Abstract
Focused Clinical Question
Can emerging technologies for periodontal regeneration become clinical reality?
Summary
Emerging technologies are presenting options to hopefully improve the outcomes of regeneration in challenging clinical scenarios. Cellular allografts represent a current technology in which cells and scaffolds are being delivered directly to the periodontal lesion. Recombinant human fibroblast growth factor 2 and teriparatide (parathyroid 1–34) have each been tested in controlled prospective human randomized clinical trials, and both have been shown to have potential for periodontal regeneration. These examples, as well as other emerging technologies, show promise for continued advancement in the field of periodontal regenerative therapy.
Conclusions
At present, there are indications that emerging technologies can be used successfully for periodontal regeneration. Case reports and clinical trials are being conducted with a variety of emerging technologies. However, many are yet to be approved by a regulatory agency, or there is a lack of evidence-based literature to validate their expanded use.
Keywords: Guided tissue regeneration, investigative techniques, periodontal disease, tissue engineering, wound healing
Background
Complete regeneration of the periodontium remains an elusive goal with current treatment modalities. A number of emerging technologies are being examined that can improve the outcome of periodontal regeneration. These include the application of protein and peptide therapy, cell-based therapy, genetic therapy, application of scaffolds, bone anabolics, and lasers. Other novel approaches include: 1) therapies directed at the resolution of inflammation; 2) therapies that take into account the influence of the microbiome; 3) therapies involving the local regulation of phosphate and pyrophosphate metabolism; and 4) approaches directed at harnessing current therapies used for other purposes.
As emerging technologies, by definition, most of these therapies lack high levels of evidence. Currently, there are numerous human clinical trials in varying stages of completion that are attempting to delineate the best applications of the emerging therapies.
Current clinical examples showcased in the body of this report reveal that excellent periodontal defect resolution can occur with a number of emerging technologies (Video 1).
Decision Process
Emerging science and technology and their implication in periodontal wound healing provide promising alternatives to enhance regenerative therapeutic outcomes.
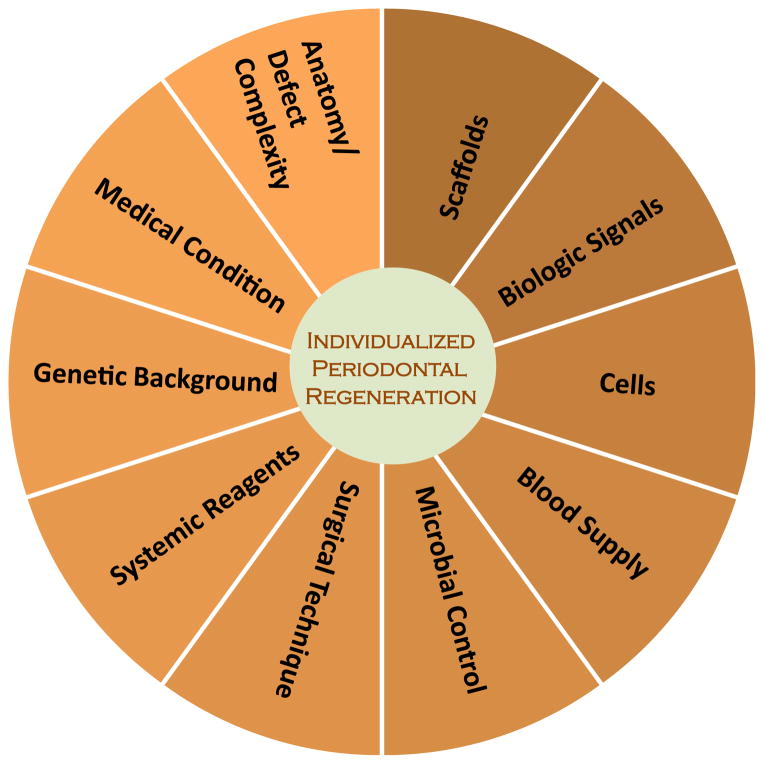
The application of new protocols to treat periodontal diseases is shaping the concept of individualized regenerative therapy; identifying the complex and heterogenic influences relative to each patient is of critical value (Fig. 1).
FIGURE 1.
Factors supporting the emergence of individualized periodontal regenerative therapy.
This report is directed toward contributing to the collective effort currently undertaken by various groups to increase the understanding of emerging therapeutic alternatives that could potentiate the ability to predictably restore normal periodontal function and structure.
