Abstract
Purpose
We performed a multi-institutional prospective phase II trial to assess late toxicities in patients with extremity soft tissue sarcoma (STS) treated with preoperative image-guided radiation therapy (IGRT) to a reduced target volume.
Patients and Methods
Patients with extremity STS received IGRT with (cohort A) or without (cohort B) chemotherapy followed by limb-sparing resection. Daily pretreatment images were coregistered with digitally reconstructed radiographs so that the patient position could be adjusted before each treatment. All patients received IGRT to reduced tumor volumes according to strict protocol guidelines. Late toxicities were assessed at 2 years.
Results
In all, 98 patients were accrued (cohort A, 12; cohort B, 86). Cohort A was closed prematurely because of poor accrual and is not reported. Seventy-nine eligible patients from cohort B form the basis of this report. At a median follow-up of 3.6 years, five patients did not have surgery because of disease progression. There were five local treatment failures, all of which were in field. Of the 57 patients assessed for late toxicities at 2 years, 10.5% experienced at least one grade ≥ 2 toxicity as compared with 37% of patients in the National Cancer Institute of Canada SR2 (CAN-NCIC-SR2: Phase III Randomized Study of Pre- vs Postoperative Radiotherapy in Curable Extremity Soft Tissue Sarcoma) trial receiving preoperative radiation therapy without IGRT (P < .001).
Conclusion
The significant reduction of late toxicities in patients with extremity STS who were treated with preoperative IGRT and absence of marginal-field recurrences suggest that the target volumes used in the Radiation Therapy Oncology Group RTOG-0630 (A Phase II Trial of Image-Guided Preoperative Radiotherapy for Primary Soft Tissue Sarcomas of the Extremity) study are appropriate for preoperative IGRT for extremity STS.
INTRODUCTION
When compared with postoperative radiation therapy (RT), the phase III National Cancer Institute of Canada SR2 (CAN-NCIC-SR2; Phase III Randomized Study of Pre- vs Postoperative Radiotherapy in Curable Extremity Soft Tissue Sarcoma) trial demonstrated that preoperative RT for extremity soft tissue sarcoma (STS) is associated with reduced late toxicities such as grade 2 or higher subcutaneous fibrosis, joint stiffness, and edema.1,2 Late radiation morbidity was reduced for preoperative RT at 2 years after treatment (preoperative v postoperative: 31.5% v 48% for fibrosis, 15.1% v 23% for edema, and 17.8% v 23% for joint stiffness).1 The decreased late toxicity in the preoperative arm likely results from lower radiation dose (50 Gy v 66 Gy) and smaller RT volumes compared with the postoperative volumes that encompass all surgically manipulated tissues, incisions, and drain sites. Other potential advantages of preoperative RT include the opportunity to facilitate resection by shrinking certain subtypes of STSs3 and to prevent tumor seeding during surgery.4 In addition, preoperative RT uses fewer treatment fractions, which decreases cost and improves convenience for patients. Therefore, although preoperative RT doubles the risk of acute major wound complications,2 it is often favored for patients with STS.
Highly conformal image-guided radiation therapy (IGRT) more precisely delivers high-dose radiation to the tumor instead of to normal tissues. This requires accurate delineation of the gross target volume (gross tumor volume [GTV]) and the adjacent areas at risk for microscopic extension of disease (ie, the clinical target volume [CTV]). However, the optimal expansion around the GTV to create the CTV has not been defined for STS. The use of IGRT allows for reduction in the target volume expansion needed to account for errors in patient setup (ie, the planning target volume [PTV]), but whether IGRT can be used in a multi-institutional setting to decrease the PTV for extremity sarcoma and decrease late toxicities without increasing marginal failures is unknown. Therefore, we performed the prospective multi-institutional phase II Radiation Therapy Oncology Group RTOG-0630 (A Phase II Trial of Image-Guided Preoperative Radiotherapy for Primary Soft Tissue Sarcomas of the Extremity) trial for preoperative IGRT for extremity STS. The primary end point was the effect of reduced radiation volume through the use of IGRT on radiation morbidity (grade ≥ 2 lymphedema, subcutaneous fibrosis, or joint stiffness) at 2 years (a window of 21 to 27 months) from the start of RT.
PATIENTS AND METHODS
Eligibility included histologically proven primary STS of the upper or lower extremity, age ≥ 18 years, and Zubrod performance status of 0 to 1. Exclusion criteria included hand or foot STS, metastatic disease, tumor size ≥ 32 cm, prior RT with overlapping fields, or concurrent or prior malignancy. Patients with intermediate- to high-grade STS with tumor size ≥ 8 cm for whom the treating physicians prescribed chemotherapy (neoadjuvant, concurrent, or adjuvant) were enrolled onto cohort A. Patients who did not receive chemotherapy were enrolled onto cohort B.
RT Planning and Target Volume Definition
Pretreatment magnetic resonance imaging (MRI) of primary STS within 8 weeks before registration was required to define the GTV. Either preoperative 3D conformal RT (3DCRT) or intensity-modulated radiation therapy (IMRT) could be used as long as the dose volume histogram constraints for critical normal structures met the protocol constraints, and the target volume was covered.
A dose of 50 Gy in 25 fractions was prescribed to cover 95% of the PTV except for some patients in cohort A in whom a reduced dose (44 Gy in 22 fractions) was prescribed with concurrent or interdigitated chemotherapy. More than 99% of the PTV received more than 97% of the prescribed dose. No more than 20% of the PTV received ≥ 110% of the prescribed dose. Beam energies of ≥ 4 MV were permitted. The limb was immobilized before computed tomography (CT) simulation for RT planning. The definition of target volumes was as follows: GTV was defined by MRI T1 plus contrast images. Coregistration of pretreatment MRI and planning CT in the same position was recommended to delineate the GTV for RT planning. CTV included gross tumor and adjacent tissue at risk for microscopic extension. For intermediate- to high-grade tumors ≥ 8 cm, CTV = GTV + 3 cm margins in the longitudinal (proximal and distal) directions to include suspicious edema as defined by MRI T2 images. For low-grade tumors or those less than 8 cm, the longitudinal margin was 2 cm beyond the GTV to include suspicious edema. The radial margin for intermediate- to high-grade tumors ≥ 8 cm was 1.5 cm beyond the GTV; for low-grade tumors or those less than 8 cm, the radial margin was 1 cm. The CTV was expanded to cover the suspicious edema if it extended beyond the CTV margin and was constrained by anatomic barriers, including fascia, bone, or compartment. Details of GTV and CTV definitions were described in a recent consensus report.5 PTV included CTV plus 5 mm for all patients.
