Abstract
Oral bacterial hydrogen sulfide (H2S) production was estimated comparing two different colorimetric methods in microtiter plate format. High H2S production was seen for Fusobacterium spp., Treponema denticola, and Prevotella tannerae, associated with periodontal disease. The production differed between the methods indicating that H2S production may follow different pathways.
Keywords: bacterial metabolites, hydrogen sulfide, oral bacteria, cysteine, methylene blue, bismuth sulfide, periodontitis, Fusobacterium spp
Periodontal disease is believed to be associated with an anaerobic and proteolytic bacterial metabolism but the pathogenesis is still largely unknown. Hydrogen sulfide (H2S) is a toxic, bacterial waste product in the subgingival pocket and up to 1.9 mM H2S has been detected in gingival crevicular fluid (1, 2). Due to its proinflammatory properties, it has been suggested that H2S may participate in the bacteria-induced inflammatory response in the periodontal diseases (3–6).
Previous studies have reported bacterial H2S production from various species, for example, Fusobacterium spp., by the degradation of cysteine (7), homocysteine (8), and glutathione (9) and from Parvimonas micra, Tannerella forsythia and Filifactor alocis (1). More research is, however, needed to elucidate the rate and the amount of H2S produced by various species/strains under various conditions both in vitro and in vivo.
The H2S-producing capacity is commonly tested by blackening of lead acetate paper (10) or with gas chromatography (1, 11) and sensors (12–14). These methods are either rough or require complex equipment and are therefore expensive. Simple chair-side methods for semi-quantification of bacterial H2S, which could further facilitate the investigation of H2S production and presence, are lacking.
The aim of the present study is to examine oral bacterial H2S production in vitro comparing two colorimetric methods in microtiter plate format.
The bacterial species tested for H2S-producing capacity are given in Table 1. The species were grown on appropriate agar plates under optimal conditions.
Table 1.
Bacterial hydrogen sulfide (H2S) production from cysteine measured with two colorimetric methods in microtiter plate format, recorded as black bismuth sulfide (BS) precipitation and methylene blue (MB) formation
| H2S productiona | |||
|---|---|---|---|
|
|
|||
| Species | Strain | BS method | MB method |
| Aggregatibacter actinomycetemcomitans | ATCCb 43718 | + | − |
| Actinomyces naeslundii | ATCC 12104 | − | − |
| Actinomyces odontolyticus | ATCC 17929 | − | − |
| Actinomyces oris | ATCC 15987 | − | − |
| Bifidobacterium dentium | ATCC 11863 | − | − |
| Eikenella corrodens | ATCC 23834 | + | − |
| Enterococcus faecalis | ATCC 19433 | + | − |
| Filifactor alocis | ATCC 35896 | − | − |
| Fusobacterium necrophorum | ATCC 51357 | + + + + + | + + + + + |
| Fusobacterium necrophorum | CCUGc 48192 | + + + + + | + + + + + |
| Fusobacterium nucleatum | ATCC 10953 | + + + + + | + + + + + |
| Fusobacterium periodonticum | ATCC 33693 | + + + + + | + + + + |
| Lactobacillus casei | CCUG 31610 | − | − |
| Lactobacillus gasseri | ATCC 33323 | − | − |
| Lactobacillus rhamnosus | ATCC 7469 | − | − |
| Lactobacillus salivarius | CCUG 55845 | − | − |
| Parvimonas micra | ATCC 33270 | + + + | + |
| Porphyromonas endodontalis | OMGSd 1205 | − | + + |
| Porphyromonas gingivalis 381F | OMGS 2860 | + | + + + |
| Porphyromonas gingivalis W83 | OMGS 197 | + | + |
| Prevotella intermedia | ATCC 25611 | + | − |
| Prevotella nigrescens | ATCC 33563 | − | − |
| Prevotella tannerae | ATCC 51259 | + + | + + + + |
| Rothia dentocariosa | CCUG 17835 | + | − |
| Staphylococcus aureus | OMGS 3871 | − | − |
| Streptococcus anginosus | ATCC 12395 | + + | − |
| Streptococcus gordonii | ATCC 33399 | − | − |
| Streptococcus intermedius | ATCC 27335 | − | − |
| Streptococcus oralis | ATCC 35037 | − | − |
| Streptococcus mitis | ATCC 49456 | − | − |
| Streptococcus mutans | ATCC 25175 | − | − |
| Streptococcus salivarius | ATCC 7073 | − | − |
| Streptococcus sanguinis | ATCC 10556 | − | − |
| Streptococcus sobrinus | CCUG 27507 | − | − |
| Tannerella forsythia | ATCC 43037 | − | − |
| Treponema denticola | OMGS 3271e | + + + + | + + + + + |
| Veillonella parvula | ATCC 10790 | + | − |
Visual color change, scored from no color production (−) to maximum black or blue color production (+ + + + +) by three individuals. The results of the bismuth method are shown after 7 h of incubation in the bismuth solution. Data shown are median values of triplicate wells for three repetitions of the experiment.