Today, the lessons learned on wound healing and periodontal development incorporate gene, protein, and metabolite data into dynamic biologic networks that include disease initiating, susceptibility, and resolving mechanisms. These ongoing efforts of describing the basic elements involved in the regeneration of the periodontal organ have unraveled novel pathways that could be targeted in the treatment of inflammatory periodontal diseases.
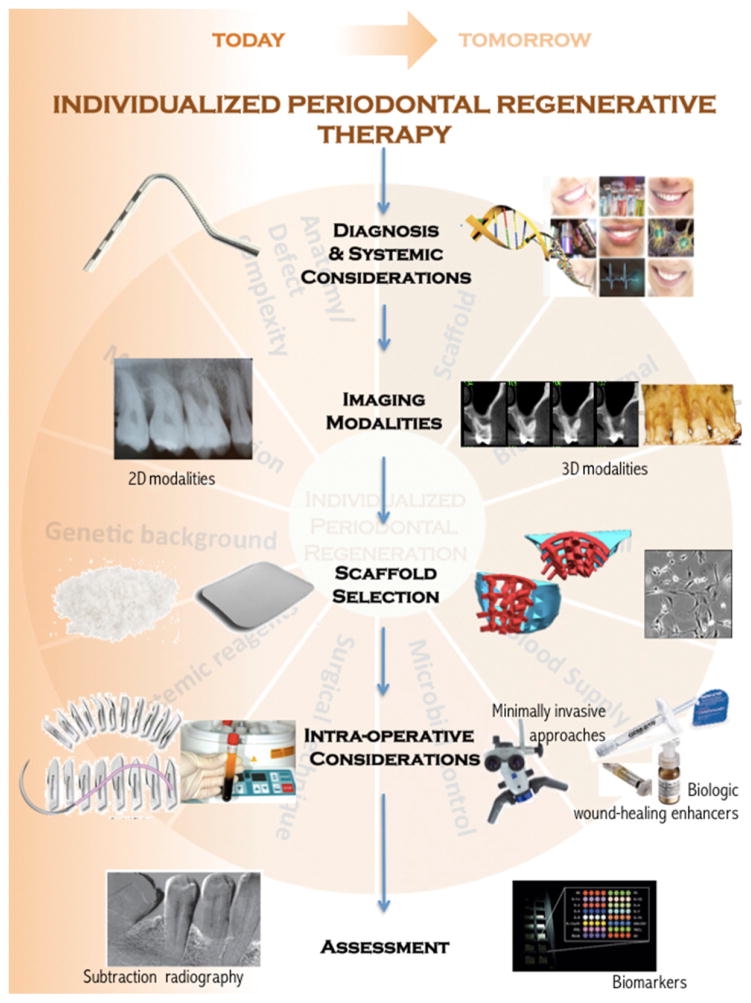
The potential for novel regenerative therapies is supported by five key promising technologies that assist the processes of gathering comprehensive information and selecting the optimal approach that will enhance each patient’s intrinsic regenerative capacity and help to define what constitutes a long-term successful outcome (Fig. 2). The availability of advanced diagnostic methods, the use of three-dimensional imaging modalities, the increasing access to optimized scaffold fabrication technology, and new surgical protocols and tools that minimize trauma and enhance wound healing are paving the road to a more predictable future in periodontal regenerative sciences.
FIGURE 2.
Pivotal emerging science and technology supporting future periodontal regenerative therapeutic protocols. 2D = two-dimensional; 3D = three-dimensional.
