Abstract
Human herpesvirus 6B (HHV-6B) frequently reactivates after cord blood transplantation (CBT). We previously reported an association between HHV-6B reactivation and delirium after hematopoietic cell transplantation. In this prospective study, 35 CBT recipients underwent twice-weekly plasma PCR testing for HHV-6 and thrice-weekly delirium assessment until day 84. There was a quantitative association between HHV-6 reactivation and delirium in univariable (odds ratio, 2.88; 95% confidence interval [CI], 0.97–8.59) and bivariable models. In addition, intensified prophylaxis with high-dose valacyclovir mitigated HHV-6 reactivation (adjusted hazard ratio, 0.39; 95% CI, 0.14–1.08). Larger trials are needed to explore the utility of HHV-6 prophylaxis after CBT.
Keywords: Herpes, HHV-6, cord blood, transplant, delirium, neurologic
INTRODUCTION
Human herpesvirus 6B (HHV-6B) reactivates in 60–90% of cord blood transplant (CBT) recipients and is associated with significant morbidity, particularly due to limbic encephalitis, which occurs in up to 10% of CBT recipients1–4. With a prospective study of 315 hematopoietic cell transplantation (HCT) recipients, we previously demonstrated that HHV-6 viremia is also associated with less fulminant central nervous system (CNS) dysfunction as measured by delirium and neurocognitive decline5. This prospective study examines whether HHV-6 reactivation is associated with an increased risk for delirium specifically after CBT. Additionally, our center changed antiviral prophylaxis strategies for cytomegalovirus (CMV) seropositive CBT recipients during enrollment, and we analyze the effect of intensified prophylaxis on HHV-6 reactivation.
SUBJECTS AND METHODS
Patients
Thirty-one patients undergoing CBT from April 2005 through August 2008 were enrolled as part of a larger study5. An additional cohort of 30 CBT recipients was enrolled between July 2009 and August 2011 following the same protocol. Patients with evidence of inherited chromosomally integrated (ci)HHV-6, defined as increasing HHV-6 plasma DNA levels in the first two weeks after HCT and persistent levels ≥100 copies per/mL in ≥80% of subsequent plasma samples, were excluded6. Of the 61 patients enrolled, 14 withdrew before contributing data, one was excluded due to suspected inherited ciHHV-6, and two were excluded for peri-HCT use of foscarnet, leaving a final cohort of 44 patients. Demographic and clinical data, including use of medications known to cause delirium and antivirals active against HHV-6, were collected from clinical records and databases and defined as previously described5.
Antiviral Prophylaxis
Antiviral prophylaxis strategies for CMV seropositive patients changed in June 2008 from valacyclovir 500 mg twice daily for herpes simplex and varicella zoster viruses to an intensified strategy using ganciclovir 5 mg/kg daily on days -8 to -2 during conditioning followed by valacyclovir 2 g every 8 hours for the first 100 days after CBT7.
HHV-6 Testing
Patients had twice-weekly plasma specimens tested for HHV-6 through day 84 after CBT. Care teams and investigators were blinded to results, which were obtained after finalization of endpoints. Routine testing for HHV-6 was not performed at our center except in the setting of neurologic symptoms. HHV-6 was quantified using PCR as previously described5. The lower limit of detection was 1 copy of HHV-6 DNA/reaction (25 copies/mL of plasma). A conserved region of the U94 gene was amplified to distinguish between species HHV-6A and HHV-6B.
Delirium Testing
Neuropsychiatric assessments for delirium were obtained thrice-weekly through day 56 and once weekly days 57 to 84 after CBT. Delirium was assessed using the Delirium Rating Scale (DRS) 8, a 10-item scale assessing delirium symptoms over 24 hours using information from patient interview, family reports, and clinical and laboratory data. Patients too ill to undergo DRS assessment were assessed using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for delirium9. A delirium episode was defined as a DRS score more than 12 or delirium based on the DSM-IV checklist on at least 2 of 3 consecutive assessments10. Overall, 35 (80%) of 44 patients were assessed for delirium; those lacking assessments were either <3 years old or unable to communicate. The protocol did not include neuroimaging or cerebrospinal fluid (CSF) testing; results from these studies were collected when they were obtained clinically.
