Abstract
We report the design and development of redox-responsive chain-shattering polymeric therapeutics (CSPTs). CSPTs were synthesized by condensation polymerization and further modified with poly(ethylene glycol) (PEG) via “Click” reaction. Size-controlled CSPT nanoparticles (NPs) were formed through nanoprecipitation with high drug loading (up to 18%); the particle size increased in a concentration dependent manner. Drug release from particles was well controlled over 48 h upon redox triggering. The anticancer efficacy of the CSPT NPs was validated both in vitro and in vivo.
Polymeric nanomedicine has attracted much attention as a new modality for potentially improved cancer treatment that may change the landscape of oncology.1–6 Efforts in the area of therapeutic polymeric nanomedicine have been largely devoted to the development of polymer-drug conjugates and polymer/drug encapsulates.7–11 For polymer-drug conjugates, drug molecules are usually grafted to the pendant functional groups of a hydrophilic polymer via cleavable linkages. Controlled drug loading and sustained drug release can be achievable, but their structure and composition control are very difficult.12,13 Moreover, the polymer-drug conjugates may have uncontrolled batch-to-batch variations for their physicochemical and pharmacological properties. For polymer/drug encapsulates, on the other hand, drug molecules are embedded in hydrophobic polymer matrices in which formation of pro-drug is avoided, but such approach may result in poorly controlled drug loading and release.14,15 Although both approaches have resulted in a handful of systems in clinical development, alternative design of polymeric nanomedicines that can address drawbacks above mentioned are clearly of great interest.
We have been interested in developing new polymeric nanomedicine platform based on the assembly of hydrophobic polymer-drug conjugates. Through drug initiated controlled ring-opening polymerization of lactide and O-carboxyanhydrides,16–19 we developed a series of structurally well defined drug-polyester conjugates that can be prepared with high drug loadings, quantitative loading efficiency and sustained drug release profiles with negligible burst release effect. They can be further self-assembled to form NPs for drug delivery applications. The technique provides a highly efficient way to generate controlled polymeric drug NPs, and the drug release is dependent on ester linker hydrolysis.
An ideal polymeric drug conjugate would allow drug being released in an on-demand manner, i.e. drugs being released in response to triggers, especially those disease specific.20 A variety of trigger-responsive systems have been developed to maximize systemic efficacy in response to various triggers, such as pH,21–24 elevated temperature,25 redox,26–29 enzyme,30,31 external stimuli32 and combination of multiple triggers.33–38 Among these triggers, intracellular redox environment offers an ideal trigger condition for pro-drug design, which possesses 3 orders of magnitude higher glutathione level (~10 mM)39 as compared to extracellular environment (~10 μM). Simple disulfide bond or thioester can be cleaved efficiently by intracellular thiols40,41 and reductase42 while they are inert to many other reactions and chemical functional groups, facilitating the easy synthesis and chemical modification of disulfide containing materials.
We recently developed a polymeric delivery system termed as chain-shattering polymeric therapeutics (CSPTs) as an effective on-demand delivery system.43 Unlike post modified polymeric drug-conjugate, CSPTs have excellent control over both composition and structure, and well defined drug distribution among polymer chains. However, the hydrophobic polymer chain would self-aggregate in aqueous solution and require the assistance of amphiphilic polymers to form nano-sized particles for intracellular and in vivo delivery. The two-component nanoparticle (NP) formulation is inherently difficult to control since the particle size and drug loading are affected by both the polymer ratio and formulation condition. In this contribution, we report a thiol-responsive, PEGylated SS-CSPT prepared by ‘graft to’ strategy. The PEGylated SS-CSPT has a high drug loading (up to 18 %) and the particle size can be controlled simply by tuning the polymer concentration in organic solvent through nanoprecipitation. Drugs are released only in the presence of thiol trigger and the NPs show remarkable efficacy against cancer cells.