American Type Culture Collection.
Culture Collection University of Gothenburg.
Oral Microbiology Gothenburg Sweden.
Originally received from Dr. R. Ellen, University of Toronto, Toronto, Canada.
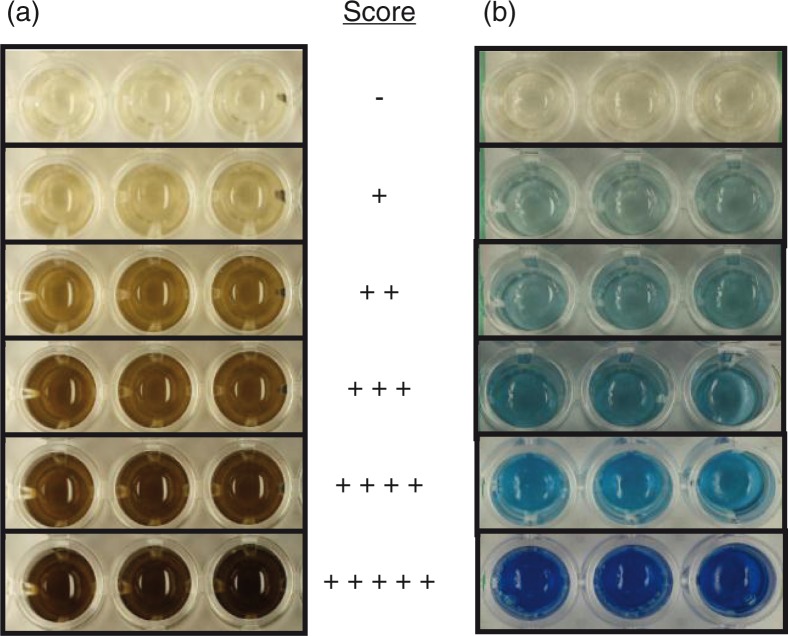
The bismuth sulfide (BS) method introduced by Yoshida et al. (8) was modified to using a 5 mM concentration of bismuth(III)chloride. Bacteria were diluted in peptone solution to 109 cells/mL. Aliquots (100 µl) of the bacterial suspension were mixed with an equal amount of newly prepared bismuth solution (0.4 M triethanolamine-HCl, pH 8.0; 10 mM bismuth(III)chloride; 20 µM pyridoxal 5-phosphate monohydrate, 20 mM EDTA and 40 mM l-cysteine) in microtiter plates. In the presence of H2S, black BS is precipitated. Three technical staff members measured the precipitation by scoring the color of the well using a visual scale, from no color production (0) to maximum black color production (5) every hour between 0 and 7 and also after 24 h (Fig. 1a). In addition to the visual scale (median), BS precipitation was determined (mean) by measuring the optical density (OD405).
Fig. 1.
(a) The BS method. The production of H2S was estimated by black BS precipitation and recorded as no color production (−; score 0) to maximum black color production (+ + + + +; score 5). (b) The methylene blue (MB) method. The production of MB in the presence of H2S was estimated from no color production (−; score 0) to maximum blue color production (+ + + + +; score 5).