Radiation avoidance structures included a longitudinal strip of skin and subcutaneous tissue of an extremity of which no more than 50% received 20 Gy. Full prescription dose to skin over areas commonly traumatized (eg, elbow, knee, shin) was avoided whenever possible. No bolus was placed over the skin unless the biopsy scar would not be resected after RT. No more than 50% of a weight-bearing bone within the radiation field received 50 Gy except when the tumor invaded bone, when there was circumferential involvement of tumor around more than a quarter of the bone, or when the bone was to be resected at surgery. For any other normal tissue structures, no dose more than the established TD5/5 limit was given. Postoperative RT was recommended for a positive margin only (see Appendix, online only).
Quality Assurance Review of RT Planning, Target Volume Definition, Pathology, and Surgery
After patients completed RT, the treatment plans were submitted to RTOG for central review. At least two members of the RTOG Sarcoma Working Group reviewed each patient's medical records for protocol variations. Patients were scored as no variation (ie, per protocol), acceptable (minor) variation, and unacceptable (major) variation. Central review was performed on the surgical reports (B.L.E. and J.M.K.) and histopathology of the pretreatment biopsy and resected tumor (D.L.).
Daily pretreatment IGRT images were coregistered with digitally reconstructed radiographs to adjust patient position before each treatment by matching the bone adjacent to the PTV. The participating institutions registered the treatment day imaging data set with the reference data set according to protocol guidelines. Daily image guidance was achieved by using orthogonal 2D kV and MV electronic images (eg, ExacTrac); linear accelerator–mounted kV and MV cone beam CT images; or linear accelerator–mounted MV CT images (eg, tomotherapy). At least one joint was included in daily pretreatment images to increase anatomic information for image registration. Setup errors were corrected by using either manual or automatic registration. To provide oversight of the image quality and registration process, one IGRT image data set per week along with a spreadsheet that included data on daily variances from the daily IGRT were submitted for central review.
Statistics and End Points
Initially, the study was designed to test for a 20% absolute improvement in the rate of grade ≥ 2 radiation morbidity (subcutaneous tissue fibrosis, joint stiffness, or edema) at 2 years (± 3 months) from 37% in the preoperative RT arm of the CAN-NCIC-SR2 study1 to 17%, which required 41 patients per cohort with 5% type I error and 90% statistical power. Allowing for 20% of patients being ineligible or not analyzable at 2 years, the total sample size was 51 patients per cohort. During accrual, the sample size for cohort B was increased to 66 (83 after accounting for 20% potential ineligibility) to test for a 15% improvement in late toxicity with 5% type 1 error and 85% statistical power. Subcutaneous tissue fibrosis and joint stiffness were scored by RTOG/European Organisation for Research and Treatment of Cancer criteria and edema by Stern's scale (Table 1). Comparison with the results from the CAN-NCIC-SR2 trial was by Fisher's exact test. Other toxicity end points were acute wound complications within 120 days of resection and adverse events per Common Terminology Criteria for Adverse Events version 3.0. Fisher's exact test was used to test for associations between grade ≥ 2 radiation morbidity at 2 years and wound complications with disease location (upper v lower extremity), tumor histology (liposarcoma v other types), tumor size (> 10 or ≤ 10 cm), radiation technique, resection status (R0 v R1 to R2), use of flap (flap v simple closure), resection of large blood vessels, postoperative RT boost, and RT central review scores.
Table 1.
Late Radiation Toxicity Grading
| Late Radiation Toxicity | Grade |
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| RTOG/EORTC Criteria | |||||
| Subcutaneous tissue (subcutaneous fibrosis) | None | Slight fibrosis; subcutaneous fat loss | Moderate fibrosis; slight field contracture | Severe fibrosis; field contracture > 10% | Necrosis |
| Joint (joint stiffness) | None | Mild stiffness; slight range of motion loss | Moderate stiffness, pain, range of motion loss | Severe stiffness, pain, range of motion loss | Necrosis; complete fixation |
| Stern's scale | |||||
| Edema | None | Mild, but definite, swelling | Moderate | Severe (considerable) swelling | Very severe (skin shiny and tight with or without skin cracking |
Abbreviations: EORTC, European Organisation for Research and Treatment of Cancer; RTOG, Radiation Therapy Oncology Group.
All local recurrences after surgery were documented on cross-sectional imaging (CT or MRI with contrast). Biopsy or resection was recommended for confirmation. Any tumor recurrence within the CTV was defined as an in-field recurrence, any tumor recurrence beyond the CTV to within 3 cm from the edge of the CTV was defined as a marginal recurrence, and any other local recurrence was defined as an out-of-field recurrence. Patients were seen in follow-up every 3 months for the first 2 years after treatment and then every 6 months in years 3 to 5. Post-treatment imaging to evaluate tumor recurrence included MRI or CT imaging of the primary site and chest CT every 6 months for the first 2 years and then once per year for 3 years. Local recurrences were confirmed by central review of pretreatment MRI, simulation CT, and post-treatment MRI imaging (D.W. and D.G.K.). Patients who did not undergo surgery because they developed metastases or patients who had an amputation as a result of treatment complications or progression were also considered to have local treatment failures.