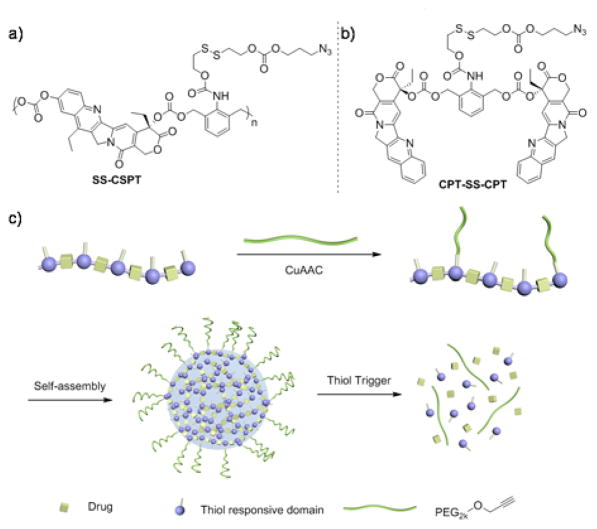
We designed a terminal azide group in the SS-CSPT side chain as a functional site for “Click” modification of PEG (Scheme 1a). The trigger-responsive domain was linked with a hydroxylethyl sulfide group, which underwent cyclization spontaneously to release an aniline structure. The unstable aniline can self-eliminate to release the conjugated drug molecules in a timely manner.43–45 SN-38, a camptothecin (CPT) derivative, was used as a model drug in our CSPT design while the mono-functional camptothecin was used to prepare a dimeric small molecule conjugate (CPT-SS-CPT) to study the release mechanism (Scheme 1b). SN-38 has been shown to be the active metabolite of clinically used irinotecan,46 but is limitedly used due to its poor water solubility and difficulty of encapsulation.
Scheme 1.
Chemical structure of thiol responsive SS-CSPT (a) and model small molecule CPT-SS-CPT (b). (c) Click PEGylation, self-assembly and triggered release of SS-CSPT.
Both CPT-SS-CPT and SS-CSPT can be prepared from the trigger responsive domain in one step (Scheme S1†). Since the backbone of the SS-CSPT polymer is inherently hydrophobic, we envisioned that incorporation of hydrophilic PEG chain into the polymer covalently would convert the polymer to be amphiphilic and assist the self-assembly thereafter. An arbitrary SS-CSPT/PEG weight ratio 2.5:1 was used in the copper-catalyzed azide-alkyne cycloaddition (CuAAC) to facilitate the further assembly as well as to maintain the high drug loading capacity. After the copper catalyst was removed by extensive dialysis in water, the obtained polymer showed bimodal distribution on GPC, which indicated the successful modification of the mPEG chain onto the polymer as the new peak had a short elution time compared with both unmodified SS-CSPT and PEG (Fig. S1†).
The self assembly behavior of the SS-CSPT-PEG was studied by nanoprecipitation method. Briefly, the SS-CSPT-PEG was first dissolved in DMF, slowly added into 40-fold water and the size of the CSPT-PEG NPs was characterized by dynamic light scattering (DLS) without further purification. As a result, the SS-CSPT-PEG readily formed NPs without any precipitation observed and was stable over 24 hours in water (Fig. S2†). Interestingly, the NP size depended linearly on the original CSPT concentration in DMF (Fig. 1a), which was similar to a drug-polylactide conjugate system reported previously.18,47 The NP size increased from 44 to 89 nm as the SS-CSPT-PEG concentration in DMF increased from 1.25 to 20 mg/mL while the polydispersity (PDI) ranged from 0.22 to 0.13. TEM image of the NPs (Fig. 1b) showed spherical morphology and slightly smaller size compared to the hydrodynamic volume measured by DLS. Drug loading of the CSPT NPs were determined to be 18% by HPLC after complete NaOH hydrolysis. For all the entries, the encapsulation efficiency ranged from 78 to 89 % (Table S1†). Salt effect on the particle formulation was also investigated using phosphate buffered saline (PBS) to replace water. Although the CSPT NPs formulated in water was stable after PBS dilution without significant size change, the particles formed in PBS directly had a larger size than that that in water at the same SS-CSPT-PEG concentration in DMF solution (112 nm compared to 65 nm). Similar observations have been reported in other polymeric NP formulations.18
Fig. 1.

(a) Nanoparticle size (hollow square) and size distribution (solid square) change as a function of SS-CSPT-PEG concentration in original DMF solution. (b) TEM image of CSPT nanoparticles formulated from 10 mg/mL DMF solution.