Statistical Analyses
The primary endpoint was delirium measured as a longitudinal binary outcome, modeled using logistic regression with generalized estimating equations to evaluate odds ratios (OR) and associated 95% confidence intervals (CI) with robust variance estimates to account for within subject correlations11. The primary risk factor of interest, any level of HHV-6 detection, was modeled as a time-dependent dichotomous variable. To evaluate a quantitative association between HHV-6 DNA detection and the endpoint, we also used the median maximum per patient (785 copies/mL) and maximum upper quartile (6,154 copies/mL) as thresholds for comparison. At each time point that delirium was assessed, HHV-6 was coded as positive if at the current or a prior time point the subject had detection at any level, >median maximum, or in the maximum upper quartile.
Multivariable Cox models were used to evaluate hazard ratios (HR) and associated CIs for the risk of HHV-6 reactivation. The effect of intensified antiviral prophylaxis on HHV-6 reactivation and delirium was assessed, and cumulative incidence curves for HHV-6 reactivation were generated, censoring at day of last contact and treating death as a competing risk event. Due to the relatively small sample size, analyses were restricted to bivariable models. Variables with a P-value <0.2 in univariable analysis were candidates for bivariable analyses. Statistical significance was defined as 2-sided P <0.05. SAS version 9.3 (SAS Institute, Cary, NC) was used for analyses.
RESULTS
Patient and virologic characteristics are presented in Table 1. HHV-6 was detected in 29 (66%) of the 44 patients by day 84 after CBT. The median maximum viral load in the whole cohort was 785 copies/mL (interquartile range [IQR], 0 - 6,154) detected at a median of 24 days after CBT (IQR, 19–27 days). Among patients who reactivated HHV-6, the median maximum viral load was 2,945 (IQR, 960–19,252). Species typing demonstrated HHV-6B in all tested patients (two were not tested).
Table 1.
Demographic, clinical, and virologic characteristics of the cohort, overall and stratified by ever having HHV-6 reactivation at any level, HHV-6 >median maximum (785 copies/mL), or delirium after CBT
| Characteristic† | Overall, no. (%)(N = 44) | HHV-6 reactivation, no. (%) (n = 44)
|
Delirium episode, no. (%) (n=35)*
|
|||
|---|---|---|---|---|---|---|
| Any level‡ (n = 29) | HHV-6 > median max (n = 22) | None (n = 15) | Yes (n = 9) | No (n = 26) | ||
| Age, y, median (IQR) | 35 (12–53) | 30 (12–45) | 33 (13–45) | 42 (2–6) | 38 (30–66) | 42 (23–59) |
| Female sex | 22 (50) | 15 (52) | 13 (59) | 7 (47) | 4 (44) | 15 (58) |
| Caucasian | 28 (64) | 19 (66) | 14 (64) | 9 (60) | 6 (67) | 17 (65) |
| Recipient CMV seropositive | 32 (73) | 24 (83) | 17 (77) | 8 (53) | 8 (89) | 18 (69) |
| High medical comorbidity | 19 (43) | 10 (34) | 8 (36) | 9 (60) | 7 (78) | 11 (42) |
| TBI dose ≥1200 cGY | 22 (50) | 15 (52) | 11 (50) | 7 (47) | 5 (56) | 12 (46) |
| More advanced underlying disease | 27 (61) | 16 (55) | 12 (55) | 11 (73) | 5 (56) | 16 (62) |
| Myeloablative conditioning regimen | 26 (59) | 19 (66) | 14 (64) | 7 (47) | 6 (67) | 12 (46) |
| Double unit CBT | 33 (75) | 23 (79) | 19 (86) | 10 (67) | 9 (100) | 23 (88) |
| HLA 4/6 Mismatch | 23 (52) | 16 (55) | 11 (50) | 7 (47) | 6 (67) | 15 (58) |
| Intensive antiviral prophylaxis | 17 (39) | 12 (41) | 6 (27) | 5 (33) | 5 (56) | 10 (38) |
| Acute GVHD, grade 3–4 | 12 (27) | 7 (24) | 7 (32) | 5 (33) | 3 (33) | 5 (19) |
| HHV-6 reactivation, any | -- | -- | -- | -- | 6 (67) | 18 (69) |
| HHV-6 reactivation >median max | -- | -- | -- | -- | 5 (56) | 13 (50) |
| HHV-6 DNA median max copies/mL (IQR) | 785 (0–6,154) | -- | -- | -- | 3,275 (0–17,224) | 1,074 (0–5,900) |
| HHV-6 day of first detection, (IQR) | -- | 20 (17–28) | 24 (19–27) | -- | -- | -- |
IQR indicates interquartile range; CMV, cytomegalovirus; TBI, total body irradiation; CBT, cord blood transplantation; HLA, human leukocyte antigen; GVHD, graft-versus-host-disease.
