Introduction
Accounting for only five percent of all renal and urothelial tumors, upper tract urothelial carcinomas (UTUC) are a rare genitourinary malignancy [1]. Although current management guidelines for UTUC advocate for radical nephroureterectomy (NUx) with formal resection of the bladder cuff as a gold standard treatment [2–4], the resultant solitary kidney status may lead to higher rates of dialysis, cardiovascular morbidity, and overall mortality [5–7]. In an effort to mitigate these attendant risks, nephron-sparing measures (NSM) have been advocated in carefully selected UTUC patients with the intention of achieving acceptable oncologic results [8–10].
While cancer staging of UTUC is commonly established with endoscopic biopsy at ureteropyeloscopy, inadequate tissue sampling and apprehensions of ureteral perforation render the accurate determination of tumor stage challenging [11]. As such, due to concerns regarding oncologic control, many clinicians are prompted to undertake radical extirpative treatment even in patients with low stage UTUC [4]. In contrast, experts advocate that UTUC patients with low grade, low stage disease may be candidates for nephron sparing strategies, including endoscopic ablation and segmental ureterectomy, provided they accept the necessity of rigorous post-procedure surveillance [2, 3, 8, 12, 13].
Contemporary studies have demonstrated the utility of individualizing patient management to arrive at the optimal UTUC treatment strategy [14–16]. Though previous reports have demonstrated that patients with low grade, low stage UTUC have been successfully managed with NSM, documentation on the oncologic efficacy of these non-extirpative measures largely has been limited to small institutional series with short-term patient follow-up [8–10, 17]. In this study, using data from a national cancer registry, our aim was to compare cancer specific and overall survival in patients treated with NUx and NSM for non-high grade, low stage UTUC. Given limitations of establishing tumor grade and stage in the clinical setting, these data afford an insightful assessment of outcomes in patients who were treated with non-extirpative measures for UTUC.
Materials and Methods
Using Surveillance, Epidemiology, and End Results (SEER) data, we identified all patients diagnosed with UTUC (codes C65.9 and C66.9) from 1992–2008. The SEER registries include those active from 1992, including Alaska natives, the metropolitan areas of San Francisco-Oakland, Detroit, Seattle, Atlanta, San Jose-Monterey, and Los Angeles county, as well as rural Georgia, Connecticut, Hawaii, Iowa, New Mexico, and Utah. The characteristics of the SEER population have been demonstrated to be a representative sample of the general population of the United States [18]. For each person diagnosed within these defined geographic areas, the SEER registries collect information of every occurrence of a primary incident cancer including the month and year of diagnosis and cancer site, stage, pathologic data including stage and histology, treatment modality, and vital status including cause of death for patients who died during follow-up.
All individuals with non-high grade, non-muscle invasive urothelial carcinoma were identified by determining all patients with localized disease through SEER Historic Staging (AJCC Stage T1). This method captures all cases of localized UTUC while excluding individuals with potentially more advanced disease observed through extent of disease codes 10 and 30. All patients with N+ and M+ disease were additionally excluded. Only patients with well differentiated (grade 1[G1]) and moderately differentiated (grade 2[G2]) tumors were included. For the purposes of this study, deaths from UTUC were coded as cancer specific events while all other deaths were considered other cause mortality.
Patients meeting inclusion criteria were stratified into two groups: those treated with NUx (pre-1998 codes 20, 30, 40, 50, 60 and 1998+ codes 40, 50, 80) and those managed through NSM (e.g. patients not undergoing nephrectomy or NUx). Demographic and clinical characteristics were compared between groups using ANOVA and chi-square tests. Cancer-specific mortality (CSM) and other-cause mortality (OCM) rates were determined using cumulative incidence estimators. Kaplan-Meier survival estimates were utilized to describe overall survival trends and compare both groups using a log-rank test for overall survival differences. Cox regressions were used for assessment of all-cause mortality (ACM) while Fine and Gray competing risks proportional hazards regressions were used to identify independent predictors of cancer specific death. Analyses were conducted using STATA version 10 (StataCorp, College Station, Texas), and R version two (The R Foundation for Statistical Computing, Vienna, Austria), with p-values of < 0.05 meeting statistical significance.
