Abstract
Purpose
Bone is a preferential site of breast cancer metastasis and models are needed to study this process at the level of the microenvironment. We have used bioluminescence imaging (BLI) and multiplex biomarker immunoassays to monitor dynamic breast cancer cell behaviors in co-culture with human bone tissue.
Procedures
Femur tissue fragments harvested from hip replacement surgeries were co-cultured with luciferase-positive MDA-MB-231-fLuc cells. BLI was performed to quantify breast cell division and track migration relative to bone tissue. Breast cell colonization of bone tissues was assessed with immunohistochemistry. Biomarkers in co-culture supernatants were profiled with MILLIPLEX® immunoassays.
Results
BLI demonstrated increased MDA-MB-231-fLuc proliferation (p<0.001) in the presence vs. absence of bones, and revealed breast cell migration toward bone. Immunohistochemistry illustrated MDA-MB-231-fLuc colonization of bone, and MILLIPLEX® profiles of culture supernatants suggested breast/bone crosstalk.
Conclusions
Breast cell behaviors that facilitate metastasis occur reproducibly in human bone tissue co-cultures and can be monitored and quantified using BLI and multiplex immunoassays.
Introduction
Bone is a frequent site of metastasis in breast cancer patients and the development of informative models to study skeletal metastasis remains an ongoing challenge [1-2]. Although in vivo mouse models enable study of the metastatic process within the context of whole body physiology, drawbacks include the prolonged time course and relative inefficiency of metastatic colonization of human breast cancer cells into the murine skeletal system, coupled with the inability to perturb and monitor detailed events at the cellular level within the microenvironment [3-6]. In vitro models based on the co-culture of breast cancer cells with bone marrow-derived stromal cells or osteoblasts facilitate the analysis of specific cell interactions, but exclude many critical components of the bone microenvironment [7-17]. Bone is a complex tissue, encompassing ossified bone and marrow compartments that house multiple cell types contributing to the metastatic niche [18-19]. Additional approaches are needed that will provide direct access to these compartments and enable the rapid quantification of dynamic interactions within them. We have established an optical imaging approach to monitor the dynamics of luciferase-expressing human breast cancer cells (MDA-MB-231-fLuc) co-cultured with human bone fragments isolated from discarded total hip replacement (THR) surgical specimens. Here we describe the use of serial bioluminescence imaging (BLI) to quantify breast cancer cell proliferation, track breast cell migration toward bones, and monitor breast cell colonization of bone tissue over time, revealing highly consistent behaviors. Because the cell-tissue interactions occur over relatively short periods of time (hours to days), the immediate quantitation afforded by BLI provides a rapid readout of cell function, enabling the direct, rapid study of dynamic processes within the metastatic niche. These co-cultures also provide ready access to factors, cells and tissues for analysis, including quantification of protein biomarkers in co-culture supernatants by MILLIPLEX® MAP magnetic bead immunoassays and post culture immunohistochemical staining. Our work demonstrates the utility for using human bone tissues in a co-culture model to characterize and target breast cancer cell behaviors within the 3-dimensional architecture of the native metastatic niche.
Methods
Human Femur Tissue
Femoral heads were collected from patients undergoing elective total hip replacement (THR) in the Department of Orthopaedic Surgery at the Stanford University Medical Center. All tissues were collected as de-identified specimens in accordance with the Stanford University Research Compliance Office. Discarded femoral head specimens were transported to the lab within 1-2 hours (h) of surgery, and placed into a Pyrex dish (VWR, Radnor, PA) (Fig. 1a). Using a sterile glove to hold the femoral head with one hand, cancellous bone fragments measuring ∼3-5 millimeter (mm)2 were dissected from the shaft using a surgical Rongeur (Fine Science Tools, Foster City, CA) for placement into co-culture experiments. Viability of the marrow compartment was determined by trypan blue exclusion at time 0, 24h, 48h, and 72h following marrow depletion of bone fragments isolated from 3 patient specimens (THR 23, 24, and 25), as described below.
Figure 1.
