Abstract
Purpose
To evaluate the relationship between race/ethnicity and breast cancer–specific survival according to subtype and explore mediating factors.
Patients and Methods
Participants were women presenting with stage I to III breast cancer between January 2000 and December 2007 at National Comprehensive Cancer Network centers with survival follow-up through December 2009. Cox proportional hazards regression was used to compare breast cancer–specific survival among Asians (n = 533), Hispanics (n = 1,122), and blacks (n = 1,345) with that among whites (n = 14,268), overall and stratified by subtype (luminal A like, luminal B like, human epidermal growth factor receptor 2 type, and triple negative). Model estimates were used to derive mediation proportion and 95% CI for selected risk factors.
Results
In multivariable adjusted models, overall, blacks had 21% higher risk of breast cancer–specific death (hazard ratio [HR], 1.21; 95% CI, 1.00 to 1.45). For estrogen receptor–positive tumors, black and white survival differences were greatest within 2 years of diagnosis (years 0 to 2: HR, 2.65; 95% CI, 1.34 to 5.24; year 2 to end of follow-up: HR, 1.50; 95% CI, 1.12 to 2.00). Blacks were 76% and 56% more likely to die as a result of luminal A–like and luminal B–like tumors, respectively. No disparities were observed for triple-negative or human epidermal growth factor receptor 2–type tumors. Asians and Hispanics were less likely to die as a result of breast cancer compared with whites (Asians: HR, 0.56; 95% CI, 0.37 to 0.85; Hispanics: HR, 0.74; 95% CI, 0.58 to 0.95). For blacks, tumor characteristics and stage at diagnosis were significant disparity mediators. Body mass index was an important mediator for blacks and Asians.
Conclusion
Racial disparities in breast cancer survival vary by tumor subtype. Interventions are needed to reduce disparities, particularly in the first 2 years after diagnosis among black women with estrogen receptor–positive tumors.
INTRODUCTION
Incidence, mortality, and survival with regard to breast cancer vary considerably according to subtype. Overall, luminal A tumors have the highest incidence but also the lowest mortality.1,2 Although basal-like and human epidermal growth factor receptor 2 (HER2) –type tumors occur less frequently, they are associated with poorer survival. In the Carolina Breast Cancer Study (CBCS), black and white women with basal-like tumors were 40% and 70% more likely to die as a result of breast cancer, respectively, compared with women of the same race with luminal A tumors.3,4 Some of the difference in survival by tumor subtype reflects availability of effective treatments. Hormone receptor–positive tumors like luminal A and luminal B can be treated with tamoxifen and aromatase inhibitors, and those that overexpress HER2 can be treated with trastuzumab.5–7 Hormone receptor–negative tumors, like triple negative and basal like, can only be treated with surgery, radiation therapy, and/or chemotherapy.8
Blacks are significantly more likely to be diagnosed with triple-negative or basal-like tumors than nonblacks.3,9 Tumor subtype distribution seems similar between Asians and whites, although there is some evidence that HER2-type tumors may be more common among Asians.10 Hispanics are less likely to be diagnosed with estrogen receptor (ER) or progesterone receptor (PR) –negative tumors than blacks but more likely to be diagnosed than whites.11 Studies have observed lower breast cancer survival among blacks and Hispanics as compared with whites, and either no difference or better survival has been observed among Asians and Pacific Islanders.12 Subtype may partially account for racial/ethnic differences in survival, and prior studies have not always been able to account for this.13 Research examining difference in survival by race has been hampered by a lack of inclusion of women from racial/ethnic groups (eg, Asian, Hispanic) that represent fast-growing segments of the US population,3,4 a lack of information on HER2 status, and inconsistent assessment of other important factors affecting survival, including treatment, socioeconomic status, body mass index (BMI), and comorbid conditions.14–17
Using prospective data from a cohort of women with breast cancer with rich clinical data, we evaluated the relationship between race/ethnicity and breast cancer–specific survival within and across breast cancer subtypes defined by ER/PR status, HER2 status, and tumor grade as proxies for gene expression markers.18 We further investigated the mediating effects of tumor characteristics, treatment, BMI, and sociodemographic factors on racial/ethnic disparities in survival.
PATIENTS AND METHODS
Study Population and Data Collection
The National Comprehensive Cancer Network (NCCN) Breast Cancer Outcomes Database has collected prospective data on patient and tumor characteristics, sociodemographic information, treatment, and outcomes for women receiving care for newly diagnosed breast cancer since 1997. The study population includes women with newly diagnosed stage I to III breast cancer19,20 who presented and received primary care at one of eight comprehensive cancer centers between January 1, 2000, and December 31, 2007: Arthur G. James Cancer Hospital at Ohio State University (Columbus, OH), City of Hope Comprehensive Cancer Center (Duarte, CA), Dana-Farber Cancer Institute (Boston, MA), Fox Chase Cancer Center (Philadelphia, PA), H. Lee Moffitt Cancer Center (Tampa, FL), University of Texas MD Anderson Cancer Center (Houston, TX), Roswell Park Cancer Institute (Buffalo, NY), and University of Michigan Comprehensive Cancer Center (Ann Arbor, MI). The institutional review board at each center approved the study, data collection, transmission methods, and storage protocols.
We identified 20,025 patients with stage I to III disease and excluded anyone with a previous cancer diagnosis (n = 1,572); those with missing racial/ethnic information, American Indian Aleutians/Eskimos, and those whose race was designated as other (n = 322); those with missing information on ER, PR, or HER2 status (n = 839); and those with missing follow-up data (n = 24). Our final sample included 17,268 women.
Exposure and Outcome Assessment
Race/ethnicity.
Race and ethnicity were self-reported. Women were classified as white, black, Asian, or Pacific Islander. Ethnicity was classified as non-Hispanic or Hispanic. If ethnicity was unknown, women were assumed to be non-Hispanic. We cross classified race and ethnicity into four categories: non-Hispanic white (white), non-Hispanic black (black), non-Hispanic Asian or Pacific Islander (Asian), and Hispanic. Hispanic participants could be of any race.
Deaths.