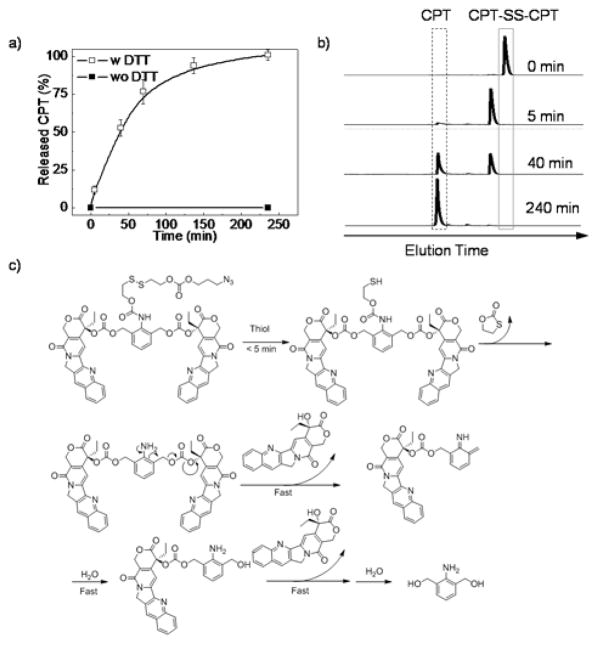
The release mechanism of the SS-CSPT was first studied using the dimeric model conjugate (CPT-SS-CPT) because the model dimeric conjugate and SS-CSPT share the same trigger-responsive domain and similar chemical structure (Scheme 1a–b). In the absence of thiol triggers, there was no drug release observed (Fig. 2a) while the disulfide bond was cleaved within 5 minutes in the presence of excessive dithiothreitol (DTT) (Fig. 2b). The intermediate gradually underwent thiol-cyclization over several hours and presumably yielded an unstable aniline structure, which was undetectable by HPLC and instantaneously release the carbonate bonded CPT through 1,4-self elimination (Fig. 2c). Overall, the original CPT was released quantitatively from the CPT-SS-CPT conjugate over 4 h without byproduct production (Fig. 2a).
Fig. 2.
(a) CPT release of CPT-SS-CPT in DMF/PBS triggered by 20 mM DTT. Data are presented as mean value±standard deviation of 3 measurements. (b) HPLC trace of DTT treated CPT-SS-CPT, λabs = 369 nm. (c) Proposed release mechanism of DTT triggered drug release.
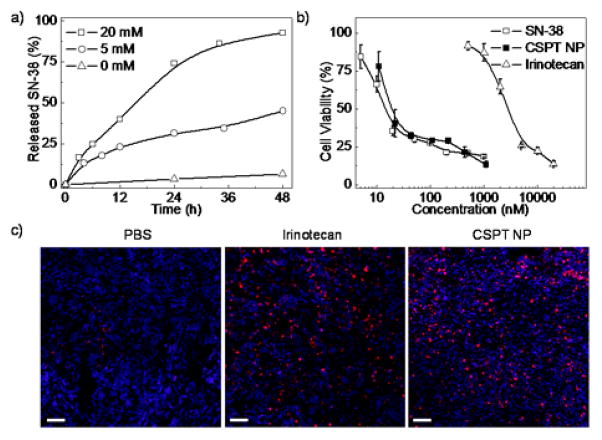
We next studied the drug release profile of the CSPT NPs upon thiol triggering. Considering the steric hindrance of the NPs, the thiol exchange step was limited by the diffusion of the thiol trigger, hence the first thiol cleavage would be much slower than that of CPT-SS-CPT, the model conjugate. Although free SN-38 drug release from the NPs was slower (Fig. 3a), the majority of the conjugated SN-38 could be released from CSPT NPs over 48 h indicating that the interior space of the NPs was accessible and sensitive to external environments. Notably, negligible SN-38 release was observed in the absence of trigger, while the release kinetics of the trigger-responsive CSPT NPs was much faster than the polymer-drug conjugates based on ester hydrolysis.48–52
Fig. 3.
(a) SN-38 release from CSPT NPs in the presence of DTT treatment. (b) Cytotoxicity of CSPT NPs in HeLa cells. Standard MTT protocol was followed by incubating drug solutions with cells for 72 hours. Data are presented as mean value±standard deviation of 3 measurements. (c) Representative images of (TUNEL) assay for apoptotic index analysis. Apoptotic cells were stained as red and cell nuclei were stained as blue. Scale bar: 100 μm.