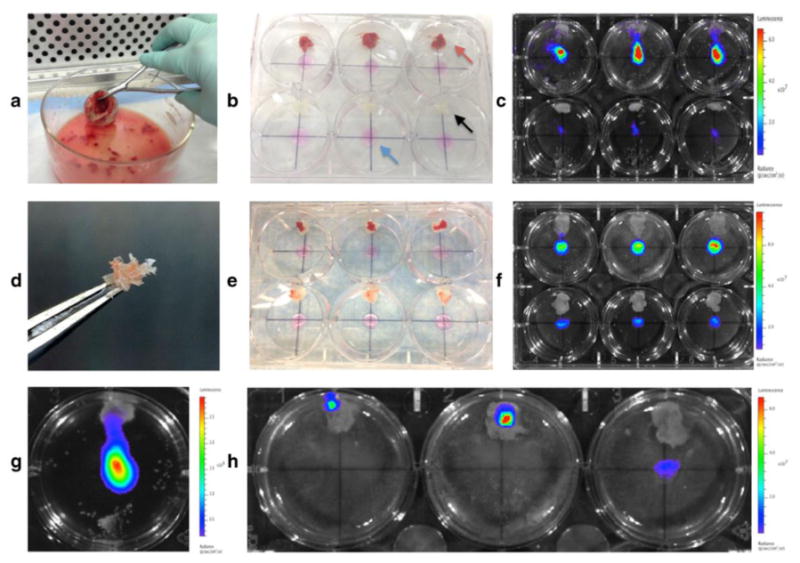
Co-culture design and bioluminescence imaging. (a) Femoral head from human total hip replacement surgery. (b) Co-culture plate before addition of media, showing MDA-MB-231-fLuc cell spots at center of all wells (blue arrow), bone wax pieces at the 12 o'clock position for immobilizing bone fragments (black arrow), and cancellous bone fragments attached to bone wax in top 3 wells (red arrow). (c) BLI of co-culture plate with (top row) vs. without (bottom row) bone fragments at 24h. (d) Marrow depleted bone fragment. (e) Co-culture plate before addition of media, showing MDA-MB-231-fLuc cell spots at center of all wells, with marrow-intact (top row) and marrow-depleted (bottom row) bone fragments attached to bone wax. (f) BLI of co-culture plate with marrow-intact (top row) and marrow-depleted (bottom row) bone fragments at 24h. (g) BLI showing migration of cells in 1 well toward bone fragment tethered at the 12 o'clock position. (h) BLI of co-culture at 96 h, showing signal associated with bone fragments in left and center wells.
Breast Cancer Cells
MDA-MB-231 breast cancer cells were purchased from the American Type Culture Collection (ATCC; Rockville, MD) and engineered for the stable expression of firefly luciferase using the Sleeping Beauty transposon plasmid pKT2/PGK-BSD:GFP_CLP-Luc (MDA-MB-231-fLuc). The original transposon was kindly provided by Drs. Scott McIvor, Univ. of Minnesota, Minneapolis, MN, and Andrew Wilber, Department of Surgery and Simmons Cancer Institute, Southern Illinois Univ. School of Medicine, Springfield, IL), and further modified in our laboratory to express the luciferase reporter and enhanced green fluorescence green protein (eGFP) [20].
Co-cultures
Co-cultures were performed in 6-well cell culture plates (Corning, Inc., NY, USA) by seeding breast cancer cells adjacent to bone fragments immobilized by bone wax (Surgical Specialties Corporation, Reading, PA) (Fig. 1b). A small (35 milligram; mg) piece of bone wax was affixed at the 12 o'clock position of all experimental and control wells as shown in Figure 1b. Breast cell spots (circumscribed areas of MDA-MB-231-fLuc cells measuring 3-mm in diameter) were established by pipetting 50 μL drops of complete cell culture medium containing 1 × 105 cells into the center of each dry well. Plates were then placed in a 37°C cell culture incubator for 45 minutes to promote cell attachment during confinement as an isolated droplet. A consistent time frame was used for all experiments to ensure uniform cell adherence in all wells, as confirmed by cell counts in preliminary experiments. Once the majority of breast cancer cells were adherent as a confined spot, bone fragments were secured onto the pieces of bone wax, using a sterile glove to apply gentle pressure. Five milliliters (mL) of (DMEM (1X) + GlutaMAX ™ supplement (Gibco, Life Technologies, USA) + 10% FBS (Gibco, Life Technologies, USA) were then pipetted into each well and plates were placed in a humidified 37°C tissue culture incubator with 5% CO2. All experiments were performed with the same lot of fetal bovine serum. For experiments set up for MILLIPLEX® analysis, additional wells containing bone fragments without breast cells were also initiated. To confirm breast cell colonization of bone tissues, 1 × 105 MDA-MB-231-fLuc cells were pipetted directly onto the top surface of bone tissues, cultured for 72 h, and processed for immunohistochemistry.