Vital status and cause of death were ascertained from medical records. Trained medical abstractors record the International Classification of Diseases (ICD) code for the underlying cause of death if the patient had died. If there was > one ICD code, they recorded the ICD code that reflected the underlying cause, as defined by the WHO. If cause of death was unknown based on the medical record, data from the National Death Index were used instead. Rigorous quality control measures were in place to ensure accuracy of the data.21
Breast cancer subtype and tumor characteristics.
Information on tumor size, lymph node status, tumor grade, ER/PR status, and HER2 status was abstracted from pathology reports. Table 1 lists the definitions of luminal A–like, luminal B–like, HER2-type, and triple-negative tumors used for this analysis.22 We used tumor grade as a surrogate for Ki-67 expression23–25 and HER2 status to differentiate between luminal A–like and luminal B–like tumors
Table 1.
Breast Cancer Subtype Definitions
| Subtype | ER/PR Status | HER2 Status | Tumor Grade |
|---|---|---|---|
| Luminal A like | ER and/or PR positive | Not overexpressed | Low or intermediate |
| Luminal B like | ER and/or PR positive | Not overexpressed | High |
| Overexpressed | Any | ||
| HER2 type | ER and PR negative | Overexpressed | Any |
| Triple negative | ER and PR negative | Not overexpressed | Any |
Data adapted.22
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Covariates.
Patient characteristics were collected via survey at first presentation at most centers, including initial sign or symptom of breast cancer, race/ethnicity, employment status at diagnosis, highest level of education completed, and menopausal status. Comorbidity at presentation was assigned using either the Charlson index or the modified version of that index using a patient survey.26,27 As part of the Breast Cancer Outcomes Database, dedicated study abstractors complete any missing elements based on medical record review. Clinical and treatment information was gathered from tumor registries, medical record review, and inpatient and outpatient records.
Statistical Analysis
We present age-standardized percentages of patient and clinical characteristics at time of first presentation to the NCCN institution, stratified by racial/ethnic group. Multinomial logistic (categorical variables) and binomial logistic (binary variables) regression models were used to generate age-adjusted P values.
Follow-up was defined as time in years from breast cancer diagnosis to date of death or last date of NCCN follow-up. We used ICD codes 174 and 174.9 to identify breast cancer death. We used multivariable Cox proportional hazards regression to estimate hazard ratios (HRs) and 95% CIs for the relationship between race/ethnicity and breast cancer–specific survival. Each subtype was modeled separately with whites as the reference group for racial/ethnic comparisons. Few deaths occurred among Asians and Hispanics; therefore, we present subtype-specific survival estimates for blacks and whites only.
We tested whether covariates met the proportional hazards assumption28 for each subtype model using a Wald test of time-dependent covariates, separately for blacks, Asians, and Hispanics as compared with whites. We found the assumption was not met for hormonal therapy, chemotherapy, or trastuzumab; therefore, we included interaction terms with log follow-up time for each treatment variable. The assumption was satisfied for comparisons of Hispanics and Asians with whites but was violated for comparison of black and white women with ER-positive tumors. Thus, we assessed the time-dependent relationship between black race and survival using two interaction terms between a binary indicator of black race and follow-up time broken into two time windows: 0 to 2 years after diagnosis and > 2 years after diagnosis to the end of the follow-up period. All models were stratified by NCCN center. Characteristics included in subtype definition were not modeled as covariates. In model one, we adjusted in steps beginning with age at diagnosis (continuous) and year of diagnosis (continuous). Model two included model one variables plus patient-related and sociodemographic factors: insurance type (Medicare, Medicaid or uninsured, managed care, indemnity, or other [eg, self-pay or foreign or military insurance] or unknown), educational attainment (≤ high school, some college, college graduate, or graduate school), employment status (employed or student, homemaker or retired, unable to work or unemployed, or other), menopausal status (pre or post), comorbidity score (≥ 1 or 0), BMI (< 18.5, 18.5 to 24.9, 25 to 29.9, or ≥ 30 kg/m2). Model three included all model two variables plus tumor characteristics: stage at diagnosis, initial sign of breast cancer (abnormal mammogram or symptom), tumor grade (high or low/intermediate), ER/PR status (positive or negative), and HER2 status (overexpressed or not overexpressed). Lastly, in model four, we additionally adjusted for initiation of adjuvant chemotherapy, hormonal therapy, or trastuzumab. Missing indicators were used to adjust for missing data in covariates.
We performed a mediation analysis of sociodemographic factors, insurance status, tumor characteristics, stage at diagnosis, BMI, and treatment on observed racial/ethnic differences in survival. We added each potential mediating variable or group of variables to the multivariable model and calculated the mediation proportion and its 95% CI using an SAS macro (SAS Institute, Cary, NC).29 The mediation proportion was the proportion of excess (or reduced) breast cancer mortality among the selected racial/ethnic group relative to whites that could be attributed to the mediator. P values are two sided, with an α level of 0.05. Analyses were performed using SAS software (version 9.2; SAS Institute).
RESULTS
Median length of follow-up was 6.2 years (Table 2). Mean age at diagnosis was youngest in Asians (51.1 years) and Hispanics (52.0 years). Blacks were most likely to be diagnosed at stage III (24.1%), with high tumor grade (64.6%) and triple-negative tumors (29.6%). Whites had the highest proportion of tumors diagnosed at stage I (45.6%) and ER-positive tumors (76.1%). Asians and whites were most likely to have luminal A–like tumors (48.0% and 47.4%, respectively). Blacks were the heaviest at time of first presentation to the NCCN center, with a mean BMI of 31.5 compared with 23.9 kg/m2 among Asians (P < .001).
Table 2.