Statistical Evaluation
Time-to-event end points were measured from the date of registration. Rates of local, regional, and distant treatment failure and second primary tumor were estimated by the cumulative incidence method6 to account for the competing risk of death without treatment failure. Distant disease-free, disease-free, and overall survival rates were estimated by the Kaplan-Meier method.7 To allow comparison with the CAN-NCIC-SR2 trial, the event-free proportions were estimated by the Kaplan-Meier method for local recurrence, regional and/or distant recurrence, and progression-free survival, after excluding patients who did not have surgery or who had an amputation to remove the primary tumor.
RESULTS
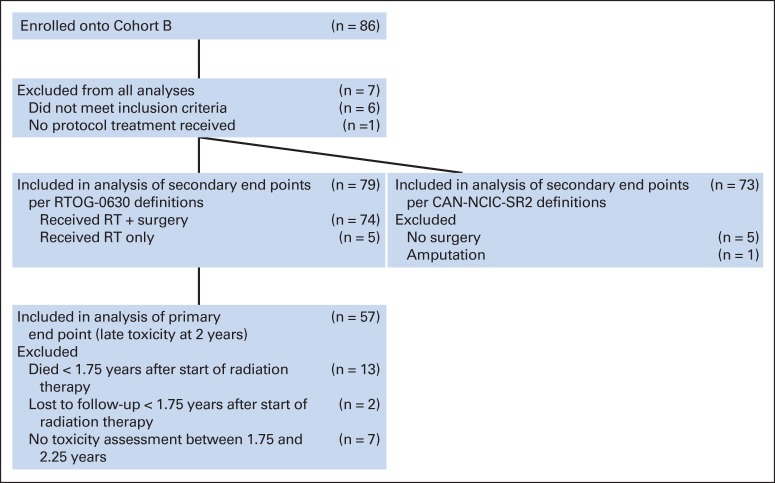
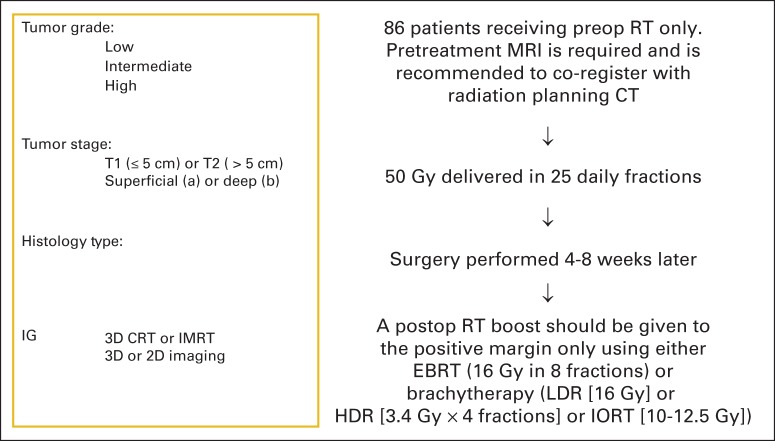
Twelve patients were enrolled onto cohort A between March 2008 and January 2010. Arm A was then closed. Results for these 12 patients are not presented. Eighty-six patients were enrolled onto cohort B between March 2008 and September 2010. Seven patients were excluded from analysis (two had nonextremity sarcoma, two had MRI scans outside of the protocol range, one had no pretreatment laboratory results, one started therapy before registration, and one did not start protocol therapy). The remaining 79 patients form the basis of this report (CONSORT diagram; Figure 1). The data set for this analysis was created on April 1, 2014. The treatment schema is shown in Figure 2.
Fig 1.
CONSORT diagram for cohort B from Radiation Therapy Oncology Group RTOG-0630 (A Phase II Trial of Image-Guided Preoperative Radiotherapy for Primary Soft Tissue Sarcomas of the Extremity). CAN-NCIC-SR2, National Cancer Institute of Canada SR2 (Phase III Randomized Study of Pre- vs Postoperative Radiotherapy in Curable Extremity Soft Tissue Sarcoma); RT, radiation therapy.
Fig 2.
Treatment schema for patients in Radiation Therapy Oncology Group RTOG-0630 (A Phase II Trial of Image-Guided Preoperative Radiotherapy for Primary Soft Tissue Sarcomas of the Extremity). Chemotherapy was allowed at the discretion of the treating medical oncologist for deep (≥ 8 cm) sarcomas in maximum dimension with intermediate- to high-grade histology (grade 3 or 4; American Joint Committee on Cancer, 6th edition). Treating centers were certified by the RTOG for sarcoma image-guided radiation therapy (RT) as well as for 3-dimensional conformal radiation therapy (3D-CRT) and/or intensity-modulated RT (IMRT). Radiation therapy technique (3DCRT v IMRT) was at the discretion of the treating radiation oncologist. CT, computed tomography; EBRT, external beam RT; HDR, high-dose rate; IORT, intraoperative RT; LDR, low-dose rate; MRI, magnetic resonance imaging.
Patient and tumor characteristics are provided in Table 2. Median age was 61 years. The most common histologies were undifferentiated pleomorphic sarcoma (22.8%), liposarcoma (21.5%), and myxofibrosarcoma (21.5%). The most common primary site was in the proximal upper thigh (41.8%). Median tumor size was 10.5 cm: 48.1% (38) were intermediate- to high-grade tumors ≥ 8 cm, 26.6% (21) were intermediate- to high-grade tumors less than 8 cm, and 16.5% were low-grade tumors.
Table 2.