Results
Demographics and clinical characteristics of the 1227 patients (mean age 70.2±11.0 yrs, 63.2% male) meeting inclusion criteria are presented in Table 1. Of the patients, 26.1% (n=320, 65.6% male) underwent conservative management (62.5% segmental ureterectomy and 37.5% endoscopic treatment/observation) of non-high grade, low stage UTUC while 73.9% (n=907) underwent NUx. Patients treated through NSM tended to be older (71.6±10.6 vs. 69.7±11.1, p=0.007) with a greater proportion (26.3% vs. 18.0%, p=0.001) of G1 tumors when compared to patients treated with NUx. More patients that underwent NSM had prior non-UTUC cancer diagnoses than NUx patients (68.4% vs. 63.8%, p < 0.001). No differences between treatment groups were observed with respect to marriage status (p=0.90), gender (p=0.23), or race (p=0.51).
Table 1.
Demographic and clinical parameters for all upper tract urothelial carcinoma patients from 1992–2008 meeting inclusion criteria.
| n (%) Mean ± SD |
Total No. Observations, n=1227 |
NSM, n=320 (26.1) |
NUx, n=907 (73.9) |
p-value |
|---|---|---|---|---|
| Age, yrs | 70.2±11.0 | 71.6±10.6 | 69.7±11.1 | 0.007 |
| Gender | 0.23 | |||
| Male | 775 (63.2) | 210 (65.6) | 565 (62.3) | |
| Female | 452 (36.8) | 110 (34.4) | 342 (37.7) | |
| Marriage Status | 0.90 | |||
| Married | 468 (38.1) | 197 (61.6) | 562 (62.0) | |
| Unmarried | 759 (61.9) | 123 (38.4) | 345 (38.0) | |
| Race | 0.51 | |||
| White | 1073 (87.5) | 283 (88.4) | 790 (87.1) | |
| Afr. Am. | 44 (3.6) | 13 (4.1) | 31 (3.4) | |
| Other | 110 (8.9) | 24 (7.5) | 86 (9.5) | |
| Tumor grade | 0.001 | |||
| G1 | 247 (20.1) | 84 (26.3) | 163 (18.0) | |
| G2 | 980 (79.9) | 236 (73.7) | 744 (82.0) | |
(nephroureterectomy (NUx), nephron-sparing measures (NSM); (endoscopic therapy, segmental ureterectomy), observation), Afr. Am: African American, G1: Grade 1 (well-differentiated), G2: Grade 2 (moderately differentiated))
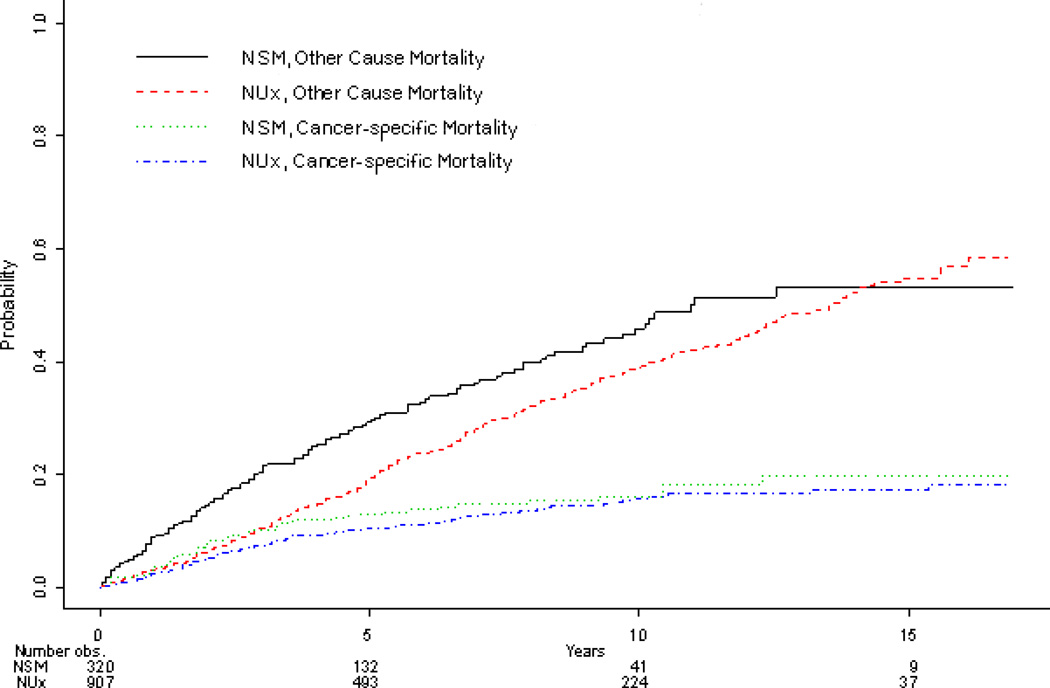
Median follow-up of all patients included for this analysis was 61 months (IQR 25-111). The cumulative incidence of death from UTUC and OCM are demonstrated in Figure 1. Although patients treated with NUx were less likely to die of other causes (p=0.009), CSM was similar (p=0.36) between treatment groups. Adjusting for clinical and pathologic characteristics, while increasing age (HR 1.02, CI 1.0-1.03, p=0.01) and female gender (HR 1.53, CI 1.11-2.11, p=0.01) were associated with CSM, no significant association was demonstrated for treatment type (HR 0.89, CI 0.63-1.26, p=0.50). Furthermore, year of diagnosis (p=0.64), marriage status (p=0.41), and race (p=0.59) were not associated with CSM.