Age-Standardized Patient Demographic and Clinicopathologic Characteristics According to Race/Ethnicity: 2000 to 2007
| Characteristic | White (n = 14,268) | Hispanic (n = 1,122) | Black (n = 1,345) | Asian (n = 533) | P* |
|---|---|---|---|---|---|
| Length of follow-up, years | < .001 | ||||
| Median | 6.3 | 6.0 | 5.7 | 6.2 | |
| SD | 2.4 | 2.3 | 2.5 | 2.3 | |
| Age at diagnosis, years† | < .001 | ||||
| Mean | 55.2 | 52.0 | 53.5 | 51.1 | |
| SD | 11.7 | 3.8 | 4.3 | 2.6 | |
| Detection with symptoms, % | 53.6 | 64.3 | 63.4 | 67.3 | < .001 |
| AJCC stage, % | < .001 | ||||
| I | 45.6 | 34.0 | 32.1 | 40.0 | |
| II | 40.7 | 44.8 | 43.8 | 45.0 | |
| III | 13.7 | 21.1 | 24.1 | 14.9 | |
| ER positive, % | 76.1 | 72.8 | 59.6 | 72.3 | < .001 |
| HER2 overexpressed, % | 16.8 | 20.1 | 18.8 | 20.6 | .04 |
| High tumor grade, % | 43.0 | 50.5 | 64.6 | 42.4 | < .001 |
| Molecular phenotype, % | < .001 | ||||
| Luminal A like | 47.4 | 42.1 | 27.1 | 48.0 | |
| Luminal B like | 29.8 | 32.6 | 34.0 | 25.8 | |
| HER2 type | 7.0 | 8.2 | 9.3 | 10.9 | |
| Triple negative | 15.9 | 17.0 | 29.6 | 15.3 | |
| Chemotherapy, % | 68.0 | 80.1 | 78.1 | 72.2 | < .001 |
| Hormonal therapy, %‡ | 95.2 | 94.1 | 92.6 | 94.7 | .004 |
| Trastuzumab, %§ | 46.4 | 55.2 | 50.0 | 46.0 | .11 |
| Postmenopausal, % | 60.1 | 52.9 | 57.9 | 46.9 | < .001 |
| Comorbidity score ≥ 1, % | 20.2 | 22.4 | 30.2 | 16.3 | < .001 |
| BMI, kg/m2 | < .001 | ||||
| Mean | 27.5 | 28.5 | 31.5 | 23.9 | |
| SD | 6.0 | 2.2 | 2.8 | 1.0 | |
| Educational attainment, % | < .001 | ||||
| ≤ High school | 24.7 | 38.8 | 30.6 | 22.1 | |
| Some college | 22.0 | 19.0 | 25.9 | 12.7 | |
| College graduate | 20.3 | 13.6 | 13.0 | 28.4 | |
| Graduate school | 15.3 | 10.6 | 9.7 | 20.5 | |
| Insurance status, % | < .001 | ||||
| Managed care | 67.0 | 59.0 | 59.3 | 72.8 | |
| Medicare | 19.8 | 9.1 | 18.9 | 8.5 | |
| Medicaid or uninsured | 3.5 | 20.8 | 15.0 | 8.3 | |
| Other or unknown | 8.4 | 10.7 | 6.0 | 10.5 | |
| Employment status, % | < .001 | ||||
| Employed or student | 50.0 | 50.3 | 50.2 | 56.0 | |
| Homemaker or retired | 34.7 | 37.4 | 24.1 | 32.3 | |
| Unable to work, unemployed, or other | 15.3 | 12.3 | 25.7 | 11.8 |
Abbreviations: AJCC, American Joint Committee on Cancer; BMI, body mass index; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; SD, standard deviation.
Age-adjusted P values calculated using multinomial (categorical variables) or binomial logistic (binary variables) regression models.
Not age adjusted.
Among women with ER-positive tumors only.
Among women with tumors that overexpressed HER2 only. Trastuzumab was indicated for adjuvant setting in 2006, so receipt of trastuzumab was lower for time period analyzed.
Table 3 lists HRs for death resulting from breast cancer comparing blacks, Asians, and Hispanics with whites. In age-adjusted models, blacks were twice as likely to die as a result of breast cancer as whites. Risk was attenuated with adjustment for sociodemographic, tumor, and treatment characteristics. In fully adjusted models, blacks were 21% more likely to die as a result of breast cancer (HR, 1.21; 95% CI, 1.00 to 1.45). Asians were at lower risk of death resulting from breast cancer compared with whites for all tumor subtypes (HR, 0.56; 95% CI, 0.37 to 0.85). A similar pattern was observed among Hispanics, who had a 22% (HR, 0.74; 95% CI, 0.58 to 0.95) lower risk of breast cancer–specific death overall. Results were similar when total mortality was examined (Appendix Table A1, online only).
Table 3.
HRs for Death Resulting From Breast Cancer According to Race/Ethnicity
| Race/Ethnicity | No. of Deaths | Model One* |
Model Two† |
Model Three‡ |
Model Four§ |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| White (reference) | 895 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black v white | 166 | 2.01 | 1.70 to 2.38 | 1.67 | 1.40 to 1.99 | 1.24 | 1.04 to 1.48 | 1.21 | 1.00 to 1.45 |
| Asian v white | 24 | 0.60 | 0.40 to 0.91 | 0.65 | 0.43 to 0.99 | 0.60 | 0.40 to 0.91 | 0.56 | 0.37 to 0.85 |
| Hispanic v white | 83 | 0.96 | 0.76 to 1.22 | 0.82 | 0.64 to 1.04 | 0.77 | 0.60 to 0.98 | 0.74 | 0.58 to 0.95 |
Abbreviation: HR, hazard ratio.
Age adjusted: age at diagnosis, National Comprehensive Cancer Network center, and year of diagnosis.
Model one plus socioeconomic factors: insurance type, educational attainment, employment status, menopausal status, comorbidity score, and body mass index.
Model two plus tumor characteristics: stage at diagnosis, triggering event, tumor grade, estrogen receptor status, progesterone receptor status, and human epidermal growth factor receptor 2 status.
Model three plus treatment: chemotherapy, hormonal therapy, and trastuzumab use.
