed] [Google Scholar]
- 23.Essock SM, Schooler NR, Stroup TS, et al. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168(7):702–708. doi: 10.1176/appi.ajp.2011.10060908. DOI: 10.1176/appi.ajp.2011.10060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goren JL, Parks JJ, Ghinassi FA, et al. When is antipsychotic polypharmacy supported by research evidence? Implications for QI. Jt Comm J Qual Patient Saf. 2008;34(10):571–582. doi: 10.1016/s1553-7250(08)34072-0. http://www.ncbi.nlm.nih.gov/pubmed/18947117. [DOI] [PubMed] [Google Scholar]
- 25.Medicaid Medical Directors Learning Network and Rutgers Center for Education and Research on Mental Health Therapeutics Antipsychotic medication use in Medicaid children and adolescents: report and resource guide from a 16-state study. MMDLN/Rutgers CERTs Publication #1. July 2010. Distributed by Rutgers CERTs at http://rci.rutgers.edu/~cseap/MMDLNAPKIDS.html.
- 26.Faries D, Ascher-Svanum H, Zhu B, et al. Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry. 2005;5:26. doi: 10.1186/1471-244X-5-26. DOI: 10.1186/1471-244X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. DOI: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 28.Ganguly R, Kotzan JA, Miller LS, et al. Prevalence, trends, and factors associated with antipsychotic polypharmacy among Medicaid-eligible schizophrenia patients, 1998–2000. J Clin Psychiatry. 2004;65(10):1377–1388. doi: 10.4088/jcp.v65n1013. http://www.ncbi.nlm.nih.gov/pubmed/15491242. [DOI] [PubMed] [Google Scholar]
- 29.Kreyenbuhl JA, Valenstein M, McCarthy JF, et al. Long-term antipsychotic polypharmacy in the VA health system: patient characteristics and treatment patterns. Psychiatr Serv. 2007;58(4):489–495. doi: 10.1176/appi.ps.58.4.489. DOI: 10.1176/appi.ps.58.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uttaro T, Finnerty M, White T, et al. Reduction of concurrent antipsychotic prescribing practices through the use of PSYCKES. Adm Policy Ment Health. 2007;34(1):57–61. doi: 10.1007/s10488-006-0075-x. DOI: 10.1007/s10488-006-0075-x. [DOI] [PubMed] [Google Scholar]
- 31.Kreyenbuhl J, Valenstein M, McCarthy JF, et al. Long-term combination antipsychotic treatment in VA patients with schizophrenia. Schizophr Res. 2006;84(1):90–99. doi: 10.1016/j.schres.2006.02.023. DOI: 10.1016/j.schres.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Essock SM, Covell NH, Leckman-Westin E, et al. Identifying clinically questionable psychotropic prescribing practices for Medicaid recipients in New York State. Psychiatr Serv. 2009;60(12):1595–1602. doi: 10.1176/ps.2009.60.12.1595. [DOI] [PubMed] [Google Scholar]
- 33.Shireman TI, Svarstad BL, Sweeney JK. Validity of claims data for long-acting injectable medications. J Pharmacoepidemiol. 1999;7(2):41–55. [Google Scholar]
- 34.Bergstralh E. Calculate exact binomial confidence intervals. Revised 2008. Available at http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros.
- 35.Masand PS, Berry SL. Switching antipsychotic therapies. Ann Pharmacother. 2000;34:200–207. doi: 10.1345/aph.18458. [DOI] [PubMed] [Google Scholar]
- 36.Lambert TJ. Switching antipsychotic therapy: what to expect and clinical strategies for improving therapeutic outcomes. J Clin Psychiatry. 2007;68(suppl 6):10–13. [PubMed] [Google Scholar]
- 37.Buckley PF, Correll CU. Strategies for dosing and switching antipsychotics for optimal clinical management. J Clin Psychiatry. 2008;69(suppl 1):4–17. [PubMed] [Google Scholar]
- 38.PSYCKES Medicaid online resources. https://www.omh.ny.gov/omhweb/psyckes_medicaid/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.