Bone Marrow Depletion
For some comparisons the marrow was flushed from the bone fragments by agitating them in three successive Petri dishes containing PBS. During each rinse, individual bone fragments were immobilized with a pair of tweezers and flushed with PBS using a 10 ml syringe and 27-guage needle until most of the red coloration disappeared (Fig. 1d). Figure 1e shows an experimental plate containing marrow-intact fragments (top row) and marrow-depleted fragments (bottom row).
Optical Imaging
Bioluminescence imaging was performed on co-culture plates in an IVIS 50 Imaging Platform (Caliper Life Sciences, Inc., Hopkinton, MA) immediately following addition of luciferin substrate (150 μg/ml) to all wells at time 0, 24, 48, 72, and 96 h. Imaging parameters were F-stop of 1, small binning, level D, 1 second exposure, and signal intensity was measured as average radiance (photons/seconcd/cm2/steradian). Average radiance was measured and quantified for all individual wells designated as regions of interest (ROI) within each plate using the Living Image Software Program (Version 4.3.1).
Culture Supernatant Protein Biomarker Analysis
Multiplex immunoassays were performed on 24-h supernatants collected from: 1) breast cell cultures, bone tissue cultures, and breast/bone co-cultures in 6-well plates, and 2) 24-h cultures of intact vs. marrow-depleted bone fragments in 24-well plates. Aliquots of 0.5 ml of conditioned media were collected into microfuge tubes and centrifuged for 5 minutes at 13,000 rpm on a tabletop micro-centrifuge at room temperature (RT). Supernatants from these centrifugations were stored at -80oC until analysis. Supernatants were analyzed using MILLIPLEX® MAP magnetic bead immunoassay kits (EMD Millipore, Billerica, MA) based on the Luminex® xMAP® technology. Protein biomarkers in breast/bone supernatants were quantitatively determined using MILLIPLEX® MAP Human Cytokine/Chemokine Panels I and II, Human Bone Panel, Human Circulating Cancer Biomarker Panel 1, Human Angiogenesis/Growth Factor Panel, and Human Cancer/Metastasis Biomarker Panel 1 (EMD Millipore, Billerica, MA) as per the manufacturer's instructions. In brief, 25 uL of supernatant samples (or standards) were incubated with 25 μL of magnetic beads conjugated with capture antibodies in a 96-well plate overnight at 4°C. On day 2, the beads were washed, 25- or 50 μL biotinylated detection antibody cocktail were added and the assay plates were incubated at RT for 1 h. Then, 25 μL streptavidin-phycoerythrin (SAPE) were added for a further 30 min incubation. The beads were then washed and resuspended in 100 uL buffer to read on a Luminex® 200™ Instrument. The sample data were analyzed with the above listed MILLIPLEX® panels using MILLIPLEX® Analyst 5.1 software.
Histological Processing
Bone tissue fragments were fixed in 10% buffered formalin, decalcified, and processed for paraffin embedding and slide preparation. Tissue sections were stained with Hematoxylin & Eosin (H &E) or processed for immunohistochemical analysis using a monoclonal mouse anti-human cytokeratin antibody (Clone AE1/AE3) (Dako Cytomation, Carpenteria, CA).
Statistical Analysis
Imaging data are expressed as average radiance (photons/second/cm2/sr) +/- SE. Values across co-culture conditions (bone vs. no bone, and marrow intact vs. marrow depleted) were compared using a one-tailed Student's t-test. P<0.05 was considered to be statistically significant.