Table 4 lists HRs for death resulting from breast cancer, comparing blacks with whites according to subtype. In age-adjusted models among women with ER-positive tumors, blacks were more than 3× as likely to die as a result of breast cancer as whites in the first 2 years after diagnosis. After multivariable adjustment, blacks were > 2× more likely to die as a result of ER-positive breast cancer in the first 2 years after diagnosis (HR, 2.65; 95% CI, 1.34 to 5.24) and 51% (HR, 1.50; 95% CI, 1.12 to 2.00) more likely to die thereafter. We saw no racial difference in breast cancer–specific survival for ER-negative tumors (HR, 1.04; 95% CI, 0.82 to 1.33). Among women with luminal A–like tumors, blacks were 77% more likely to die as compared with whites (HR, 1.76; 95% CI, 1.09 to 2.85) in our fully adjusted model. Blacks with luminal B–like breast cancer were also at increased risk of death compared with whites (HR, 1.56; 95% CI, 1.14 to 2.15). We saw no survival differences for triple-negative (HR, 1.04; 95% CI, 0.79 to 1.37) or HER2-type tumors (HR, 0.99; 95% CI, 0.57 to 1.73) in fully adjusted models. Results were similar when total mortality was examined (Appendix Table A2, online only).
Table 4.
HRs for Death Resulting From Breast Cancer According to Race/Ethnicity (non-Hispanic black v non-Hispanic white) and Tumor Subtype
| Race/Ethnicity | No. of Deaths | Model One* |
Model Two† |
Model Three‡ |
Model Four§ |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| ER positive | |||||||||
| White (reference) | 439 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 74 | 2.28 | 1.78 to 2.93 | 1.91 | 1.47 to 2.48 | 1.58 | 1.21 to 2.05 | 1.62 | 1.24 to 2.12 |
| ER positive (0 to 2 years) | |||||||||
| White (reference) | 102 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 24 | 3.73 | 1.92 to 7.26 | 3.14 | 1.61 to 6.14 | 2.53 | 1.29 to 4.93 | 2.65 | 1.34 to 5.24 |
| ER positive (≥ 2 years to end of follow-up) | |||||||||
| White (reference) | 337 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 50 | 2.13 | 1.62 to 2.79 | 1.77 | 1.34 to 2.35 | 1.46 | 1.11 to 1.94 | 1.50 | 1.12 to 2.00 |
| ER negative | |||||||||
| White (reference) | 456 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 92 | 1.38 | 1.10 to 1.73 | 1.19 | 0.94 to 1.51 | 1.14 | 0.90 to 1.45 | 0.99 | 0.77 to 1.28 |
| Luminal A like | |||||||||
| White (reference) | 162 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 22 | 2.50 | 1.60 to 3.93 | 1.98 | 1.24 to 3.16 | 1.78 | 1.12 to 2.86 | 1.76 | 1.09 to 2.85 |
| Luminal B like | |||||||||
| White (reference) | 285 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 55 | 1.79 | 1.34 to 2.41 | 1.60 | 1.18 to 2.19 | 1.58 | 1.16 to 2.15 | 1.56 | 1.14 to 2.15 |
| Triple negative | |||||||||
| White (reference) | 304 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 72 | 1.41 | 1.09 to 1.84 | 1.22 | 0.92 to 1.60 | 1.04 | 0.79 to 1.37 | 1.04 | 0.79 to 1.37 |
| HER2 type | |||||||||
| White (reference) | 121 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 16 | 1.15 | 0.68 to 1.95 | 1.04 | 0.60 to 1.79 | 1.00 | 0.57 to 1.73 | 0.99 | 0.57 to 1.73 |
Abbreviation: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio.
Age adjusted: age at diagnosis, National Comprehensive Cancer Network center, and year of diagnosis.
Model one plus socioeconomic factors: insurance type, educational attainment, employment status, menopausal status, comorbidity score, and body mass index.
Model two plus tumor characteristics: stage at diagnosis, triggering event, tumor grade (included in all subtype models except for luminal A like and luminal B like), progesterone receptor status (included in ER-positive and ER-negative models only), and HER2 status.
Model three plus treatment: chemotherapy, hormonal therapy (included in all models except for triple-negative and HER2-type models), and trastuzumab use (included in luminal B–like and HER2-type models only).
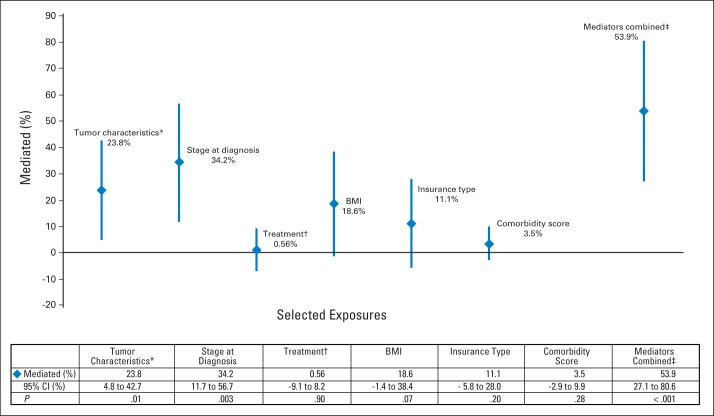
We explored factors that mediated observed racial/ethnic differences in breast cancer–specific survival (Fig 1). The estimated proportion of excess breast cancer mortality among blacks that was mediated by hormone receptor status (ER and/or PR status), HER2 status, and tumor grade was 23.8% (95% CI, 4.8% to 42.7%; P = .01). Other mediators included stage at diagnosis (34.2%; 95% CI, 11.7% to 56.7%; P = .003) and BMI (18.6%; 95% CI, −1.4% to 38.4%; P = .07). When we examined BMI, tumor characteristics, and stage at diagnosis together, the proportion of excess mortality mediated was 59.9% (95% CI, 27.1% to 80.6%). Among Asians, BMI (14.9%; 95% CI, 0.2% to 29.7%; P = .05) was a significant mediator, with lower BMI being protective (data not shown). Educational attainment, comorbidity, insurance status, and treatment were not significant mediators in either group. We found no significant mediators among Hispanics.
Fig 1.