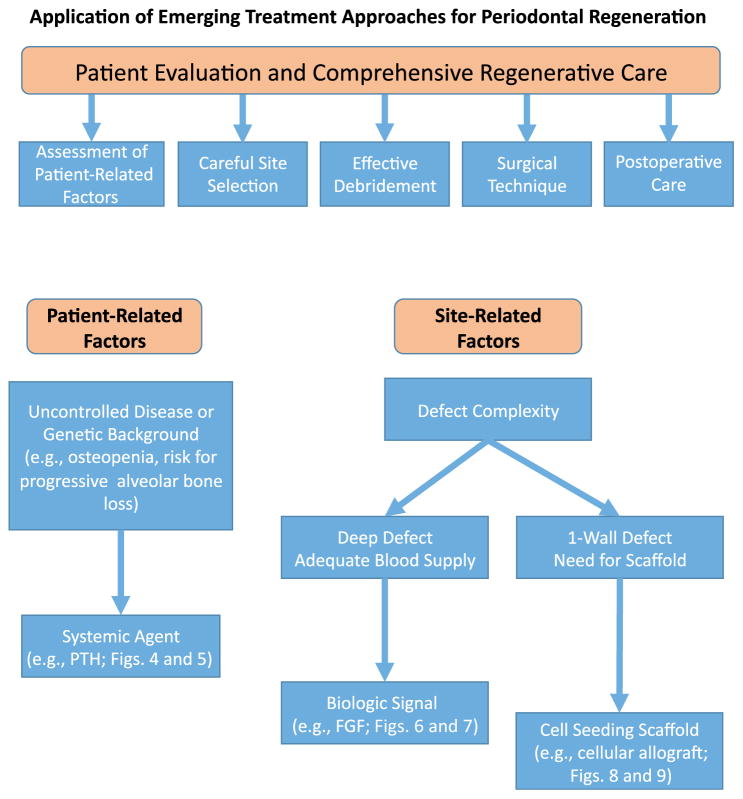
To illustrate the potential benefit of emerging regenerative protocols, this report focuses on three unique approaches: 1) the use of systemic anabolic agents; 2) local delivery of growth factors; and 3) cell therapy. Collectively, the available clinical evidence and the comprehensive understanding of their mechanism of action supports their value and assists the health care provider in the task of identifying those clinical scenarios in which their use may be particularly beneficial (Fig. 3). In the case of systemic bone anabolic agents, such as teriparatide, the literature suggests that all patients would likely benefit from the use of this novel approach. Nonetheless, it is fair to suggest that patients with known bone metabolism issues, such as osteopenia or osteoporosis, might benefit the most from this therapy. With regards to recombinant human fibroblast growth factor (rhFGF), the vast understanding of its mechanism of action positions the local delivery of this factor as a promising emerging therapy. Its role in enhancing periodontal cell proliferation and migration suggests a benefit for tipping the scale in the direction of regeneration. Furthermore, its effects on favoring angiogenesis could help overcome or ameliorate challenging clinical scenarios that are characterized by impaired wound healing. Last, in the case of cellular allografts, these alternatives tend to lend themselves to defects that do not have much in the way of bony walls, which typically supply cells during the wound healing. Examples of these types of defects include 1- and 2-wall defects and furcation defects.
FIGURE 3.
Decision tree model. An emerging treatment algorithm for the selection of viable therapeutic options.
Although the early clinical testing of these therapies is promising, its full potential and clinical significance in wound-healing biology relevant to periodontal regeneration requires the application of sound basic surgical principles along with proper case selection.
Clinical Scenarios
All patients in the following clinical scenarios provided written or oral informed consent prior to treatment.
Teriparatide
A promising anabolic agent is teriparatide,1 which is the commercially available form of parathyroid hormone (PTH 1–34). Teriparatide consists of the first 34 amino acids of the PTH molecule and is given using self-administered daily subcutaneous injections to the thigh or abdomen. Its ability to promote anabolic effects relies on low, intermittent doses. Teriparatide acts on preosteoblasts to increase proliferation and also acts indirectly on osteoblasts to decrease osteoblast apoptosis. This results in bone formation. Figures 4 and 5 show two clinical examples of patients who have received systemic teriparatide for 6 weeks after open flap debridement (OFD) surgery, and it can be observed that bone formation was achieved at 12 months, especially in the furcation regions.
FIGURE 4.