Nine patients were too young, too sick, or otherwise unable to participate in delirium assessments.
Characteristics were defined as previously described (Zerr et al, 2011).
This category includes patients in the HHV-6 >median maximum category.
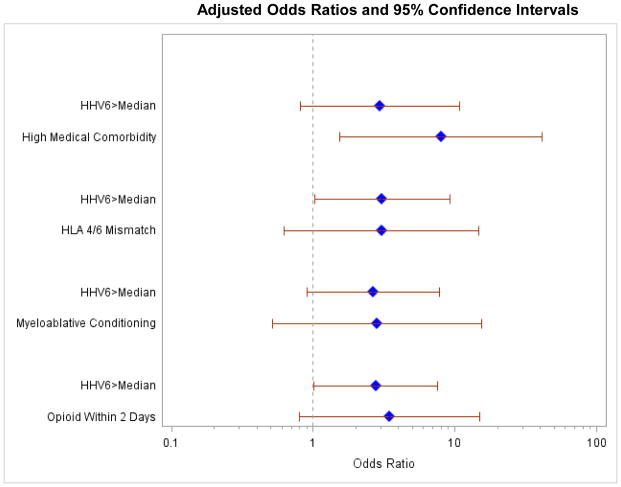
Among 35 subjects assessed for delirium, 11 (31%) had any delirium (at least 1 positive assessment), and 9 (26%) had >1 positive delirium assessment on consecutive testing (i.e. a delirium episode) lasting a median of 3 days (IQR, 3–6). In univariable logistic regression models, a delirium episode was more likely in patients with HHV-6 levels >median (OR, 2.88; 95% CI, 0.97–8.59; p=0.06) and a comorbidity score ≥3 (OR, 7.93; 95% CI, 1.53–40.99; p=0.01). Using a threshold of HHV-6 detection in the upper quartile resulted in a higher odds for delirium (OR=4.54) but wider CI due to limited events. The association between HHV-6 >median and delirium was maintained in a series of bivariable models (Figure 1).
Figure 1. Multivariable models evaluating HHV-6 as a predictor of delirium.
Bivariable logistic regression models evaluating detection of HHV-6 DNA >median maximum as a risk factor for delirium, adjusted for the other variable shown.
Cerebrospinal fluid (CSF) was obtained by care providers in 6 patients. Five of these patients had delirium assessments and 2 had a delirium episode within 1 week of CSF sampling. One of these patients did not have HHV-6 in CSF or plasma, whereas the other had HHV-6 detected in CSF samples and plasma samples within 1 week. This was the only patient with findings consistent with HHV-6 encephalitis, the incidence of which was 2.3% (1/44 patients). HHV-6 was detected in the CSF of 2 additional patients with headache but without delirium.