Figure 1.
Adjusting for clinical and pathologic characteristics, patients undergoing radical surgery were less likely (HR 0.78, CI 0.64-0.94, p=0.009) to experience ACM compared to patients managed with NSM. Similarly, females (HR 0.83, CI 0.69-0.99, p=0.04) and married patients (HR 0.83, CI 0.69-0.99, p=0.04) were less likely to die of other causes. In comparison, older patient age (HR 1.07, CI 1.06-1.08, p < 0.001), and multiple prior cancer diagnoses (HR 1.25, CI 1.01-1.54, p=0.04) were significantly associated with an increased risk of ACM. Despite no demonstrable differences in CSM, UTUC patients with non-high grade, low stage disease undergoing NUx enjoyed an overall improvement in cancer specific death (log rank p < 0.001) compared to patients undergoing NSM (Figure 1).
Discussion
The present study is the first population-based analysis demonstrating comparable CSM in patients managed conservatively for low or moderate grade, non-invasive UTUC when compared to patients undergoing NUx. Utilizing such a national cancer registry to assess mortality trends is advantageous since UTUC is a rare disease with an annual incidence of one to two cases per 100,000 individuals in western countries [3]. With a poor overall prognosis, UTUC patients with ≥pT2 disease have a five-year overall survival of <50%, while patients with non-invasive disease, in comparison, have much higher rates of cure [19].
Management of UTUC patients has evolved greatly over the past two decades and now includes nephron-sparing strategies such as endoscopic ablation/segmental ureterectomy in addition to open and minimally invasive NUx. Previous efforts to define the role of non-extirpative treatment options in UTUC patients showed promising results in small series. In a seminal report on the ureteroscopic management of UTUC in patients with low or moderate grade, low stage disease, Chen and Bagley observed acceptable oncologic outcomes in 23 patients undergoing laser ablation with strict ureteroscopic surveillance [13]. Similarly, in a larger series of the endoscopic management of upper tract tumors, Gadzinski et al. reported equivalent five-year CSM in 34 patients who underwent endoscopic management to 62 patients that underwent NUx [8]. Additionally, in a SEER analysis reviewing outcomes of 569 segmental ureterectomy patients, Jeldres et al. demonstrated comparable CSM to NUx patients when stratified by pathologic stage [20].
As a result, the utilization of nephron sparing techniques such as endoscopic ablation and segmental ureterectomy has become an acceptable alternative in select non-high grade, low stage UTUC patients who are at low risk for disease progression [21, 22]. However, while the risk of cancer progression or recurrence is estimated to be as high as 30% within five years [8, 23], patients managed conservatively mandate close endoscopic surveillance as often as every three months. As such, endoscopic management has not been shown to adversely affect postoperative disease status in the event subsequent NUx becomes necessary [9].
The invasive surgical treatment of low or moderate grade, low stage UTUC is in stark contrast to the advocated treatment regimens of low stage renal cancer. Currently, nephron sparing surgery is recommended for the management of cT1 renal masses [24], as contemporary studies have suggested the deleterious effects of solitary kidney status such as increased rates of renal insufficiency [5–7, 25]. In a large multi-institutional analysis, Kaag and associates identified a reduction in mean eGFR by approximately 24% after NUx in patients with UTUC [26]. Thus, similar to radical nephrectomy in patients with parenchymal renal masses, patients undergoing NUx have notable decreases in renal function and resulting chronic kidney disease [26, 27]. Importantly, such a decline in renal function after radical surgery in UTUC patients may affect eligibility for adjuvant chemotherapy in the event of disease progression. Furthermore, given multifocal nature of urothelial carcinoma, patients following NUx are at significant life-long risk for tumor recurrence in the remaining solitary renal unit [28].