The in vitro anticancer effect of the CSPT NPs was next evaluated by the standard MTT (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay to demonstrate the therapeutic potential of the delivery system. By incubating HeLa cells with the CSPT NPs for 72 h, significant cancer cell proliferation inhibition was observed (Fig. 3b). The IC50 value of the CSPT NPs was 19 nM, while free SN-38 and irinotecan, a clinically used CPT derivative, showed IC50 values of 14 nM and 2800 nM, respectively. The significant cytotoxicity of CSPT NPs indicated that the intracellular thiols and thiol reductase actively reduced the disulfide bond within the NPs, releasing SN-38 in a timely manner. We also did preliminary evaluation of the in vivo anticancer efficacy of the CSPT NPs against MCF-7 human breast cancer xenografts in athymic nude mice. MCF-7 cells were subcutaneously injected and three injections of PBS, irinotecan (50 mg/kg) and CSPT NPs (equivalent 20 mg/kg SN-38) were administered every four days after the tumor volume reached 100 mm3. Mice were sacrificed 3 days after the last injection (time course of the in vivo study see Fig S4a†) and tumors were collected to assess the antitumor efficacy of NPs. The apoptotic index of the tumors was analyzed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. CSPT NPs showed equivalent efficacy (8.6% vs. 5.3% of irinotecan) as compared to higher dose of irinotecan (Fig 3c and Fig. S4c).
In summary, we report a novel redox trigger-responsive CSPT. By copper catalyzed “Click” reaction post polymerization, we were able to introduce hydrophilic PEG chain onto the hydrophobic CSPT. The amphiphilic polymer could self-assemble into NPs. The size of CSPT NPs can be precisely controlled by the concentration of the CSPT in DMF prior to nanoprecipitation over a range of 40 to 90 nm; NPs are also very stable under physiological conditions. The formulated NPs can efficiently release the conjugated drugs through self elimination and chain shattering mechanism in the presence of thiol trigger. As revealed by MTT and TUNEL assay, the NPs showed cytotoxicity in vitro comparable to SN38 and anticancer efficacy in vivo over clinically used irinotecan. As the azide attached polymer side chain can be used via “Click” chemistry, targeting group can be readily introduced to the CSPT NPs. Our previous studies using silica NPs showed that ~50 nm could be most optimal size of nanomedicine;53,54 it is subject to further studies to demonstrate whether such size is optimal in other nanomedicine system, such as polymeric nanomedicine. Therefore, CSPT NP not only is a trigger-responsive nanomedicine for translational applications, but could also be an excellent system for fundamental studies, such as size-dependent in vitro and in vivo drug delivery, given its controlled size (Fig 1a).
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation (Career Program DMR-0748834 and DMR-1309525) and the National Institute of Health (NIH Director’s New Innovator Award 1DP2OD007246-01; 1R21CA152627). K. C. and Q. Y. were funded at UIUC from NIH National Cancer Institute Alliance for Nanotechnology in Cancer ‘Midwest Cancer Nanotechnology Training Center’ Grant R25 CA154015A.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details and characterizations of compounds, HPLC calibration curve of SN-38, TUNEL assay analysis of in vivo tumor cell apoptosis. See DOI: 10.1039/b000000x/
References
- 1.Ma L, Kohli M, Smith A. ACS Nano. 2013;7:9518–9525. doi: 10.1021/nn405674m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greco F, Vicent MJ. Adv Drug Deliv Rev. 2009;61:1203–1213. doi: 10.1016/j.addr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini A, Chen P. J Controlled Release. 2013;166:182–194. doi: 10.1016/j.jconrel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Pearson RM, Lee O, Lee CW, Chatterton RT, Khan SA, Hong S. Adv Funct Mater. 2014;24:2442–2449. [Google Scholar]