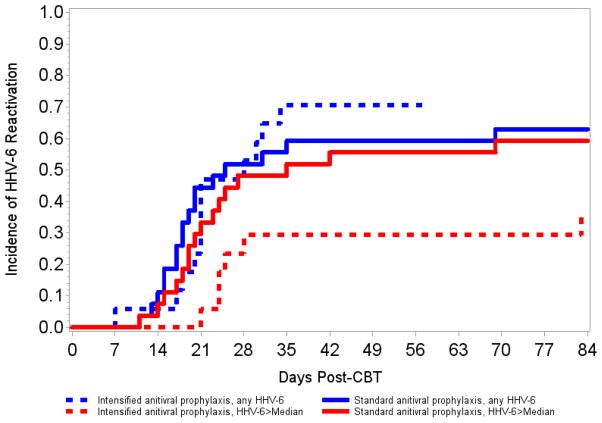
There were no patient characteristics associated with any level HHV-6 reactivation. Risk of HHV-6 >median was increased by double CBT (vs. single; hazard ratio [HR], 3.45; 95% CI, 1.01–11.76; p=0.05) and acute graft-versus-host disease grades 3–4 (HR, 2.41; 95% CI, 0.94–6.19; p=0.07) but reduced in patients receiving intensified antiviral prophylaxis (HR, 0.46; 95% CI, 0.18–1.17; p=0.10; Figure 2). A multivariable model adjusted for these variables demonstrated that intensified antiviral prophylaxis maintained its effect on mitigating HHV-6 reactivation >median (aHR, 0.39; 95% CI, 0.14–1.08; p=0.07; Table 2). Intensified prophylaxis had an even more apparent effect on reducing HHV-6 detection in the upper quartile (HR, 0.13; 95% CI 0.02–1.03; p=0.05). We could not meaningfully analyze the effect of intensified antiviral prophylaxis on delirium or death given the number of subjects and events.
Figure 2. Cumulative incidence curves of HHV-6 reactivation stratified by antiviral prophylaxis strategy.
Cumulative incidence curves for detection of HHV-6 DNA at any or >median maximum level, stratified by antiviral prophylaxis strategy.
Table 2.
Cox proportional hazards model for risk factors for HHV-6 reactivation >median maximum (785 copies/mL)
| Variable | HR (95% CI) | P value | aHR (95% CI) | P value |
|---|---|---|---|---|
| Double CBT (vs. single) | 3.45 (1.01–11.76) | 0.05 | 5.03 (1.43–17.72) | 0.01 |
| Acute GVHD grade 3–4 | 2.41 (0.94–6.19) | 0.07 | 2.06 (0.75–5.69) | 0.16 |
| Intensified antiviral prophylaxis | 0.46 (0.18–1.17) | 0.10 | 0.39 (0.14–1.08) | 0.07 |
HHV-6 indicates human herpesvirus 6; HR, hazard ratio; CI, confidence interval; aHR, adjusted hazard ratio; CBT, cord blood hematopoietic cell transplantation; GVHD, graft-versus-host disease.
Discussion
We demonstrate a quantitative association between HHV-6 reactivation and delirium in a prospective cohort of CBT recipients. We also report that intensified antiviral prophylaxis with high-dose valacylovir may mitigate HHV-6 reactivation. The results are of particular importance to CBT recipients who reactivate HHV-6 more frequently and at higher levels with an attendant increase in morbidity, likely due to the immunologically immature allograft1–3,12. This study helps to establish an objective clinical endpoint and further impetus for investigating the benefit of HHV-6 screening, prophylaxis, and treatment after CBT.