For the methylene blue (MB) method, adapted after Cline (15), bacterial colonies from the agar plates were incubated in broth with 20 mM l-cysteine until approximately 109 cells/mL were obtained. Thereafter, 10 µl was transferred to 72 µl of distilled water (pH 9.6 + 0.1 mM diethylenetriaminepentaacetic acid) (16) in a microtiter well. A volume of 18 µl of a solution (17.1 mM N,N-dimethyl-p-phenylenediamine sulfate salt and 37.0 mM FeCl3 in 6 M HCl) was immediately added (17). The MB method measures the H2S produced during bacterial growth in the broth and the formation was measured 30 min after the addition of the solution (17) (Fig. 1b). Similar to the BS method, visual-scale estimations (median) and optical density (OD668) measurements (mean) were performed.
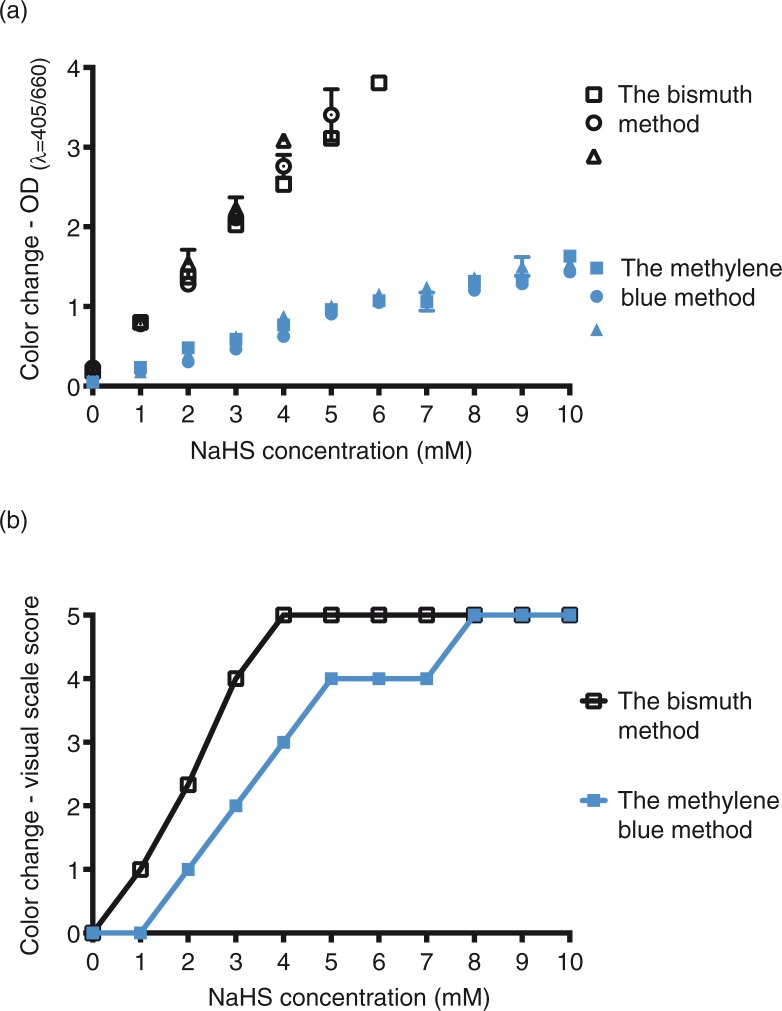
To test the sensitivity of the methods, different concentrations of sodium hydrosulfide (NaHS) were mixed with the BS and MB solutions. In both cases, a linear correlation between the color intensity and the HS−/S2− ion concentration was found with the spectrophotometric and the visual determination (Fig. 2). The visual detection limit for H2S was 0.6 mM for the BS method and 2 mM for the MB method.
Fig. 2.