Patient and Tumor Characteristics for Cohort B of RTOG-0630 (n = 79)
| Characteristic | No. | % | Tumor Size (cm) |
|||
|---|---|---|---|---|---|---|
| < 8 | % | ≥ 8 | % | |||
| Age, years | ||||||
| Median | 61 | |||||
| Range | 24-88 | |||||
| Sex | ||||||
| Male | 42 | 53.2 | ||||
| Female | 37 | 46.8 | ||||
| Race/ethnicity | ||||||
| American Indian or Alaskan native | 1 | 1.3 | ||||
| Black or African American | 4 | 5.1 | ||||
| White | 71 | 89.9 | ||||
| More than one race | 1 | 1.3 | ||||
| Unknown | 2 | 2.5 | ||||
| Zubrod performance status | ||||||
| 0 | 61 | 77.2 | ||||
| 1 | 18 | 22.8 | ||||
| Histology (study chair review) | ||||||
| Extraskeletal myxoid chondrosarcoma | 1 | 1.3 | ||||
| Leiomysarcoma | 6 | 7.6 | ||||
| Epitheliod | 1 | 1.3 | ||||
| Pleomorphic | 1 | 1.3 | ||||
| Liposarcoma | ||||||
| Dedifferentiated | 1 | 1.3 | ||||
| Myxoid | 10 | 12.7 | ||||
| Myxoid/round cell | 3 | 3.8 | ||||
| Pleomorphic | 1 | 1.3 | ||||
| Well differentiated | 2 | 2.5 | ||||
| Low-grade fibromyxoid sarcoma | 1 | 1.3 | ||||
| Malignant peripheral nerve sheath tumor, spindle cell | 1 | 1.3 | ||||
| Myxofibrosarcoma | 17 | 21.5 | ||||
| Pleomorphic rhabdomyosarcoma | 1 | 1.3 | ||||
| Sclerosing epithelioid fibrosarcoma | 1 | 1.3 | ||||
| Synovial sarcoma | ||||||
| Biphasic | 1 | 1.3 | ||||
| Monophasic spindle cell | 3 | 3.8 | ||||
| Unclassified | 3 | 3.8 | ||||
| Undifferentiated pleomorphic sarcoma | 18 | 22.8 | ||||
| Unknown | 7 | 8.9 | ||||
| Disease location | ||||||
| Upper extremity | 11 | 13.9 | ||||
| Shoulder | 2 | 2.5 | ||||
| Proximal arm | 5 | 6.3 | ||||
| Distal arm | 2 | 2.5 | ||||
| Proximal forearm | 1 | 1.3 | ||||
| Distal forearm | 1 | 1.3 | ||||
| Lower extremity | 62 | 78.5 | ||||
| Proximal thigh | 33 | 41.8 | ||||
| Distal thigh | 13 | 16.5 | ||||
| Proximal leg | 10 | 12.7 | ||||
| Distal leg | 6 | 7.6 | ||||
| Hip | 2 | 2.5 | ||||
| Buttocks | 4 | 5.1 | ||||
| Disease size (longest diameter, cm) | ||||||
| Median | 10.5 | |||||
| Range | 3.5-30 | |||||
| T stage | ||||||
| T1a | 4 | 5.1 | ||||
| T1b | 5 | 6.3 | ||||
| T2a | 10 | 12.7 | ||||
| T2b | 60 | 75.9 | ||||
| Histologic grade (central review) | ||||||
| G1 | 13 | 16.5 | 2 | 2.5 | 11 | 13.9 |
| G2 | 21 | 26.6 | 7 | 8.9 | 14 | 17.7 |
| G3 | 38 | 48.1 | 14 | 17.7 | 24 | 30.4 |
| Unknown | 7 | 8.9 | 2 | 2.5 | 5 | 6.3 |
| Biopsy type (central review) | ||||||
| Core needle | 62 | 78.5 | ||||
| Incisional | 8 | 10.1 | ||||
| Excisional | 1 | 1.3 | ||||
| Unknown | 8 | 10.1 | ||||
Abbreviation: RTOG-0630, Radiation Therapy Oncology Group RTOG-0630 (A Phase II Trial of Image-Guided Preoperative Radiotherapy for Primary Soft Tissue Sarcomas of the Extremity) trial.
All 79 eligible patients received a median dose of 50 Gy in 25 fractions by IMRT (74.7%) or 3DCRT (25.3%). Seventy-four patients (93.7%) underwent protocol surgery whereas five did not because of metastatic tumor progression. Fifty-six (76%) had an R0 resection. Only 11 patients (15%) received postoperative RT boost for an R1 resection. IMRT was used in seven (63.6%) of 11 patients, 3DCRT in two (18.2%) of 11, high-dose radiation brachytherapy in one (9.1%) of 11, and intraoperative RT in one (9.1%) of 11. Treatment-related adverse events that occurred in more than 5% of patients are provided in Appendix Table A1 (online only). At post-treatment RT central review, 44 patients (55.7%) received treatment per protocol, 26 (32.9%) received treatment with acceptable variations, and eight (10.1%) received treatment with unacceptable variations. One patient (1.3%) was not evaluated.
Analysis of Late Toxicities (primary end point)
Fifty-seven (72.2%) of 79 patients were evaluable for the 2-year late toxicity end point as used in the preoperative RT arm of the CAN-NCIC-SR2 study.1 Six (10.5%) of 57 experienced grade ≥ 2 late toxicity (subcutaneous tissue fibrosis, joint stiffness, or edema), a significantly lower number of patients than in the preoperative arm of the CAN-NCIC-SR2 trial (six [10.5%] of 57 v 27 [37%] of 73; P < .001). Rates of individual grade ≥ 2 late toxicity compared favorably with those in the CAN-NCIC-SR2 trial preoperative arm: three (5.3%) of 57 versus 23 (31.5%) of 73 for fibrosis, two (3.5%) of 57 versus 13 (17.8%) of 73 for joint stiffness, and three (5.3%) of 57 versus 11 (15.1%) of 73 for edema. Resection of major blood vessels was associated with increased late subcutaneous fibrosis (two of 10 v one of 46; P = .08) and joint stiffness (two of 10 v zero of 46; P = .03), but not edema (zero of 10 v three of 47; P = 1.0; Table 3).
Table 3.