The association of HHV-6 reactivation with delirium expands our appreciation for the potential impact of this virus beyond the 2–10% of CBT recipients affected by overt HHV-6 encephalitis1,2,4,13. Nevertheless, our understanding of the significance of HHV-6 DNA detection in the blood or CSF, how well this correlates with end-organ disease, and the mechanisms underlying associated morbidity remains incomplete and continues to evolve. For example, not all instances of CSF HHV-6 DNA detection are associated with significant CNS morbidity, as we have previously reported in our HCT patient population14. However, viral detection in the CSF and blood compartments may underestimate the true incidence of tissue-level reactivation and pathogenicity. HHV-6 is a neurotropic virus with latency in astrocytes and glial cells15,16, and brain tissue from patients with HHV-6 encephalitis has been shown to have higher levels and prolonged detection of HHV-6 DNA compared to blood or CSF samples17,18. Indeed, the detection of herpesvirus DNA in liquid compartments (e.g. blood, CSF, bronchoalveolar lavage fluid) may not be a sensitive or specific biomarker for end-organ disease. Mechanistically, one can hypothesize that direct effects of tissue-level reactivation and/or indirect effects of systemic reactivation, such as the induction of pro-inflammatory cytokines20,21, may contribute to delirium in affected patients. Interestingly, some of these same cytokines have been associated with delirium in critically ill patients22.
The incidence of HHV-6 encephalitis after CBT in this cohort (2.3%) is similar to that recently reported at Memorial Sloan-Kettering Cancer Center4 but lower than incidence rates of 8–10% at other centers1,2,13. This finding may be due to differences in strategies for antiviral prophylaxis (such as we report here) or preemptive therapy (such as reported at Sloan-Kettering). Variability in conditioning regimens may also play a role. Neither our center nor Sloan-Kettering use antithymocyte globulin for CBT, which has been variably associated with HHV-6 reactivation2,25.
Intensified antiviral prophylaxis with high-dose valacyclovir has been shown to similarly diminish HHV-6 reactivation in one other study of HCT recipients receiving 2 grams of valacyclovir four times a day23. Whether or not this intervention may reduce HHV-6-associated complications is unknown, and our study was not powered to address this question. Ogata and colleagues demonstrated that low-dose foscarnet prophylaxis did not significantly reduce high-level HHV-6 DNA detection or HHV-6 encephalitis in a population of unrelated and cord blood HCT recipients24; however, the results were suggestive and indicate that further study is required.
The findings in this and our previous study of HHV-6-associated delirium in a diverse cohort of allogeneic HCT recipients5 are clinically meaningful in light of studies linking delirium to morbidity after HCT10. Whether routine screening for HHV-6 reactivation after CBT is warranted remains to be determined. The development of new broad-spectrum antiviral agents with limited side effects, such as brincidofovir (CMX001)26, provide unique opportunities for clinical trials exploring whether prophylactic or preemptive approaches to mitigating HHV-6 reactivation can improve HCT outcomes.
This study’s strengths include a prospective design, frequent quantitative HHV-6 assessments, and standardized neuropsychiatric testing blinded to HHV-6 reactivation. The relatively small sample size limited our analyses and conclusions, and this may explain the lack of a statistically significant association with delirium after adjusting for comorbidity and conditioning (Figure 1). Despite this, our findings support a quantitative association between HHV-6 viral load and the endpoints of interest. The lack of an international standard for HHV-6 DNA measurement precludes extrapolation of quantitative levels to other studies, as inter-laboratory correlation is known to be poor27. Lack of CSF HHV-6 testing in all patients with delirium was another limitation.
In conclusion, HHV-6 reactivation after CBT is quantitatively associated with delirium, and antiviral prophylaxis with high-dose valacyclovir may mitigate HHV-6 reactivation. Larger interventional studies are needed to assess the utility of low-toxicity HHV-6 prevention strategies for reducing delirium and other adverse outcomes in high-risk HCT recipients.
Key Points.
HHV-6 reactivation after cord blood transplantation is associated with delirium.
High-dose valacyclovir may mitigate HHV-6 reactivation.
Larger studies are needed to assess the impact of HHV-6 prevention on outcomes.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (grant numbers R01 AI057639-05S1, D.M.Z.; CA018029 and K24 HL093294, M.B.). The funding source had no involvement in the study design, collection, analysis, and interpretation of data.
The authors thank the patients and their families who participated in this study, without whose patience and dedication this study could not have been undertaken.
Footnotes
Ethical approval
All patients provided written consent, and the protocol was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board.