- 6.Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 7.Tanner P, Baumann P, Enea R, Onaca O, Palivan C, Meier W. Acc Chem Res. 2011;44:1039–1049. doi: 10.1021/ar200036k. [DOI] [PubMed] [Google Scholar]
- 8.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumura Y. Adv Drug Deliv Rev. 2008;60:899–914. doi: 10.1016/j.addr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Bromberg L. J Controlled Release. 2008;128:99–112. doi: 10.1016/j.jconrel.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Matsumura Y. Jpn J Clin Oncol. 2008;38:793–802. doi: 10.1093/jjco/hyn116. [DOI] [PubMed] [Google Scholar]
- 12.Christie RJ, Grainger DW. Adv Drug Deliv Rev. 2003;55:421–437. doi: 10.1016/s0169-409x(02)00229-6. [DOI] [PubMed] [Google Scholar]
- 13.Duncan R, Vicent MJ. Adv Drug Deliv Rev. 2010;62:272–282. doi: 10.1016/j.addr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Byrne JD, Napier ME, DeSimone JM. Adv Drug Deliv Rev. 2012;64:1021–1030. doi: 10.1016/j.addr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kita K, Dittrich C. Expert Opin Drug Deliv. 2011;8:329–342. doi: 10.1517/17425247.2011.553216. [DOI] [PubMed] [Google Scholar]
- 16.Tong R, Cheng J. Angew Chem Int Ed. 2008;47:4830–4834. doi: 10.1002/anie.200800491. [DOI] [PubMed] [Google Scholar]
- 17.Tong R, Cheng J. Bioconjugate Chem. 2010;21:111–121. doi: 10.1021/bc900356g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong R, Yala L, Fan TM, Cheng J. Biomaterials. 2010;31:3043–3053. doi: 10.1016/j.biomaterials.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong R, Cheng J. J Am Chem Soc. 2009;131:4744–4754. doi: 10.1021/ja8084675. [DOI] [PubMed] [Google Scholar]
- 20.Wang YP, Byrne JD, Napier ME, DeSimone JM. Adv Drug Deliv Rev. 2012;64:1021–1030. doi: 10.1016/j.addr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du JZ, Du XJ, Mao CQ, Wang J. J Am Chem Soc. 2011;133:17560–17563. doi: 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]
- 22.Chen CY, Kim TH, Wu WC, Huang CM, Wei H, Mount CW, Tian Y, Jang SH, Pun SH, Jen AK. Biomaterials. 2013;34:4501–4509. doi: 10.1016/j.biomaterials.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon YJ, James E, Shastri N, Frechet JMJ. Proc Natl Acad Sci U S A. 2005;102:18264–18268. doi: 10.1073/pnas.0509541102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aryal S, Hu CMJ, Zhang LF. ACS Nano. 2010;4:251–258. doi: 10.1021/nn9014032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owens DE, Jian YC, Fang JE, Slaughter BV, Chen YH, Peppas NA. Macromolecules. 2007;40:7306–7310. [Google Scholar]
- 26.Xu H, Cao W, Zhang X. Acc Chem Res. 2013;46:1647–1658. doi: 10.1021/ar4000339. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Tang L, Tu C, Song Z, Yin Q, Yin L, Zhang Z, Cheng J. Biomacromolecules. 2013;14:3706–3712. doi: 10.1021/bm401086d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei H, Volpatti LR, Sellers DL, Maris DO, Andrews IW, Hemphill AS, Chan LW, Chu DS, Horner PJ, Pun SH. Angew Chem, Int Ed. 2013;52:5377–5381. doi: 10.1002/anie.201301896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei H, Schellinger JG, Chu DS, Pun SH. J Am Chem Soc. 2012;134:16554–16557. doi: 10.1021/ja3085803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu L, Wang T, Perche F, Taigind A, Torchilin VP. Proc Natl Acad Sci U S A. 2013;110:17047–17052. doi: 10.1073/pnas.1304987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang S, Shao K, Liu Y, Kuang Y, Li J, An S, Guo Y, Ma H, Jiang C. ACS Nano. 2013;7:2860–2871. doi: 10.1021/nn400548g. [DOI] [PubMed] [Google Scholar]