Comparisons of Potential Variables With Late Morbidities
| Potential Variables | Grade 2+ Fibrosis, Joint Stiffness, or Edema at 2 Years* | P† |
|---|---|---|
| Upper extremity v lower extremity | 0/7 v 6/50 | 1.00 |
| Liposarcoma v other | 2/15 v 4/42 | .65 |
| Tumor > 10 v ≤ 10 cm | 4/30 v 2/27 | .67 |
| IMRT v 3DCRT | 5/42 v 1/15 | 1.00 |
| R0 v R1 to R2 | 3/41 v 3/16 | .33 |
| Simple v flap reconstruction | 3/28 v 3/29 | 1.00 |
| Resection of blood vessels (no v yes) | 3/47 v 3/10 | .06 |
| Postoperative RT (no v yes) | 5/48 v 1/9 | 1.00 |
| RT review score | ||
| Target volume: per protocol/acceptable variation v unacceptable deviation | 4/47 v 2/10 | .28 |
| Organs at risk: per protocol v acceptable variation | 5/50 v 1/7 | .56 |
| Target volume dose volume analysis: per protocol/acceptable variation v unacceptable deviation | 6/41 v 0/13 | .32 |
| Overall: per protocol/acceptable variation v unacceptable deviation | 6/51 v 0/6 | 1.00 |
Abbreviations: 3DCRT, 3-dimensional conformal RT; IMRT, intensity-modulated RT; RT, radiation therapy.
No. of patients with grade 2 or greater fibrosis or joint stiffness or edema at 2 years of a total No. of patients with each potential variable.
Fisher's exact test.
Analysis of Acute Major Wound Complications as Defined in the CAN-NCIC-SR2 Study
Of 71 patients assessed for wound complications, 36.6% (26 of 71) experienced at least one wound complication. The most common were those requiring secondary operative debridement (25.4%), prolonged dressing changes (23.9%), and readmission for wound care (21.1%). All wound complications occurred in lower-extremity tumors (26 [41.9%] of 62) versus zero of nine for upper extremity tumors; P = .02) and were more common in the proximal than the distal lower extremity (19 of 41 v six of 17; P = .56).
Disease-Associated Outcome End Points
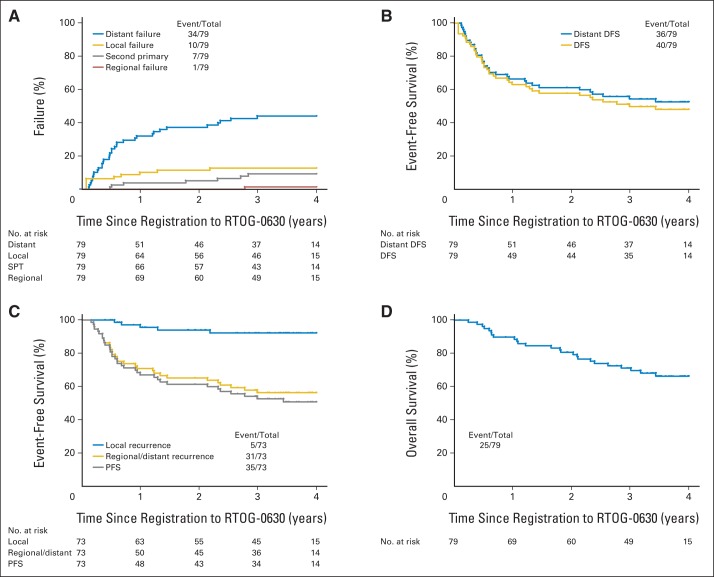
Twenty-five of 79 patients died, 22 from tumor progression. Median follow-up for surviving patients was 3.6 years (range, 0.1 to 5.0 years). There were five local treatment failures; all were in-field as confirmed by central review (Table 4). Among patients undergoing protocol surgery, local recurrence occurred in three of 17 patients with positive margins versus two of 56 with negative margins (P = .08). Time-to-event end points according to both RTOG-0630 and CAN-NCIC-SR2 definitions are shown in Figure 3. Twenty-nine of 40 first disease-free survival events were distant (72.5%). Scoring failure to undergo surgery because of distant disease progression as local failure, the estimated 2-year rates of local, regional, and distant treatment failure and second primary are 11.4% (95% CI, 5.6% to 19.6%), 0.0%, 37.3% (95% CI, 26.6% to 48.0%), and 5.1% (95% CI, 1.7% to 11.7%), respectively. Estimated 2-year disease-free and distant disease-free survival rates are 58.1% (95% CI, 47.1% to 69.0%) and 61.4% (95% CI, 50.6% to 72.2%), respectively. When failure to undergo surgery because of distant disease progression was not scored as local failure, the estimated 2-year local recurrence-free, regional and/or distant recurrence-free, and progression-free survival rates per the CAN-NCIC-SR2 trial definitions are 94.0% (95% CI, 88.2% to 99.7%), 65.3% (95% CI, 54.2% to 76.3%), and 61.5% (95% CI, 50.3% to 72.7%), respectively. The estimated 2-year survival rate is 80.6% (95% CI, 71.8% to 89.4%).
Table 4.
Treatment Parameters for Five In-Field Local Recurrences
| Tumor Size (cm) | Central Review |
Postoperative Radiation Boost | Radiation Review Score |
|||
|---|---|---|---|---|---|---|
| Histologic Grade | Histology | Resection Status | Target Volume | Target Volume Dose Volume Analysis | ||
| 3.7 | 3 | Leiomyosarcoma | 0 | No | Acceptable variation | Per protocol |
| 6.1 | 3 | Malignant peripheral nerve sheath tumor, spindle cell | 1 | No | Acceptable variation | Unacceptable variation |
| 7.2 | 3 | Undifferentiated pleomorphic sarcoma | 1 | Yes | Per protocol | Per protocol |
| 12.0 | 3 | Undifferentiated pleomorphic sarcoma | 1 | Yes | Unacceptable variation* | Unacceptable variation |
| 16.5 | 3 | Pleomorphic leiomyosarcoma | 0 | No | Unacceptable variation† | Per protocol |
Abbreviations: GTV, gross tumor volume.