NaHS was tested with two colorimetric tests: the BS method (black) and the methylene blue (MB) method (blue). The color production for known concentrations of NaHS was a) determined (mean) with optical density analysis (λ=405 nm for the bismuth method and λ=668 nm for the MB method), b) scored on a visual scale by three individuals (median). Data shown are individual values for triplicates of the test.
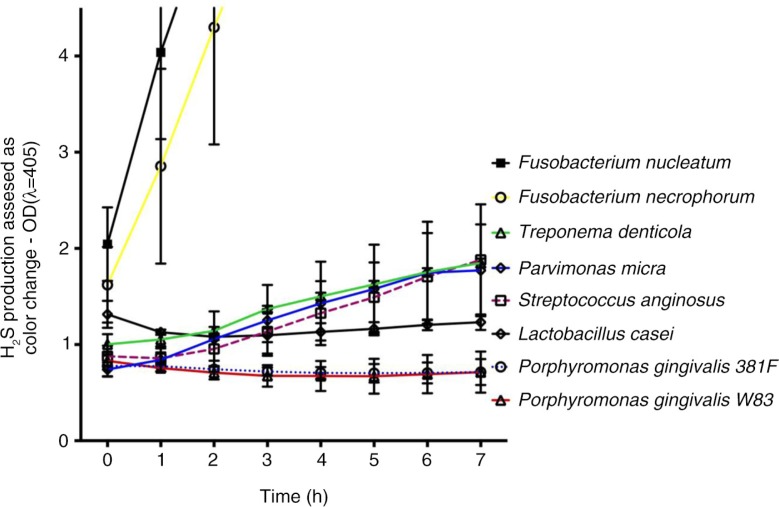
The most rapid H2S production was seen for Fusobacterium spp. reaching the maximum color production (Fig. 3). Treponema denticola, P. micra, and Streptococcus anginosus also showed changes in OD, while Lactobacillus casei and both strains of Porphyromonas gingivalis failed to do so. Also, the highest production was seen for Fusobacterium spp. (Table 1). T. denticola showed a clear color change with both methods but at a slower rate. Interestingly, Prevotella tannerae had a high capacity to produce H2S reaching a concentration of approximately 3 mM according to both methods. To our knowledge, this strain has not previously been tested for its H2S-producing capacity. Also, P. micra and P. gingivalis produced H2S but the color production differed between the BS and MB methods and between the two P. gingivalis strains tested. Although P. gingivalis W83 is believed to be more virulent than 381F (18, 19), the latter showed higher H2S production in broth (MB method). This could result from more proteins being available for degradation in the broth than in the BS solution. A preference for proteins before amino acids as source of nutrients (20) may also be true for P. endodontalis. The results indicate that H2S production may follow different pathways in the two methods.
Fig. 3.
Bacterial H2S production was determined by BS precipitation and optical density (OD405) measurements up to 7 h at different time points. The bacteria were analyzed at a concentration of approximately 5×108 cells/mL. Data shown are mean values for triplicate wells for three repetitions of the experiment.
Both methods, based on BS precipitation and MB formation, respectively, had a high reproducibility, reliability, and simplicity. The highest rate to maximal detectable production of H2S was found for Fusobacterium spp. Different findings from the two methods for some bacteria may reflect different pathways used for H2S production and, therefore, the BS and MB methods may complement one another. The BS method was more sensitive than the MB method and may be suitable for in vivo estimation of H2S production, using, for example, plaque samples from bacterial infections, such as in the periodontal pockets and other anaerobic infection sites. The production of H2S is complex and needs more attention in future studies to enhance the knowledge of the mechanisms involved in H2S production and its impact in vivo.
Conflict of interest and funding
This study was supported by TUA-Grant (TUAGBG-67191) and by grants from Göteborgs Tandläkare Sällskap (GTS). Special thanks to Gunilla Hjort and Lisbeth Bengtsson for visual scale scoring. There is no conflict of interest in the present study for any of the authors.