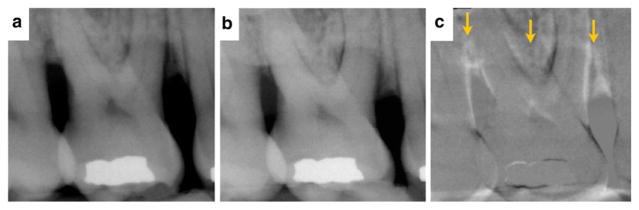
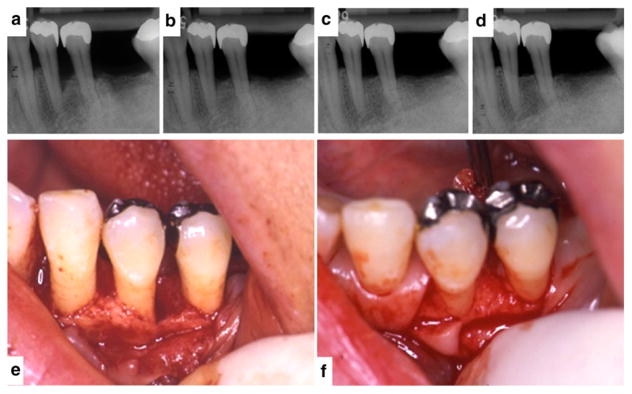
A 45-year-old female former smoker. Radiographic evidence of new bone formation for tooth #3 suggesting periodontal regeneration in the area of the furcation after OFD surgery in conjunction with teriparatide treatment (case courtesy of JDB). 4a Day of surgery. 4b One-year postoperative reevaluation. 4c Digital subtraction radiography of before and after. Arrows localize the enhanced signal reflective of new bone formation.
FIGURE 5.
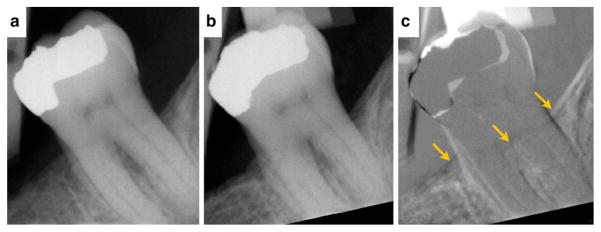
A 48-year-old male current smoker (half pack per day). Radiographic evidence of new bone formation for tooth #18 suggesting periodontal regeneration in the area of the furcation after OFD surgery in conjunction with teriparatide treatment (case courtesy of JDB). 5a Day of surgery. 5b One-year postoperative reevaluation. 5c Digital subtraction radiography of before and after. Arrows localize the enhanced signal reflective of new bone formation.
rhFGF-2
Another emerging approach to regeneration is the controlled release, local delivery of growth factors, such as FGF-2.2–4 rhFGF-2 acts by binding heparin, which results in broad mitogenic and angiogenic actions. Similar to other commercially available regenerative agents, rhFGF-2 also acts early in the differentiation of osteoprogenitor cells to increase bone formation. Figures 6 and 7 represent clinical and radiographic evidence of two patients who received rhFGF-2 combined with cellulose from an exploratory Food and Drug Administration Phase IIa study demonstrating that this growth factor and carrier significantly improved the percentage of bone fill compared with carrier alone at 36 weeks after treatment.
FIGURE 6.
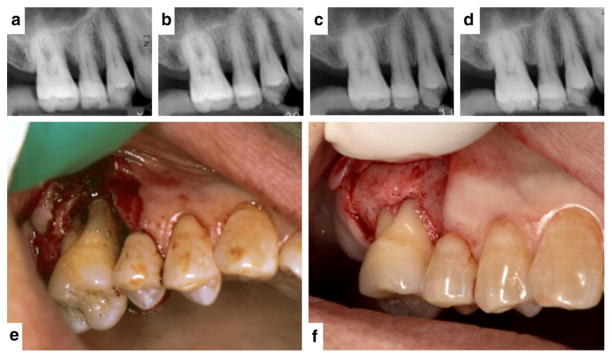
A 41-year-old female diagnosed with chronic periodontitis. She had no smoking habit and no systemic complications. Radiographic and clinical evidence of new bone formation for tooth #21 suggesting periodontal regeneration after 9 months of local delivery of 0.3% rhFGF-2/cellulose into a periodontal defect (case courtesy of Drs. M. Ninomiya and T. Nagata, Tokushima University, Tokushima, Japan). 6a through 6d Standardized periapical radiographs before surgery (6a) and at the 3-month (6b), 6-month (6c), and 9-month (6d) reevaluations. 6e and 6f The periodontal defect observed at baseline (6e) contrasted with the evidence of bone fill at clinical reentry (6f).
FIGURE 7.