Unacceptable variation: gross tumor volume deviation unacceptable (includes edema). Clinical tumor volume deviation unacceptable (includes excessive subcutaneous tissue). Planning target volume deviation unacceptable (not adequate expansion per protocol), overall score not acceptable.
Unacceptable variation: GTV deviation unacceptable; slice 4.5, 3.5, and 2.5 cm GTV too tight anteriorly; GTV not covering tumor anteriorly compared with magnetic resonance imaging; overall score not acceptable.
Fig 3.
(A) Cumulative incidence of local, regional, and distant failure and second primary tumor (SPT) per Radiation Therapy Oncology Group RTOG-0630 (A Phase II Trial of Image-Guided Preoperative Radiotherapy for Primary Soft Tissue Sarcomas of the Extremity) definitions of failure. (B) Kaplan-Meier estimates of disease-free survival (DFS) and distant disease-free survival per RTOG-0630 definitions of failure. (C) Kaplan-Meier estimates of local recurrence, regional/distant recurrence, and progression-free survival (PFS) per National Cancer Institute of Canada SR2 (CAN-NCIC-SR2; Phase III Randomized Study of Pre- vs Postoperative Radiotherapy in Curable Extremity Soft Tissue Sarcoma) definitions of failure. (D) Kaplan-Meier estimates of overall survival. In the CAN-NCIC-SR2 trial, patients who did not have surgery or had an amputation to remove the primary tumor were not included in analysis, so (C) has fewer patients (73) than (A), (B), and (D), which have 79. Event No. indicates distant, local, or regional failure or second primary.
DISCUSSION
There have been only two reports about late toxicity in patients with extremity STS treated with sophisticated RT, such as IMRT or IGRT. Alektiar et al8 retrospectively reviewed their institutional experience with 41 patients with STS who were treated with IMRT (83% received postoperative IMRT to a median dose of 63 Gy). With a median follow-up of 35 months, 12.2% (four of 41) had moderate edema, 4.9% (two of 41) had bone fracture, and 17.1% (seven of 41) had moderate joint stiffness. Data for subcutaneous tissue fibrosis were not reported. More recently, O'Sullivan et al9 completed a single-institution phase II prospective study in 70 patients with extremity STS by using preoperative IG-IMRT with 4-cm longitudinal expansion of GTV for the CTV. Rates of grade 2 or higher late toxicity in the 54 patients with lower-extremity STS that survived at least 2 years were low: five (9.3%) of 54 for moderate fibrosis, three (5.6%) of 54 for moderate joint stiffness, and six (11.1%) of 54 for moderate edema. The results of this prospective multi-institutional study of preoperative IGRT similarly show low rates of late toxicity compared with patients who received preoperative RT in the CAN-NCIC-SR2 trial (six of 57 v 27 of 73; P < .001). A statistical comparison between this study and the recent phase II IG-IMRT trial9 cannot be performed because of different time points for the late toxicity assessment (assessment time points not reported v a window period between 21 and 27 months following treatment in RTOG-0630). Taken together, however, results from these two prospective phase II clinical trials independently demonstrate a significant reduction of late toxicities after IGRT, likely as a result of the use of reduced target volumes compared with those in the CAN-NCIC-SR2 trial.
The local treatment failure rate in RTOG-0630 was similar to that in previous studies of patients with extremity STS treated with adjuvant RT and limb-sparing surgery (Fig 3).2,10–12 Although 5 of the 74 patients who received preoperative IGRT and limb-sparing surgery had local recurrences, central review confirmed that each of these five local recurrences were inside the CTV (in-field recurrence), and three of the five had positive margins, known to be an adverse risk factor for local recurrence. The low local treatment failure rate, the absence of marginal recurrences, and the favorable late toxicity profile suggest that reduction of longitudinal CTV margins as used in this protocol is appropriate for extremity sarcoma IGRT.
As in other studies of preoperative RT,2,13–15 a high rate of wound complications was observed in this study, with 36.6% overall and 42% in lower-extremity STS. This is similar to the results from the CAN-NCIC-SR2 trial preoperative arm in which 35% of all patients and 43% with a lower-extremity STS developed a wound complication after surgery.2 In RTOG-0630, acute major wound complications occurred exclusively in patients with lower-extremity STS. Similarly, in the CAN-NCIC-SR2 trial, almost all of the wound complications after preoperative RT and surgery occurred in the lower extremities. Of note, O'Sullivan et al9 recently reported a 30.5% rate of wound complications in patients with lower-extremity STS treated with preoperative IG-IMRT when the radiation dose was minimized to tissues used in wound closure. This wound complication rate was lower than the rate of 43% in patients with lower-extremity STS treated in the CAN-NCIC-SR2 trial preoperative arm, but the difference was not statistically different. Taken together, these results suggest that high-dose radiation should be limited to the uninvolved tissues as much as possible.
In conclusion, in this multi-institutional prospective phase II clinical trial (RTOG-0630), we find a significant reduction in late toxicity in patients with extremity STS treated with preoperative IGRT to a reduced target volume when compared with patients in the CAN-NCIC-SR2 trial. Excellent local control, the absence of marginal-field recurrences, and the favorable late toxicity profile suggest that the parameters used, namely reductions of longitudinal CTV margin to 3 cm for intermediate- or high-grade sarcomas of ≥ 8 cm or to 2 cm for low-grade sarcomas or tumors of less than 8 cm, are appropriate for preoperative IG-IMRT for extremity STS. The rate of major wound complications remains high, and further efforts are needed to reduce acute major wound complications in patients with lower-extremity STS undergoing preoperative RT.