Our study demonstrates equivalent cancer specific outcomes in a large cohort of patients with low or moderate grade, low stage UTUC managed through nephron preservation compared to patients undergoing radical NUx. We employed a competing risks analysis for appropriate risk adjustment, given the fact that patients who underwent NSM were significantly older and were more likely to die of non-UTUC causes. In fact, our data suggest a strong selection bias for patients with shorter life expectancy undergoing NSM over NUx. In contrast, administrative datasets suggest a selection bias for non-nephron sparing approaches in patients with renal cell carcinoma [29]. This difference is likely due to increased expected perioperative morbidity for patients undergoing nephron-sparing surgery for renal cell carcinoma, while in UTUC it is in fact the radical resection, which exposes patients to highest perioperative risks [30]. Previous studies examining the oncologic efficacy of patients managed conservatively have been limited by small sample sizes, lack of generalizability, and inclusion of patients with aggressive disease characteristics. Using national registry data, our study suggests equivalent CSM in UTUC patients with low or moderate grade, low stage disease (n=1227 patients) undergoing NSM compared to patients undergoing NUx.
Our retrospective cohort study has important limitations that must be acknowledged when integrating these data into clinical management decisions. Characteristics inherent to SEER-based studies include a lack of patient specific comorbidity data, concomitant malignancy data, peri-procedural complication data, tumor anatomic data, baseline renal function, and surgeon preferences for treatment. Due to the limits of utilizing SEER coding data for non-extirpative UTUC surgical treatments, we relied on stratifying our cohorts solely through the performance of extirpative kidney surgery for the management of UTUC. As such, a small portion of patients who were included in the endoscopy group may not have received treatment. Further, SEER only captures and records the most advanced pathologic stage along a patient’s disease course, which limits our cohort to patients with documented low or moderate grade, low stage disease managed with conservative techniques who did not progress to more intensive therapy. Also, patients who were upstaged at segmental ureterectomy of NUx were excluded, while patients who underwent endoscopic management may have harbored more advanced grade/stage pathology. Despite these important limitations, our finding of acceptable oncologic control in patients who underwent nephron sparing management for low or moderate grade, low stage UTUC is relevant, since selection of patients for these non-extirpative treatments appears to have been appropriate even in a large administrative dataset.
Conclusions
We report that nephron sparing strategies (endoscopic ablation and segmental ureterectomy) in non-high grade, low stage UTUC in a population based analysis are more often performed for older patients who are more likely to die of other causes, but have acceptable cancer specific survival outcomes. These data may be useful in counseling patients with UTUC regarding treatment options. In the absence of level I evidence, non-extirpative management of UTUC should be informed by prudent clinical judgment.
Acknowledgments
This publication was supported in part by grant number P30 CA006927 from the National Cancer Institute (RGU). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The authors were supported in part through the National Institutes of Health R03CA152388 (BLE), and Department of Defense, Physician Research Training Award (AK).
Footnotes
All authors deny any financial and/or conflict of interest with the presented subject matter.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Raman JD, Scherr DS. Management of patients with upper urinary tract transitional cell carcinoma. Nat Clin Pract Urol. 2007;4(8):432–443. doi: 10.1038/ncpuro0875. [DOI] [PubMed] [Google Scholar]
- 3.Roupret M, Zigeuner R, Palou J, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011;59(4):584–594. doi: 10.1016/j.eururo.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115(6):1224–1233. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 5.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7(9):735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKiernan J, Simmons R, Katz J, Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59(6):816–820. doi: 10.1016/s0090-4295(02)01501-7. [DOI] [PubMed] [Google Scholar]
- 7.Zini L, Perrotte P, Capitanio U, et al. Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer. 2009;115(7):1465–1471. doi: 10.1002/cncr.24035. [DOI] [PubMed] [Google Scholar]
- 8.Gadzinski AJ, Roberts WW, Faerber GJ, Wolf JS Jr. Long-term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma. J Urol. 2010;183(6):2148–2153. doi: 10.1016/j.juro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Lucas SM, Svatek RS, Olgin G, et al. Conservative management in selected patients with upper tract urothelial carcinoma compares favourably with early radical surgery. BJU Int. 2008;102(2):172–176. doi: 10.1111/j.1464-410X.2008.07535.x. [DOI] [PubMed] [Google Scholar]