A 52-year-old female non-smoker. Radiographic and clinical evidence of new bone formation for tooth #3 suggesting periodontal regeneration after 9 months of local delivery of 0.3% rhFGF-2/cellulose into the furcation and vertical periodontal defect. The baseline radiograph shows vertical bone loss on the mesial side and the furcation area (case courtesy of SM). 7a through 7d Standardized periapical radiographs before surgery (7a) and at the 3-month (7b), 6-month (7c), and 9-month (7d) reevaluations. 7e and 7f The advanced furcation lesion observed at baseline (7e) contrasted with the evidence of bone fill at clinical reentry (7f). Figure 7 was previously published in Clinical Advances in Periodontics.4
Cellular Allograft
Cell therapy has also been studied extensively as a valuable therapeutic option in regenerative medicine.5,6 In the context of periodontal regeneration, the cells seeded into periodontal defects should be easy to harvest, non-immunogenic, and highly proliferative and have the ability to differentiate into the various types of cells comprising the periodontal tissues. Adult mesenchymal stem cells can become osteoblasts, fibroblasts, and cementoblasts. In turn, these cells can all accelerate and enhance periodontal wound healing and aid in periodontal regeneration. Figures 8 and 9 show two clinical examples of patients who received a commercially available allograft cellular bone matrix.
FIGURE 8.
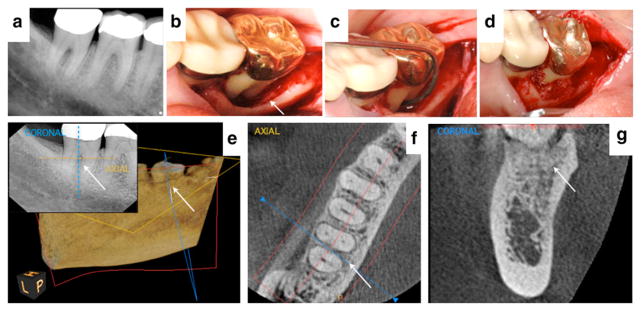
A healthy 55-year-old female with 9-mm probing depths and Class II furcation involvement on the lingual aspect of tooth #31 (case courtesy of BSM). 8a Preoperative radiograph showing bone loss in the furcation. 8b and 8c The extensive size of the periodontal defect can be visualized after flap reflection. 8d The cellular allograft|||| containing mesenchymal stem cells and osteoprogenitor cells was delivered into the periodontal defect. 8e A 6-month two-dimensional radiograph and three-dimensional cone-beam computed tomorgraphy¶¶ (CBCT) view of the site after 3 years suggests significant bone fill. Color dotted lines on the cross-sectional radiograph assist location of the coronal and axial views from the CBCT. 8f and 8g Axial and coronal slices from a 3-year postoperative CBCT reflect complete bone fill of the furcation and supportive evidence of a normal periodontal ligament space (5 × 5 cm scan set at 90-μm resolution). Figures 8a, 8c, and 8d reproduced with permission from Quintessence (McAllister6).
FIGURE 9.
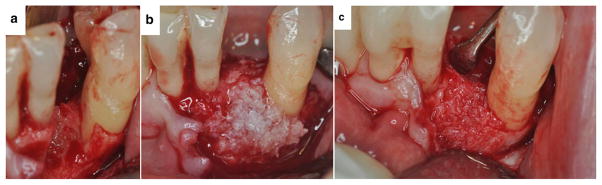
A healthy 57-year-old female with significant periodontal breakdown on tooth #22 (case courtesy of BSM). 9a After flap reflection and degranulation, the periodontal breakdown can be seen. 9b The cellular allograft## containing mesenchymal stem cells and osteoprogenitor cells was added to the defect. 9c Periodontal osseous healing on the mesial aspect of tooth #22 can be seen coronal to the original native bone at 4 months after graft placement when the site was entered for implant placement.
Discussion
Periodontal diseases affect a large proportion of the population, and the desire to restore health through regenerative approaches has been hampered by the technical difficulties and often unpredictable outcomes. Traditionally, the current standard of care has allowed for the identification, diagnosis, and treatment of patients with periodontal disease based on distinct clinical features that support a common treatment protocol. However, the clinical reality reflects a heterogeneity and complexity of factors that influence wound healing.
Current advances in science and technology will enable clinicians to seek a more comprehensive understanding of the processes that occur within the susceptible patient’s periodontium that favor the initiation and progression of disease and will allow an individualized approach based on biologically driven therapies that could help overcome existing challenges.
Therefore, deciphering the biologic phenotype that determines tissue function and structure within an individual is important.7 Acknowledging and incorporating this information as clinicians will support not only the emergence of novel therapeutic targets but also better individualized methods to promote successful regenerative periodontal therapy.