Acknowledgment
Presented in part as an oral presentation at the 55th Annual Meeting of the American Society of Radiation Oncology, Atlanta, GA, September 22-25, 2013, and the 18th Annual Meeting of the Connective Tissue Oncology Society, New York, NY, October 30-November 2, 2013.
Glossary Terms
- image-guided radiation therapy:
a technique of radiation therapy delivery in which the location of the tumor is monitored by imaging on a daily basis to ensure the precise delivery of the radiation therapy dose to the predefined volume of interest.
- intensity-modulated radiation therapy:
radiation treatment using beams with nonuniform fluence profiles that shape the dose distribution in the target volume and adjacent normal structures. Beam modulation is typically achieved via multileaf collimators or custom-milled compensators to achieve the appropriate fluence profiles calculated by inverse optimization algorithms. The radiation beam is divided into beamlets of varying intensity such that the sum from multiple beams via inverse planning results in improved tumor targeting and normal tissue sparing. A technique of radiation therapy delivery in which the intensity of each beamlet of radiation coming from a specific angle can be adjusted to provide a desired dose distribution when the doses delivered from all beamlets are added from a single angle and from all dose delivery angles. An advanced type of high-precision radiation therapy, which aims to improve the coverage of the radiation therapy target and/or minimize radiation dose to surrounding normal tissue.
Appendix
Description of Postoperative Radiation Boost to the Positive Margin Only
Postoperative radiation therapy boost (external beam or brachytherapy or intraoperative radiation therapy boost).
Postoperative radiation therapy (RT) will be given to the positive tumor margin (residual tumor) only plus a margin of 1 cm within 2 weeks after surgery or after adequate wound healing has occurred. The patient can receive postoperative external beam, low-dose-rate (LDR) or high-dose-rate (HDR) brachytherapy, or intraoperative RT as a boost to the residual tumor bed (positive margin). Metallic clips or gold seeds are recommended to be placed during surgery to aid in defining the residual tumor bed for a positive margin. The target volume for postoperative RT will be the residual tumor bed as defined by the surgical and pathologic findings.
External beam RT boost.
Postoperative external beam boost dose is 16 Gy in eight fractions (once per day). Postoperative external beam RT boost will begin 2 weeks after resection if the healing of the surgical wound is satisfactory at the discretion of surgeon. Bolus should be avoided unless positive margins occur in cutaneous or subcutaneous tissues. Because preoperative RT has been delivered, it is not necessary to include the entire surgical bed, drain sites, and wound. Unless brachytherapy or intraoperative RT is to be used, postoperative RT should be consistent with the technique used for the patient's preoperative RT (ie, if image-guided 3-dimensional conformation RT was used for preoperative RT, it should be used for postoperative RT; if image-guided intensity-modulated RT was used for preoperative RT, it should be used for postoperative RT. The boost can be started more than 2 weeks after surgery if wound healing requires it. The reasons for a delay in boost treatment should be documented and reported.
Brachytherapy boost.
Either LDR or HDR brachytherapy as a boost to the positive tumor margin is acceptable as an alternative to external beam RT. Brachytherapy should not start until day 5 after the surgery (day 0) and must be completed within 2 weeks after surgery. Typically, brachytherapy catheters are placed at an interval of 0.5 to 1.0 cm on the residual tumor bed (positive margin) plus a margin of 1 cm during surgery. Skin surface dose should be kept below 50% of the prescription dose unless positive margins occur in cutaneous or subcutaneous tissues. It is not necessary to include the entire surgical bed, drain sites, and wound. For LDR brachytherapy, the dose is 16 Gy at no more than 0.8 Gy per hour. For HDR brachytherapy, four fractions of 3.4 Gy are delivered in a twice-per-day fashion, with an interval of at least 6 hours between fractions.
Intraoperative RT boost.
For those institutions that deliver intraoperative RT (electron therapy or HDR interstitial brachytherapy), the dose is 10 to 12.5 Gy in a single fraction to a margin that is microscopically positive at the time of resection. Note that a frozen section diagnosis of positive margin must be obtained before intraoperative RT. Typically the dose is prescribed to 1 cm depth or 90% isodose line. However, prescription depth or isodose line coverage should be decided at the discretion of the treating radiation oncologist on the basis of the consideration of boost target volume, surgical and/or pathologic findings, and adjacent normal tissue structure tolerance.
Table A1.
Treatment-Related Adverse Events Occurring in At Least 5% of Patients in Cohort B of RTOG-0630 (n = 79)
| Adverse Event | Grade |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | All (%) | 3 to 4 (%) | |
| Blood/bone marrow | ||||||
| Decreased hemoglobin | 7 | 2 | 2 | 1 | 15.2 | 3.8 |
| Constitutional symptoms | ||||||
| Fatigue | 11 | 7 | 4 | 0 | 27.8 | 5.1 |
| Weight loss | 4 | 0 | 0 | 0 | 5.1 | 0.0 |
| Dermatology/skin | ||||||
| Skin induration | 25 | 11 | 1 | 0 | 46.8 | 1.3 |
| Radiation dermatitis | 10 | 10 | 2 | 1 | 29.1 | 3.8 |
| Skin hyperpigmentation | 14 | 0 | 0 | 0 | 17.7 | 0.0 |
| Dermatologic radiation recall reaction | 4 | 4 | 1 | 0 | 11.4 | 1.3 |
| Skin disorder | 5 | 3 | 0 | 0 | 10.1 | 0.0 |
| Desquamating rash | 4 | 1 | 0 | 0 | 6.3 | 0.0 |
| Wound dehiscence | 2 | 0 | 2 | 0 | 5.1 | 2.5 |
| Gastrointestinal | ||||||
| Nausea | 6 | 1 | 0 | 0 | 8.9 | 0.0 |
| Anorexia | 4 | 0 | 0 | 0 | 5.1 | 0.0 |
| Infection with normal or grade 1 to 2 ANC | ||||||
| Wound infection | 0 | 1 | 8 | 0 | 11.4 | 10.1 |
| Skin infection | 0 | 1 | 3 | 0 | 5.1 | 3.8 |
| Lymphatics | ||||||
| Limb edema | 30 | 18 | 2 | 0 | 63.3 | 2.5 |
| Musculoskeletal/soft tissue | ||||||
| Joint disorder | 14 | 12 | 2 | 0 | 35.4 | 2.5 |
| Seroma | 7 | 0 | 3 | 0 | 12.7 | 3.8 |
| Abnormal gait | 4 | 1 | 0 | 0 | 6.3 | 0.0 |
| Neurology | ||||||
| Peripheral sensory neuropathy | 12 | 1 | 1 | 0 | 17.7 | 1.3 |
| Peripheral motor neuropathy | 1 | 2 | 3 | 0 | 7.6 | 3.8 |
| Neurologic disorder NOS | 4 | 0 | 0 | 0 | 5.1 | 0.0 |
| Pain | ||||||
| In extremity | 16 | 17 | 1 | 0 | 43.0 | 1.3 |
| Other | 5 | 3 | 1 | 0 | 11.4 | 1.3 |
| Skin | 1 | 5 | 0 | 0 | 7.6 | 0.0 |
NOTE. Treatment related adverse event could be definitely, probably, or possibly related to protocol treatment (or with unknown relationship). Adverse events were classified according to Common Terminology Criteria for Adverse Events, version 3.0.