Estimated proportion of excess breast cancer mortality and 95% CIs among blacks relative to whites, mediated by selected exposures. (*) Estrogen and/or progesterone receptor status, human epidermal growth factor receptor 2 status, and tumor grade. (†) Receipt of chemotherapy, hormonal therapy, or trastuzumab. (‡) Includes tumor characteristics, stage at diagnosis, and body mass index (BMI).
DISCUSSION
Racial disparities in breast cancer–specific survival are well documented, and this study confirms and expands on prior findings in a large cohort with detailed information on tumor characteristics, including HER2 status and treatment. Our mediation analyses show that factors such as stage at diagnosis, tumor characteristics, and BMI are significant contributors to racial differences in survival. However, black/white disparities persisted after accounting for those factors. Although black women were more likely to be diagnosed with poor-prognosis tumors, we observed the greatest black/white survival disparities among women with luminal tumors. This was particularly evident in the first 2 years after diagnosis among those with ER-positive tumors. Importantly, these disparities existed even among women treated at National Cancer Institute–designated comprehensive cancer centers, where we could discern no differences in treatment.
A meta-analysis of 20 studies with 14,103 black and 76,111 white women found that black women were 19% more likely to die as a result of breast cancer than white women, after adjustment for age, stage, and socioeconomic status.30 However, the authors did not account for breast cancer subtype. Lund et al31 found no differences in overall survival between blacks and whites for HER2-type, luminal A, or luminal B tumors but did find higher breast cancer mortality among blacks for triple-negative tumors. In contrast, the CBCS found that blacks were approximately twice as likely to die as a result of luminal A and HER2-type tumors as whites but found no differences for luminal B or basal-like tumors.4 Notably, this study stratified time into two groups: 0 to 5 years and > 5 years since diagnosis. Differences in findings may reflect time and place of diagnosis. Lund et al included only 106 black and 360 white women in their study, all in Atlanta, Georgia, whereas our study included > 14,000 white and > 1,300 black women from across the country. We included women diagnosed from 2000 to 2007, whereas the CBCS included women diagnosed from 1993 to 2001. Availability of treatments such as trastuzumab has changed over time, and recent findings show wide variation in magnitude of survival disparities between white and black patients across US metropolitan areas. The age-adjusted HR ranged from 2.11 in Memphis, Tennessee, to 0.95 in Sacramento, California.32
Blacks with ER-positive breast cancer were more than twice as likely to die in the first 2 years after diagnosis as whites. Some potential explanations include incomplete or delayed receipt of locoregional therapy, chemotherapy, or endocrine therapy or less effective therapy among blacks because of toxicity or underdosing. Blacks experience delays in diagnosis and treatment initiation,33–37 and delays are associated with worse survival.38,39 We previously reported that time to diagnosis was longer for nonwhite patients,40 and other work involving this database has shown that black and Hispanic patients had longer times to adjuvant chemotherapy compared with whites.41 Other work has shown that blacks with breast cancer are more likely to receive non–guideline-adherent primary treatment, including inappropriate primary surgical or radiation treatment, as compared with whites.12,42 Endocrine therapy, a key part of treatment for ER-positive tumors, is generally prescribed for ≥ 5 years. Several studies have shown that nonadherence and early discontinuation of adjuvant endocrine therapy is relatively common overall43–45 and more so among nonwhites.46–49 Thus, differences in timing of treatment initiation, persistence, and adherence to endocrine therapy may contribute to racial survival disparities among women with ER-positive tumors, particularly in the early period after diagnosis. In addition, black and lower–socioeconomic status patients may receive less than the standard dose of adjuvant therapies as a result of underdosing and use of nonstandard regimens.50,51 In this analysis, we observed no racial disparity in the receipt of adjuvant chemotherapy or endocrine therapy, with blacks and whites equally likely to initiate treatment. However, we were not able to assess the role of treatment delay, early discontinuation, or nonadherence48,52 in this study.
We observed the greatest disparities between black and white women for the tumor type considered least aggressive, for which we have the greatest breadth of effective treatment options. That black women are disproportionately dying as a result of ER-positive tumors represents a system failure. Our findings further suggest that the early period after diagnosis is a window of susceptibility where disparities in survival may promulgate. Interventions to reduce treatment delays, improve endocrine therapy adherence, and increase receipt of guideline-adherent care are needed. Programs such as patient navigation have been implemented to increase the timeliness of diagnosis and, to a lesser extent, treatment among vulnerable populations. Results of these programs have been mixed but suggest that navigation services are effective when targeted to high-risk populations59,60 and aimed at increasing receipt of antiestrogen therapy.61 We also must acknowledge the potential role of biologic differences in ER expression, sensitivity to endocrine therapy, or toxicity in these findings. In one report, black women with ER-positive tumors had greater risk of recurrence or death compared with whites, even in a randomized clinical trial setting, where many barriers to and inequities in care had been removed.62
Asians and Hispanics were less likely to die as a result of breast cancer as compared with whites. Asians and Hispanics are the fastest growing racial/ethnic groups in the United States.63 When disaggregated into subgroups defined by country of origin, primary language, and geographic location, there is significant heterogeneity in breast cancer survival within Asian and Hispanic populations.64 For example, most of the Hispanic women in this cohort identified their race as white or Caucasian (93%). Because of this heterogeneity, the relationship between Asian race or Hispanic ethnicity and breast cancer survival depends on the distribution of these factors in each study population.65 We were unable to account for this in our study.
There are several important limitations to our study. NCCN is not a population-based database, and it only examines care of those who had access to and received treatment at major academic cancer centers. The median age of patients with breast cancer in the NCCN database is almost 10 years younger than the national median. Therefore, the experiences of women in this study may not be generalizable to all women with breast cancer. However, the NCCN subtype distribution is similar to that in population-based registries,66 and if anything, we believe that our results may underestimate the magnitude of racial disparities outside of comprehensive cancer centers. Power was limited by small numbers of deaths for some subtype/race combinations, particularly among Asians and Hispanics. We used reported receptor status and grade as surrogates for molecular subtype, and without information on cytokeratins or epidermal growth factor receptor, we could not disaggregate triple-negative and basal subtypes with different prognoses.67 We had limited characterization of socioeconomic status, with educational attainment and employment status used as surrogates, leaving potential for residual confounding.