- 10.Roupret M, Hupertan V, Traxer O, et al. Comparison of open nephroureterectomy and ureteroscopic and percutaneous management of upper urinary tract transitional cell carcinoma. Urology. 2006;67(6):1181–1187. doi: 10.1016/j.urology.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Smith AK, Stephenson AJ, Lane BR, et al. Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: implications for conservative management. Urology. 2011;78(1):82–86. doi: 10.1016/j.urology.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Iborra I, Solsona E, Casanova J, et al. Conservative elective treatment of upper urinary tract tumors: a multivariate analysis of prognostic factors for recurrence and progression. J Urol. 2003;169(1):82–85. doi: 10.1016/S0022-5347(05)64041-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen GL, Bagley DH. Ureteroscopic management of upper tract transitional cell carcinoma in patients with normal contralateral kidneys. J Urol. 2000;164(4):1173–1176. [PubMed] [Google Scholar]
- 14.Clements T, Messer JC, Terrell JD, et al. High-grade ureteroscopic biopsy is associated with advanced pathology of upper-tract urothelial carcinoma tumors at definitive surgical resection. J Endourol. 2012;26(4):398–402. doi: 10.1089/end.2011.0426. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann J, Suttmann H, Kovac I, et al. Transitional cell carcinoma of the ureter: prognostic factors influencing progression and survival. Eur Urol. 2007;51(5):1281–1288. doi: 10.1016/j.eururo.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Tavora F, Fajardo DA, Lee TK, et al. Small endoscopic biopsies of the ureter and renal pelvis: pathologic pitfalls. Am J Surg Pathol. 2009;33(10):1540–1546. doi: 10.1097/PAS.0b013e3181aec42a. [DOI] [PubMed] [Google Scholar]
- 17.Silberstein JL, Power NE, Savage C, et al. Renal function and oncologic outcomes of parenchymal sparing ureteral resection versus radical nephroureterectomy for upper tract urothelial carcinoma. J Urol. 2012;187(2):429–434. doi: 10.1016/j.juro.2011.09.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surveillance, Epidemiology, and End Results (SEER) Research Cancer Data (1973 – 2007), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2010, based on the November 2009 submission. Program ( www.seer.cancer.gov).
- 19.Abouassaly R, Alibhai SM, Shah N, Timilshina N, Fleshner N, Finelli A. Troubling outcomes from population-level analysis of surgery for upper tract urothelial carcinoma. Urology. 2010;76(4):895–901. doi: 10.1016/j.urology.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Jeldres C, Lughezzani G, Sun M, et al. Segmental ureterectomy can safely be performed in patients with transitional cell carcinoma of the ureter. J Urol. 2010;183(4):1324–1329. doi: 10.1016/j.juro.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Pohar KS, Sheinfeld J. When is partial ureterectomy acceptable for transitional-cell carcinoma of the ureter? J Endourol. 2001;15(4):405–408. doi: 10.1089/089277901300189439. [DOI] [PubMed] [Google Scholar]
- 22.Elliott DS, Segura JW, Lightner D, Patterson DE, Blute ML. Is nephroureterectomy necessary in all cases of upper tract transitional cell carcinoma? Long-term results of conservative endourologic management of upper tract transitional cell carcinoma in individuals with a normal contralateral kidney. Urology. 2001;58(2):174–178. doi: 10.1016/s0090-4295(01)01109-8. [DOI] [PubMed] [Google Scholar]
- 23.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52(4):594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 24.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Go AS, Chertow GM, Fan D, McCullouch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 26.Kaag MG, O'Malley RL, O'Malley P, et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 2010;58(4):581–587. doi: 10.1016/j.eururo.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane BR, Smith AK, Larson BT, et al. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer. 2010;116(12):2967–2973. doi: 10.1002/cncr.25043. [DOI] [PubMed] [Google Scholar]
- 28.Kang CH, Yu TJ, Hsieh HH, et al. The development of bladder tumors and contralateral upper urinary tract tumors after primary transitional cell carcinoma of the upper urinary tract. Cancer. 2003;98(8):1620–1626. doi: 10.1002/cncr.11691. [DOI] [PubMed] [Google Scholar]
- 29.Smaldone MC, Egleston B, Uzzo RG, Kutikov A. Does partial nephrectomy result in a durable overall survival benefit in the medicare population? J Urol. 2012;188(6):2089–2094. doi: 10.1016/j.juro.2012.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutikov A, Smaldone MC, Egleston BL, Uzzo RG. Should partial nephrectomy be offered to all patients whenever technically feasible? Eur Urol. 2012;61(4):732–734. doi: 10.1016/j.eururo.2011.12.014. [DOI] [PubMed] [Google Scholar]