Authorship
Contribution: D.M.Z., M.B., J.A.H., A.L.A, M.-L.H., J.R.F., C.D., and W.M.L. participated in the study design, data analysis and interpretation, and critical review of the report; J.H. wrote the first draft and all revisions of the report; and H.X. and W.M.L. performed the statistical analyses and participated in revision of the report.
Conflict-of-interest disclosure: M.B. received research funding for clinical trials and consulting fees from Chimerix Inc., Genentech/Roche, and Gilead in addition to consulting for Clinigen. D.M.Z received research funding from Chimerix Inc. All other authors report no potential conflicts.
References
- 1.Ogata M, Satou T, Kadota J-I, Saito N, Yoshida T, Okumura H, et al. Human Herpesvirus 6 (HHV-6) Reactivation and HHV-6 Encephalitis After Allogeneic Hematopoietic Cell Transplantation: A Multicenter, Prospective Study. Clin Infect Dis. 2013;57:671–81. doi: 10.1093/cid/cit358. [DOI] [PubMed] [Google Scholar]
- 2.Hill Ja, Koo S, Guzman Suarez BB, Ho VT, Cutler C, Koreth J, et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant. 2012;18:1638–48. doi: 10.1016/j.bbmt.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chevallier P, Hebia-Fellah I, Planche L, Guillaume T, Bressolette-Bodin C, Coste-Burel M, et al. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: a comparison with matched unrelated donors as stem cell source. Bone Marrow Transplant. 2010;45:1204–11. doi: 10.1038/bmt.2009.326. [DOI] [PubMed] [Google Scholar]
- 4.Olson AL, Dahi PB, Zheng J, Devlin SM, Lubin M, Gonzales AM, et al. Frequent Human Herpesvirus-6 Viremia But Low Incidence of Encephalitis in Double-Unit Cord Blood Recipients Transplanted Without Antithymocyte Globulin. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zerr DM, Fann JR, Breiger D, Boeckh M, Adler AL, Xie H, et al. HHV-6 reactivation and its effect on delirium and cognitive functioning in hematopoietic cell transplantation recipients. Blood. 2011;117:5243–9. doi: 10.1182/blood-2010-10-316083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark Da, Nacheva EP, Leong HN, Brazma D, Li YT, Tsao EHF, et al. Transmission of integrated human herpesvirus 6 through stem cell transplantation: implications for laboratory diagnosis. J Infect Dis. 2006;193:912–6. doi: 10.1086/500838. [DOI] [PubMed] [Google Scholar]
- 7.Milano F, Pergam Sa, Xie H, Leisenring WM, Gutman Ja, Riffkin I, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood. 2011;118:5689–96. doi: 10.1182/blood-2011-06-361618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Res. 1988;23:89–97. doi: 10.1016/0165-1781(88)90037-6. [DOI] [PubMed] [Google Scholar]
- 9.Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 10.Basinski JR, Alfano CM, Katon WJ, Syrjala KL, Fann JR. Impact of delirium on distress, health-related quality of life, and cognition 6 months and 1 year after hematopoietic cell transplant. Biol Blood Marrow Transplant. 2010;16:824–31. doi: 10.1016/j.bbmt.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang BYK, Zeger SL. Longitudinal data analysis using generalized linear models. 1986;73:13–22. [Google Scholar]
- 12.Safdar A, Rodriguez GH, De Lima MJ, Petropoulos D, Chemaly RF, Worth LL, et al. Infections in 100 cord blood transplantations: spectrum of early and late posttransplant infections in adult and pediatric patients 1996–2005. Medicine (Baltimore) 2007;86:324–33. doi: 10.1097/MD.0b013e31815c52b0. [DOI] [PubMed] [Google Scholar]
- 13.Scheurer ME, Pritchett JC, Amirian ES, Zemke NR, Lusso P, Ljungman P. HHV-6 encephalitis in umbilical cord blood transplantation: a systematic review and meta-analysis. Bone Marrow Transplant. 2013;48:574–80. doi: 10.1038/bmt.2012.180. [DOI] [PubMed] [Google Scholar]