Despite these limitations, our study used recent data from more than 17,000 women across the United States to examine racial variation in breast cancer survival according to subtype. Few previous studies have examined white, black, Asian, and Hispanic women concurrently or included HER2 status in their analyses. This is one of the first studies to formally test for mediators of observed racial disparities in breast cancer survival. This is an important step toward ameliorating—not just describing—differences. In summary, we found that black women with breast cancer had poorer survival relative to whites and that this difference was greatest in the first 2 years after diagnosis among women with ER-positive tumors. Further work is necessary to understand the early period after diagnosis among black women with ER-positive disease to understand potential interventions to reduce observed disparities in breast cancer mortality. We encourage additional studies in Hispanics and Asians to understand the mechanisms associated with their equal or better survival relative to whites.
Acknowledgment
Presented in part at the American Association for Cancer Research Fifth Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved, San Diego, CA, October 27-30, 2012.
Glossary Terms
- aromatase inhibitors:
inhibitors used in treating breast cancer in postmenopausal women. Aromatase inhibitors inhibit the conversion of androgens to estrogens by the enzyme aromatase, thus depriving the tumor of estrogenic signals. Because of decreased production of estrogen, estrogen receptors, which are important in the progression of breast cancer, cannot be activated.
- Cox proportional hazards regression model:
a statistical model for regression analysis of censored survival data, examining the relationship of censored survival distribution to one or more covariates. This model produces a baseline survival curve, covariate coefficient estimates with their standard errors, risk ratios, 95% CIs, and significance levels.
- cytokeratins:
members of a large family of intermediate filament cytoskeletal proteins. Intermediate filament proteins are expressed in a tissue-specific manner and are assembled as filamentous arrays. Intermediate filament proteins have diverse biologic functions and their association with a wide array of human diseases resulting from aberrant post-translational modifications, limited proteolysis, and cross linking.
- epidermal growth factor receptor (EGFR):
a member of a family of receptors (HER2, HER3, HER4 are other members of the family) that binds to the EGF, TGF-α, and other related proteins, leading to the generation of proliferative and survival signals within the cell. EGFR (also known as HER1) also belongs to the larger family of tyrosine kinase receptors and is generally overexpressed in several solid tumors of epithelial origin.
- HER2/neu (human epidermal growth factor receptor 2):
also called ErbB2. HER2/neu belongs to the epidermal growth factor receptor (EGFR) family and is overexpressed in several solid tumors. Like EGFR, it is a tyrosine kinase receptor whose activation leads to proliferative signals within the cells. On activation, the human epidermal growth factor family of receptors are known to form homodimers and heterodimers, each with a distinct signaling activity. Because HER2 is the preferred dimerization partner when heterodimers are formed, it is important for signaling through ligands specific for any members of the family. It is typically overexpressed in several epithelial tumors.
- trastuzumab:
a humanized anti-ErbB2 monoclonal antibody approved for treating patients whose breast cancers overexpress the ErbB2 protein or demonstrate ErbB2 gene amplification. It is currently being tested in combination with other therapies.
Appendix
Table A1.
HRs for Death According to Race/Ethnicity
| Race/Ethnicity | No. of Deaths | Model One* |
Model Two† |
Model Three‡ |
Model Four§ |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| White (reference) | 2,004 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black v white | 308 | 1.83 | 1.62 to 2.07 | 1.10 | 0.77 to 1.59 | 1.19 | 0.83 to 1.72 | 1.11 | 0.97 to 1.27 |
| Asian v white | 39 | 0.62 | 0.45 to 0.85 | 0.64 | 0.46 to 0.88 | 0.60 | 0.43 to 0.83 | 0.56 | 0.37 to 0.85 |
| Hispanic v white | 139 | 1.04 | 0.87 to 1.25 | 0.86 | 0.71 to 1.03 | 0.80 | 0.67 to 0.97 | 0.59 | 0.42 to 0.82 |
Abbreviation: HR, hazard ratio.
Age adjusted: age at diagnosis, National Comprehensive Cancer Network center, and year of diagnosis.
Model one plus socioeconomic factors: insurance type, educational attainment, employment status, menopausal status, comorbidity score, and body mass index.
Model two plus tumor characteristics: stage at diagnosis, triggering event, tumor grade, estrogen receptor status, progesterone receptor status, and human epidermal growth factor receptor 2 status.
Model three plus treatment: chemotherapy, hormonal therapy, and trastuzumab use.
Table A2.
HRs for Death According to Race/Ethnicity (non-Hispanic black v white) and Tumor Subtype
| Race/Ethnicity | No. of Deaths | Model One* |
Model Two† |
Model Three‡ |
Model Four§ |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| ER positive | |||||||||
| White (reference) | 1,229 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 160 | 1.91 | 1.62 to 2.26 | 1.53 | 1.28 to 1.81 | 1.36 | 1.14 to 1.63 | 1.34 | 1.12 to 1.61 |
| ER positive (0 to 2 years) | |||||||||
| White (reference) | 146 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 32 | 2.80 | 1.90 to 4.13 | 2.22 | 1.50 to 3.28 | 1.95 | 1.32 to 2.87 | 1.89 | 1.25 to 2.85 |
| ER positive (≥ 2 years to end of follow-up) | |||||||||
| White (reference) | 1,083 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 128 | 1.77 | 1.47 to 2.13 | 1.42 | 1.17 to 1.71 | 1.27 | 1.05 to 1.53 | 1.25 | 1.02 to 1.53 |
| ER negative | |||||||||
| White (reference) | 775 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 148 | 1.35 | 1.13 to 1.62 | 1.17 | 0.97 to 1.41 | 1.11 | 0.92 to 1.34 | 1.00 | 0.83 to 1.20 |
| Luminal A like | |||||||||
| White (reference) | 563 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 51 | 1.69 | 1.26 to 2.26 | 1.38 | 1.03 to 1.86 | 1.29 | 0.95 to 1.73 | 1.36 | 0.99 to 1.87 |
| Luminal B like | |||||||||
| White (reference) | 623 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 114 | 1.87 | 1.52 to 2.29 | 1.49 | 1.20 to 1.83 | 1.46 | 1.19 to 1.81 | 1.34 | 1.07 to 1.68 |
| Triple negative | |||||||||
| White (reference) | 534 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 115 | 1.36 | 1.11 to 1.67 | 1.18 | 0.95 to 1.46 | 0.98 | 0.79 to 1.22 | 0.97 | 0.78 to 1.21 |
| HER2 type | |||||||||
| White (reference) | 199 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Black | 24 | 1.01 | 0.96 to 1.56 | 0.84 | 0.54 to 1.31 | 0.79 | 0.51 to 1.23 | 0.81 | 0.52 to 1.26 |
Abbreviation: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio.