- 32.Timko BP, Arruebo M, Shankarappa SA, McAlvin JB, Okonkwo OS, Mizrahi B, Stefanescu CF, Gomez L, Zhu J, Zhu A, Santamaria J, Langer R, Kohane DS. Proc Natl Acad Sci U S A. 2014;111:1349–1354. doi: 10.1073/pnas.1322651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Sun X, Mao W, Sun W, Tang J, Sui M, Shen Y, Gu Z. Adv Mater. 2013;25:3670–3676. doi: 10.1002/adma.201300929. [DOI] [PubMed] [Google Scholar]
- 34.Dai J, Lin S, Cheng D, Zou S, Shuai X. Angew Chem Int Ed. 2011;50:9404–9408. doi: 10.1002/anie.201103806. [DOI] [PubMed] [Google Scholar]
- 35.Wong S, Shim MS, Kwon YJ. J Mater Chem B. 2014;2:595–615. doi: 10.1039/c3tb21344g. [DOI] [PubMed] [Google Scholar]
- 36.Pornpattananangkul D, Zhang L, Olson S, Aryal S, Obonyo M, Vecchio K, Huang CM, Zhang LF. J Am Chem Soc. 2011;133:4132–4139. doi: 10.1021/ja111110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mo R, Jiang T, DiSanto R, Tai W, Gu Z. Nature communications. 2014;5:3364. doi: 10.1038/ncomms4364. [DOI] [PubMed] [Google Scholar]
- 38.Mo R, Jiang T, Gu Z. Angew Chem Int Ed. 2014;53:5815–5820. doi: 10.1002/anie.201400268. [DOI] [PubMed] [Google Scholar]
- 39.Schafer FQ, Buettner GR. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Zhao M, Feng F, Sizovs A, Wang J. J Am Chem Soc. 2013;135:10938–10941. doi: 10.1021/ja405261u. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, Lee JH, Jeon HM, Han JH, Park N, He Y, Lee H, Hong KS, Kang C, Kim JS. J Am Chem Soc. 2013;135:11657–11662. doi: 10.1021/ja405372k. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Duan D, Liu Y, Ge C, Cui X, Sun J, Fang J. J Am Chem Soc. 2014;136:226–233. doi: 10.1021/ja408792k. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Yin Q, Yin L, Ma L, Tang L, Cheng J. Angew Chem Int Ed. 2013;52:6435–6439. doi: 10.1002/anie.201300497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Ma L, Deng X, Cheng J. Polym Chem. 2013;4:224–228. [Google Scholar]
- 45.Miller K, Erez R, Segal E, Shabat D, Satchi-Fainaro R. Angew Chem Int Ed. 2009;48:2949–2954. doi: 10.1002/anie.200805133. [DOI] [PubMed] [Google Scholar]
- 46.Herben VM, Ten Bokkel Huinink WW, Schellens JH, Beijnen JH. Pharm World Sci. 1998;20:161–172. doi: 10.1023/a:1008613806051. [DOI] [PubMed] [Google Scholar]
- 47.Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, Levy-Nissenbaum E, Radovic-Moreno AF, Langer R, Farokhzad OC. Biomaterials. 2007;28:869–876. doi: 10.1016/j.biomaterials.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin Q, Tong R, Xu Y, Baek K, Dobrucki LW, Fan TM, Cheng J. Biomacromolecules. 2013;14:920–929. doi: 10.1021/bm301999c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin Q, Tong R, Yin LC, Fan TM, Cheng J. Polym Chem. 2014;5:1581–1585. doi: 10.1039/C3PY01245J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L, Sinclair A, Cao Z, Ella-Menye JR, Xu X, Carr LR, Pun SH, Jiang S. Small. 2013;9:3439–3444. doi: 10.1002/smll.201202727. [DOI] [PubMed] [Google Scholar]
- 51.Chan JM, Zhang LF, Tong R, Ghosh D, Gao WW, Liao G, Yuet KP, Gray D, Rhee JW, Cheng JJ, Golomb G, Libby P, Langer R, Farokhzad OC. Proc Natl Acad Sci U S A. 2010;107:2213–2218. doi: 10.1073/pnas.0914585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turturro S, Sunoqrot S, Ying HY, Hong S, Yue BYJT. Mol Pharmaceutics. 2013;10:3023–3032. doi: 10.1021/mp4001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang L, Yang X, Yin Q, Cai K, Wang H, Chaudhury I, Yao C, Zhou Q, Kwon M, Hartman JA, Dobrucki IT, Dobrucki LW, Borst LB, Lezmi S, Helferich WG, Ferguson AL, Fan TM, Cheng J. Proc Natl Acad Sci U S A. 2014;111:15344–15349. doi: 10.1073/pnas.1411499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang L, Gabrielson NP, Uckun FM, Fan TM, Cheng J. Mol Pharm. 2013;10:883–892. doi: 10.1021/mp300684a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.