- 14.Hill JA, Boeckh MJ, Sedlak RH, Jerome KR, Zerr DM. Human herpesvirus 6 can be detected in cerebrospinal fluid without associated symptoms after allogeneic hematopoietic cell transplantation. J Clin Virol. 2014 doi: 10.1016/j.jcv.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassiani-Ingoni R, Greenstone HL, Donati D, Fogdell-Hahn A, Martinelli E, Refai D, et al. CD46 on glial cells can function as a receptor for viral glycoprotein-mediated cell-cell fusion. Glia. 2005;52:252–8. doi: 10.1002/glia.20219. [DOI] [PubMed] [Google Scholar]
- 16.He J, McCarthy M, Zhou Y, Chandran B, Wood C. Infection of primary human fetal astrocytes by human herpesvirus 6. J Virol. 1996;70:1296–300. doi: 10.1128/jvi.70.2.1296-1300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wainwright MS, Martin PL, Morse RP, Lacaze M, Provenzale JM, Coleman RE, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001;50:612–9. doi: 10.1002/ana.1251. [DOI] [PubMed] [Google Scholar]
- 18.Fotheringham J, Akhyani N, Vortmeyer A, Donati D, Williams E, Oh U, et al. Detection of active human herpesvirus-6 infection in the brain: correlation with polymerase chain reaction detection in cerebrospinal fluid. J Infect Dis. 2007;195:450–4. doi: 10.1086/510757. [DOI] [PubMed] [Google Scholar]
- 19.Ishiyama K, Katagiri T, Hoshino T, Yoshida T, Yamaguchi M, Nakao S. Preemptive therapy of human herpesvirus-6 encephalitis with foscarnet sodium for high-risk patients after hematopoietic SCT. Bone Marrow Transplant. 2011;46:863–9. doi: 10.1038/bmt.2010.201. [DOI] [PubMed] [Google Scholar]
- 20.Fujita A, Ihira M, Suzuki R, Enomoto Y, Sugiyama H, Sugata K, et al. Elevated serum cytokine levels are associated with human herpesvirus 6 reactivation in hematopoietic stem cell transplantation recipients. J Infect. 2008;57:241–8. doi: 10.1016/j.jinf.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Ichiyama T, Ito Y, Kubota M, Yamazaki T, Nakamura K, Furukawa S. Serum and cerebrospinal fluid levels of cytokines in acute encephalopathy associated with human herpesvirus-6 infection. Brain Dev. 2009;31:731–8. doi: 10.1016/j.braindev.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Sobbi SC, van den Boogaard M. Inflammation biomarkers and delirium in critically ill patients: new insights? Crit Care. 2014;18:153. doi: 10.1186/cc13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang FZ, Dahl H, Linde A, Brytting M, Ehrnst A, Ljungman P. Lymphotropic herpesviruses in allogeneic bone marrow transplantation. Blood. 1996;88:3615–20. [PubMed] [Google Scholar]
- 24.Ogata M, Satou T, Inoue Y, Takano K, Ikebe T, Ando T, et al. Foscarnet against human herpesvirus (HHV)-6 reactivation after allo-SCT: breakthrough HHV-6 encephalitis following antiviral prophylaxis. Bone Marrow Transplant. 2012;48:257–64. doi: 10.1038/bmt.2012.121. [DOI] [PubMed] [Google Scholar]
- 25.Wang L-R, Dong L-J, Lu D-P. Surveillance of active human herpesvirus 6 infection in chinese patients after hematopoietic stem cell transplantation with 3 different methods. Int J Hematol. 2006;84:262–7. doi: 10.1532/IJH97.A10607. [DOI] [PubMed] [Google Scholar]
- 26.Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369:1227–36. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 27.De Pagter PJ, Schuurman R, de Vos NM, Mackay W, van Loon AM. Multicenter external quality assessment of molecular methods for detection of human herpesvirus 6. J Clin Microbiol. 2010;48:2536–40. doi: 10.1128/JCM.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]