Age adjusted: age at diagnosis, National Comprehensive Cancer Network center, and year of diagnosis.
Model one plus socioeconomic factors: insurance type, educational attainment, employment status, menopausal status, comorbidity score, and body mass index.
Model two plus tumor characteristics: stage at diagnosis, triggering event, tumor grade (included in all subtype models except for luminal A like and luminal B like), progesterone receptor status (included in ER-positive and ER-negative models only), and HER2 status.
Model three plus treatment: chemotherapy, hormonal therapy (included in all models except for triple-negative and HER2-type models), and trastuzumab use (included in luminal B–like and HER2-type models only).
Footnotes
Supported by the National Cancer Institute (NCI) Specialized Program of Research Excellence in Breast Cancer Grant No. NIH P50 CA089393; by the National Comprehensive Cancer Network; and by NCI Grants No. 5T32CA009001-36 and K01CA188075-01 (E.T.W.).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Erica T. Warner, Rulla M. Tamimi, Ann H. Partridge
Provision of study materials or patients: Stephen B. Edge, Richard Theriault
Collection and assembly of data: Melissa E. Hughes, Rebecca A. Ottesen, Yu-Ning Wong, Stephen B. Edge, Richard Theriault, Douglas W. Blayney, Joyce C. Niland, Eric P. Winer, Jane C. Weeks, Ann H. Partridge
Data analysis and interpretation: Erica T. Warner, Rulla M. Tamimi, Melissa E. Hughes, Rebecca A. Ottesen, Ann H. Partridge
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Erica T. Warner
No relationship to disclose
Rulla M. Tamimi
No relationship to disclose
Melissa E. Hughes
No relationship to disclose
Rebecca A. Ottesen
No relationship to disclose
Yu-Ning Wong
Research Funding: Pfizer (Inst), Janssen Pharmaceuticals (Inst), Medivation (Inst), Millennium Pharmaceuticals (Inst), Bayer (Inst)
Travel, Accommodations, Expenses: Eviti
Stephen B. Edge
No relationship to disclose
Richard Theriault
Patents, Royalties, Other Intellectual Property: Royalty from UpToDate
Douglas W. Blayney
Stock or Other Ownership: UnitedHealth Group, Johnson & Johnson, Express Scripts, Covidien, Bristol-Myers Squibb, Amgen, Abbott Laboratories, Oracle, International Business Machine, Google, Citrix Systems, Clinical Oncology Advisory Group, Physician Resource Management
Consulting or Advisory Role: AVEO Pharmaceuticals, Prometheus, Clinical Oncology Advisory Group, GlaxoSmithKline, Sobi, UnitedHealthcare, TEVA Pharmaceuticals Industries, Cephalon, Bristol-Myers Squibb, AstraZeneca/MedImmune, Genentech/Roche, Pfizer, Taiho Pharmaceutical, Physician Resource Management
Joyce C. Niland
No relationship to disclose
Eric P. Winer
No relationship to disclose
Jane C. Weeks
No relationship to disclose
Ann H. Partridge
No relationship to disclose
REFERENCES
- 1.Clarke CA, Keegan TH, Yang J, et al. Age-specific incidence of breast cancer subtypes: Understanding the black-white crossover. J Natl Cancer Inst. 2012;104:1094–1101. doi: 10.1093/jnci/djs264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engstrøm M, Opdahl S, Hagen A, et al. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res Treat. 2013;140:463–473. doi: 10.1007/s10549-013-2647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien KM, Cole SR, Tse CK, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platet N, Cathiard AM, Gleizes M, et al. Estrogens and their receptors in breast cancer progression: A dual role in cancer proliferation and invasion. Crit Rev Oncol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 7.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 8.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: Therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 9.Parise CA, Bauer KR, Caggiano V. Variation in breast cancer subtypes with age and race/ethnicity. Crit Rev Oncol Hematol. 2010;76:44–52. doi: 10.1016/j.critrevonc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J. 2009;15:593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 11.Banegas MP, Li CI. Breast cancer characteristics and outcomes among Hispanic black and hispanic white women. Breast Cancer Res Treat. 2012;134:1297–1304. doi: 10.1007/s10549-012-2142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Gomez SL, Clarke CA, Shema SJ, et al. Disparities in breast cancer survival among Asian women by ethnicity and immigrant status: A population-based study. Am J Public Health. 2010;100:861–869. doi: 10.2105/AJPH.2009.176651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroenke CH, Chen WY, Rosner B, et al. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 15.Senie RT, Rosen PP, Rhodes P, et al. Obesity at diagnosis of breast carcinoma influences duration of disease-free survival. Ann Intern Med. 1992;116:26–32. doi: 10.7326/0003-4819-116-1-26. [DOI] [PubMed] [Google Scholar]
- 16.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 17.Newman LA, Mason J, Cote D, et al. African-American ethnicity, socioeconomic status, and breast cancer survival. Cancer. 2002;94:2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 18.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes: Dealing with the diversity of breast cancer—Highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, et al. (eds) AJCC Cancer Staging Manual (ed 6) New York, NY: Springer Verlag; 2002. [Google Scholar]
- 20.Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Christian CK, Niland J, Edge SB, et al. A multi-institutional analysis of the socioeconomic determinants of breast reconstruction: A study of the National Comprehensive Cancer Network. Ann Surg. 2006;243:241–249. doi: 10.1097/01.sla.0000197738.63512.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnitt SJ. Classification and prognosis of invasive breast cancer: From morphology to molecular taxonomy. Mod Pathol. 2010;23(suppl 2):S60–S64. doi: 10.1038/modpathol.2010.33. [DOI] [PubMed] [Google Scholar]
- 23.Trihia H, Murray S, Price K, et al. Ki-67 expression in breast carcinoma: Its association with grading systems, clinical parameters, and other prognostic factors—A surrogate marker. Cancer. 2003;97:1321–1331. doi: 10.1002/cncr.11188. [DOI] [PubMed] [Google Scholar]
- 24.Weidner N, Moore DH, 2nd, Vartanian R. Correlation of ki-67 antigen expression with mitotic figure index and tumor grade in breast carcinomas using the novel “paraffin”-reactive MIB1 antibody. Hum Pathol. 1994;25:337–342. doi: 10.1016/0046-8177(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 25.Loi S, Haibe-Kains B, Desmedt C, et al. Definition of clinically distinct molecular subtypes in estrogen receptor–positive breast carcinomas through genomic grade. J Clin Oncol. 2007;25:1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 26.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Ng'andu NH. An empirical comparison of statistical tests for assessing the proportional hazards assumption of Cox's model. Stat Med. 1997;16:611–626. doi: 10.1002/(sici)1097-0258(19970330)16:6<611::aid-sim437>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Hertzmark E, Pazaris M, Spiegelman D. The SAS MEDIATE macro. http://www.hsph.harvard.edu/faculty/spiegelman/mediate/mediate.pdf.