References
- 1.Persson S, Edlund MB, Claesson R, Carlsson J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol Immunol. 1990;5:195–201. doi: 10.1111/j.1399-302x.1990.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 2.Persson S. Hydrogen sulfide and methyl mercaptan in periodontal pockets. Oral Microbiol Immunol. 1992;7:378–9. doi: 10.1111/j.1399-302x.1992.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo AA. The possible role of hydrogen sulfide in human periodontal disease. I. Hydrogen sulfide production in periodontal pockets. Periodontics. 1967;5:233–6. [PubMed] [Google Scholar]
- 4.Morhart RE, Mata LJ, Sinskey AJ, Harris RS. A microbiological and biochemical study of gingival crevice debris obtained from Guatemalan Mayan Indians. J Periodontol. 1970;41:644–9. doi: 10.1902/jop.1970.41.11.644. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz A, Folke LEA. Hydrogen sulfide production in the periodontal environment. J Periodontol. 1973;44:390–5. doi: 10.1902/jop.1973.44.7.390. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Kajiya M, Giro G, Ouhara K, Mackler HE, Mawardi H, et al. Bacteria-derived hydrogen sulfide promotes IL-8 production from epithelial cells. Biochem Biophys Res Commun. 2010;391:645–50. doi: 10.1016/j.bbrc.2009.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pianotti R, Lachette S, Dills S. Desulfuration of cysteine and methionine by Fusobacterium nucleatum . J Dent Res. 1986;65:913–17. doi: 10.1177/00220345860650061101. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida A, Yoshimura M, Ohara N, Yoshimura S, Nagashima S, Takehara T, et al. Hydrogen sulfide production from cysteine and homocysteine by periodontal and oral bacteria. J Periodontol. 2009;80:1845–51. doi: 10.1902/jop.2009.090012. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson J, Larsen JT, Edlund MB. Peptostreptococcus micros has a uniquely high capacity to form hydrogen sulfide from glutathione. Oral Microbiol Immunol. 1993;8:42–5. doi: 10.1111/j.1399-302x.1993.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 10.Lennette E, Balows A, Hausler W, Shadomy H. fourth ed. Washington, DC: American Society for Microbiology; 1985. Manual of clinical microbiology; p. 385. [Google Scholar]
- 11.Blanchette AR, Cooper AD. Determination of hydrogen sulfide and methyl mercaptan in mouth air at the parts-per-billion level by gas chromatography. Anal Chem. 1976;48:729–31. doi: 10.1021/ac60368a002. [DOI] [PubMed] [Google Scholar]
- 12.Searcy DG, Peterson MA. Hydrogen sulfide consumption measured at low steady state concentrations using a sulfidostat. Anal Biochem. 2004;324:269–75. doi: 10.1016/j.ab.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, et al. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Torresyap G, Haffajee AD, Uzel NG, Socransky SS. Relationship between periodontal pocket sulfide levels and subgingival species. J Clin Periodontol. 2003;30:1003–10. doi: 10.1034/j.1600-051x.2003.00377.x. [DOI] [PubMed] [Google Scholar]
- 15.Cline J. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr. 1969;14:454–8. [Google Scholar]
- 16.Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide. The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47:1346–53. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Reese BK, Finneran DW, Mills HJ, Zhu MX, Morse JW. Examination and refinement of the determination of aqueous hydrogen sulfide by the methylene blue method. Aquat Geochem. 2011;17:567–82. [Google Scholar]
- 18.van Steenbergen TJ, Kastelein P, Touw JJ, de Graaff J. Virulence of black-pigmented Bacteroides strains from periodontal pockets and other sites in experimentally induced skin lesions in mice. J Periodontal Res. 1982;17:41–9. doi: 10.1111/j.1600-0765.1982.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 19.Grenier D, Mayrand D. Selected characteristics of pathogenic and nonpathogenic strains of Bacteroides gingivalis . J Clin Microbiol. 1987;25:738–40. doi: 10.1128/jcm.25.4.738-740.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah HN, Williams RAD. Utilization of glucose and amino acids by Bacteroides intermedius and Bacteroides gingivalis . Curr Microbiol. 1987;15:241–6. [Google Scholar]