Abbreviations: ANC, absolute neutrophil count; NOS, not otherwise specified; RTOG-0630, Radiation Therapy Oncology Group RTOG-0630 (A Phase II Trial of Image-Guided Preoperative Radiotherapy for Primary Soft Tissue Sarcomas of the Extremity) trial.
Footnotes
Processed as a Rapid Communication manuscript.
Supported by Grants No. U10CA21661, U10CA180868, U10CA180822, U10CA37422, and U24CA180803 from the National Cancer Institute.
Clinical trial information: NCT00589121
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Dian Wang, Qiang Zhang, Burton L. Eisenberg, John M. Kane, Thomas F. DeLaney, David G. Kirsch
Provision of study materials or patients: George Dundas
Collection and assembly of data: Dian Wang, X. Allen Li, David Lucas, Ivy A. Petersen, Thomas F. DeLaney, Carolyn R. Freeman, Steven E. Finkelstein, Ying J. Hitchcock, Manpreet Bedi, Anurag K. Singh, George Dundas, David G. Kirsch
Data analysis and interpretation: Dian Wang, Qiang Zhang, X. Allen Li, David Lucas, Ivy A. Petersen, Thomas F. DeLaney, David G. Kirsch
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Dian Wang
No relationship to disclose
Qiang Zhang
No relationship to disclose
Burton L. Eisenberg
No relationship to disclose
John M. Kane
No relationship to disclose
X. Allen Li
No relationship to disclose
David Lucas
No relationship to disclose
Ivy A. Petersen
No relationship to disclose
Thomas F. DeLaney
Stock or Other Ownership: GlaxoSmithKline
Honoraria: UpToDate, Wolters Kluwer Health
Carolyn R. Freeman
No relationship to disclose
Steven E. Finkelstein
Employment: 21st Century Oncology
Consulting or Advisory Role: Bayer/Algeta, Dendreon, Medivation/Astellas, Blue Earth
Speakers' Bureau: Bayer/Algeta, Dendreon
Research Funding: Dendreon (Inst), Bayer/Algeta (Inst)
Ying J. Hitchcock
No relationship to disclose
Manpreet Bedi
No relationship to disclose
Anurag K. Singh
No relationship to disclose
George Dundas
No relationship to disclose
David G. Kirsch
Stock or Other Ownership: Lumicell
Honoraria: UpToDate
Research Funding: Lumicell
REFERENCES
- 1.Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 3.Chung PW, Deheshi BM, Ferguson PC, et al. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: A comparison with other soft tissue sarcomas. Cancer. 2009;115:3254–3261. doi: 10.1002/cncr.24375. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JA, Kainz K, Li XA, et al. The role of image guided radiotherapy in the treatment of soft tissue sarcoma. Curr Cancer Ther Rev. 2010;6:207–213. [Google Scholar]
- 5.Wang D, Bosch W, Roberge D, et al. RTOG sarcoma radiation oncologists reach consensus on gross tumor volume and clinical target volume on computed tomographic images for preoperative radiotherapy of primary soft tissue sarcoma of extremity in Radiation Therapy Oncology Group studies. Int J Radiat Oncol Biol Phys. 2011;81:e525–e528. doi: 10.1016/j.ijrobp.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons; 1980. [Google Scholar]
- 7.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 8.Alektiar KM, Brennan MF, Healey JH, et al. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol. 2008;26:3440–3444. doi: 10.1200/JCO.2008.16.6249. [DOI] [PubMed] [Google Scholar]
- 9.O'Sullivan B, Griffin AM, Dickie CI, et al. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer. 2013;119:1878–1884. doi: 10.1002/cncr.27951. [DOI] [PubMed] [Google Scholar]
- 10.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 11.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 12.Kraybill WG, Harris J, Spiro IJ, et al. Long-term results of a phase 2 study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. Cancer. 2010;116:4613–4621. doi: 10.1002/cncr.25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bujko K, Suit HD, Springfield DS, et al. Wound healing after preoperative radiation for sarcoma of soft tissues. Surg Gynecol Obstet. 1993;176:124–134. [PubMed] [Google Scholar]
- 14.Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93:980–987. doi: 10.1097/00006534-199404001-00012. [DOI] [PubMed] [Google Scholar]
- 15.Cheng EY, Dusenbery KE, Winters MR, et al. Soft tissue sarcomas: Preoperative versus postoperative radiotherapy. J Surg Oncol. 1996;61:90–99. doi: 10.1002/(SICI)1096-9098(199602)61:2<90::AID-JSO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]