- 30.Newman LA, Griffith KA, Jatoi I, et al. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342–1349. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- 31.Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: A population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113:357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 32.Hunt BR, Whitman S, Hurlbert MS. Increasing black:white disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol. 2014;38:118–123. doi: 10.1016/j.canep.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Gorin SS, Heck JE, Cheng B, et al. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166:2244–2252. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 34.Balasubramanian BA, Demissie K, Crabtree BF, et al. Black Medicaid beneficiaries experience breast cancer treatment delays more frequently than whites. Ethn Dis. 2012;22:288–294. [PubMed] [Google Scholar]
- 35.McGee SA, Durham DD, Tse CK, et al. Determinants of breast cancer treatment delay differ for African American and white women. Cancer Epidemiol Biomarkers Prev. 2013;22:1227–1238. doi: 10.1158/1055-9965.EPI-12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fedewa SA, Ward EM, Stewart AK, et al. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: A national cohort study 2004-2006. J Clin Oncol. 2010;28:4135–4141. doi: 10.1200/JCO.2009.27.2427. [DOI] [PubMed] [Google Scholar]
- 37.Livaudais JC, Hershman DL, Habel L, et al. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012;131:607–617. doi: 10.1007/s10549-011-1762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hershman DL, Wang X, McBride R, et al. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys. 2006;65:1353–1360. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 39.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warner ET, Tamimi RM, Hughes ME, et al. Time to diagnosis and breast cancer stage by race/ethnicity. Breast Cancer Res Treat. 2012;136:813–821. doi: 10.1007/s10549-012-2304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in national comprehensive cancer network institutions. J Natl Cancer Inst. 2013;105:104–112. doi: 10.1093/jnci/djs506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 43.Livaudais JC, Lacroix AZ, Chlebowski RT, et al. Racial/ethnic differences in use and duration of adjuvant hormonal therapy for breast cancer in the women's health initiative. Cancer Epidemiol Biomarkers Prev. 2013;22:365–373. doi: 10.1158/1055-9965.EPI-12-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 46.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534–2542. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magai C, Consedine NS, Adjei BA, et al. Psychosocial influences on suboptimal adjuvant breast cancer treatment adherence among African American women: Implications for education and intervention. Health Educ Behav. 2008;35:835–854. doi: 10.1177/1090198107303281. [DOI] [PubMed] [Google Scholar]
- 48.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 49.Friese CR, Pini TM, Li Y, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138:931–939. doi: 10.1007/s10549-013-2499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25:2522–2527. doi: 10.1200/JCO.2006.10.2749. [DOI] [PubMed] [Google Scholar]
- 51.Wu XC, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30:142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 52.Nurgalieva ZZ, Franzini L, Morgan RO, et al. Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Med Oncol. 2013;30:419. doi: 10.1007/s12032-012-0419-1. [DOI] [PubMed] [Google Scholar]
- 53. Reference deleted.
- 54. Reference deleted.
- 55. Reference deleted.
- 56. Reference deleted.
- 57. Reference deleted.
- 58. Reference deleted.
- 59.Freund KM, Battaglia TA, Calhoun E, et al. Impact of patient navigation on timely cancer care: The patient navigation research program. J Natl Cancer Inst. 2014;106:dju115. doi: 10.1093/jnci/dju115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paskett ED, Harrop JP, Wells KJ. Patient navigation: An update on the state of the science. CA Cancer J Clin. 2011;61:237–249. doi: 10.3322/caac.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ko NY, Darnell JS, Calhoun E, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Navigation Research Program. J Clin Oncol. 2014;32:2758–2764. doi: 10.1200/JCO.2013.53.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sparano JA, Wang M, Zhao F, et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104:406–414. doi: 10.1093/jnci/djr543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoeffel EM, Rastogi S, Kim MO, et al. The Asian population: 2010. http://www.census.gov/prod/cen2010/briefs/c2010br-11.pdf.
- 64.Yi M, Liu P, Li X, et al. Comparative analysis of clinicopathologic features, treatment, and survival of Asian women with a breast cancer diagnosis residing in the United States. Cancer. 2012;118:4117–4125. doi: 10.1002/cncr.27399. [DOI] [PubMed] [Google Scholar]
- 65.Keegan TH, Quach T, Shema S, et al. The influence of nativity and neighborhoods on breast cancer stage at diagnosis and survival among California Hispanic women. BMC Cancer. 2010;10:603. doi: 10.1186/1471-2407-10-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the national comprehensive cancer network. Cancer. 2012;118:5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clinical Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]