Conclusions
Today’s challenges facing periodontal regenerative therapy continue to stimulate important research and clinical development, which, in turn, shape the current concept of periodontal tissue engineering. Most of the protocols illustrated in this report are under development and not currently available for this specific application. However, it is already clear that these emerging technologies, such as stem cell therapy, local delivery of growth factors, and bone anabolic agents, offer unique opportunities to enhance the predictability of current regenerative surgical approaches and inspire development of novel treatment strategies. Nonetheless, even with these emerging technologies, basic surgical skills of excellent flap management and proper root preparation are paramount to obtaining a successful regenerative outcome.
Acknowledgments
Dr. Rios has received research funding from Osteology Foundation (Lucerne, Switzerland), has served as a consultant for Geitslich Pharma (Wolhusen, Switzerland) and Organogenesis (Canton, Massachusetts), and his research has been supported by the National Institutes of Health/National Institute of Dental and Craniofacial Research (1K23DE019872, R21DE023845-01A1, and R56DE022787-01A1). Dr. Bashutski has received research funding from Eli Lilly (Indianapolis, Indiana), Biomet 3i (Palm Beach Gardens, Florida), and Osteology Foundation. Dr. McAllister has received research funding and consulting, advisor, and lecture fees from ACE Surgical Supply (Brockton, Massachusetts), Nobel Biocare (Zürich, Switzerland), Institute Straumann (Basel, Switzerland), Osteohealth (Shirley, New York), Cytograft Tissue Engineering (Novato, California), and Organogenesis as well as advisor fees from Imaging Sciences International (Hatfield, Pennsylvania). Dr. Murakami has received research funding and consulting fees from Kaken Pharmaceutical (Tokyo, Japan). Dr. Cobb has received research funding from OraPharma (Horsham, Pennsylvania) and served as an unpaid consultant for Hu-Friedy (Chicago, Illinois) and Livionex (Los Gatos, California). Research by Dr. Chun was supported by the National Institutes of Health/National Institute of Dental and Craniofacial Research (K08DE022800) and National Center for Advancing Translational Sciences (UL1TR001120). Dr. Cochran has received research funding and consulting fees from Sunstar Americas (Chicago, Illinois). Drs. Lin and Mandelaris report no conflicts of interest related to this study. The 2014 Regeneration Workshop was hosted by the American Academy of Periodontology (AAP) and supported in part by the AAP Foundation, Geistlich Pharma North America, Colgate-Palmolive, and the Osteology Foundation.
Footnotes
Osteocel, ACE Surgical Supply, Brockton MA.
CS 9300 CBCT system, Carestream Health, Rochester, NY.
Osteocel, ACE Surgical Supply.
References
- 1.Bashutski JD, Eber RM, Kinney JS, et al. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 2010;363:2396–2405. doi: 10.1056/NEJMoa1005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitamura M, Akamatsu M, Machigashira M, et al. FGF-2 stimulates periodontal regeneration: Results of a multi-center randomized clinical trial. J Dent Res. 2011;90:35–40. doi: 10.1177/0022034510384616. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura M, Nakashima K, Kowashi Y, et al. Periodontal tissue regeneration using fibroblast growth factor-2: Randomized controlled phase II clinical trial. PLoS One. 2008;3:e2611. doi: 10.1371/journal.pone.0002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ninomiya M, Azuma T, Kido J, Murakami S, Nagata T. Successful case of periodontal tissue repair with fibroblast growth factor-2: Long-term follow-up and comparison to enamel matrix derivative. Clin Adv Periodontics. 2013;3:215–221. [Google Scholar]
- 5.Gonshor A, McAllister BS, Wallace SS, Prasad H. Histologic and histomorphometric evaluation of an allograft stem cell-based matrix sinus augmentation procedure. Int J Oral Maxillofac Implants. 2011;26:123–131. [PubMed] [Google Scholar]
- 6.McAllister BS. Stem cell-containing allograft matrix enhances periodontal regeneration: Case presentations. Int J Periodontics Restorative Dent. 2011;31:149–155. [PubMed] [Google Scholar]
- 7.Offenbacher S, Barros SP, Beck JD. Rethinking periodontal inflammation. J Periodontol. 2008;79(Suppl 8):1577–1584. doi: 10.1902/jop.2008.080220. [DOI] [PubMed] [Google Scholar]