Results
Growth of MDA-MB-231-fLuc Cells in Co-Cultures
MDA-MB-231-fLuc cell proliferation in the presence vs. absence of bone tissues was determined by levels of luciferase activity in each well. A reproducibly higher BLI signal (indicating higher cell numbers) was observed when breast cancer cells were co-cultured with bone, as shown in Figure 1c. While BLI signal was equivalent at time zero (Figure 2c), reflecting uniform breast cell seeding, quantitative BLI analysis at 24 h confirmed significantly increased luciferase activity from breast cells in the presence vs. absence of bones, as illustrated for THR specimens 2, 3 and 4 in Figure 2a (P < 0.001). As shown in Figure 2f, MDA-MB-231-fLuc cell proliferation was also significantly greater, as assessed by bioluminescent signal intensity, in the presence of intact vs. marrow-depleted bone fragments in co-cultures with THR specimens 5 (P <0.05), 9 and 10 (P<0.01). Increased cell division in the presence of bone fragments was observed in co-cultures maintained with, and without, regular media changes. Figure 2c shows quantitative analysis of BLI generated by co-cultures with THR specimens 13 and 14 at 48 h, illustrating growth enhancement patterns in cultures with (+) and without (-) media change at 24 h.
Figure 2.
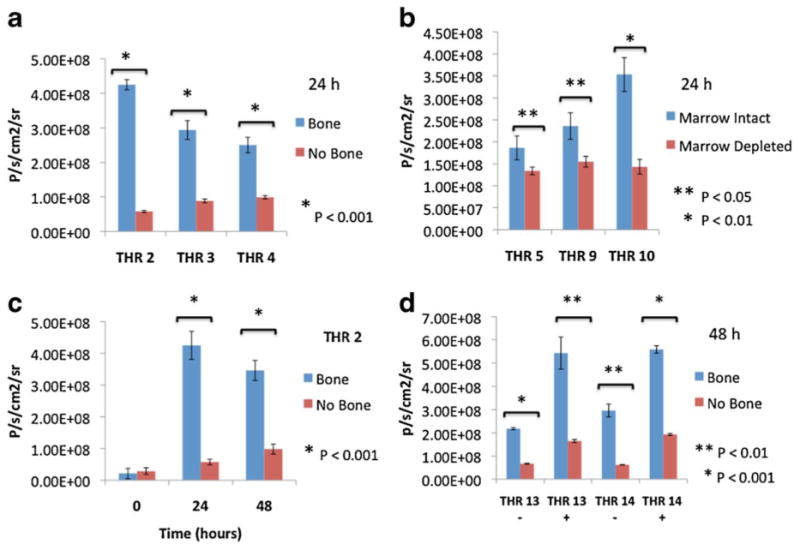
Quantitative BLI analysis of co-cultures. (a) Quantitative analysis of co-culture BLI signal at 24 h illustrating a significant increase in MDA-MB-231-fLuc breast cancer cell proliferation when grown in the presence vs. absence of human bone fragments from 3 different total hip replacement patients (THR 2, THR3 and THR 4) (P < 0.001). (b) Quantitative analysis of co-culture BLI signal at 24 h illustrating significant reduction of MDA-MB-231-fLuc cell proliferation in the presence of marrow-depleted vs. intact bone fragments from 3 different THR patients (P <0.05 and P< 0.01). (c) Quantitative BLI analysis of THR 2 co-culture over time showing significant differences in signal at 24h and 48h (P < 0.001), but not at time 0, illustrating uniform seeding of MDA-MB-231 cells. (d) Quantitative analysis of BLI signal for 2 co-cultures (THR 13 and THR 14) at 48 h without (-) and with (+) media replacement at 24 h. The demonstration of growth enhancement in the presence of bone following media replacement suggests mediation by factors produced during co-culture. Each bar represents average BLI values measured across 9 (a and c) or 3(b and d) separate supernatant/bone fragments/wells for a given condition or time point. Error bars represent standard error and asterisks denote statistically significant differences.
Migration of MDA-MB-231-fLuc Cells Toward Bone Fragments
BLI of co-culture dishes suggested the directed migration of MDA-MB-231-fLuc cells toward the bone fragments, as shown in Figures 1g and h. Figure 1g shows BLI of THR specimen 11 at 48 h, and f shows BLI of THR specimen 11 at 96 h illustrating dramatic relocation of signal from the central location of the plastic dish to the 12 o'clock position harboring the bone fragments in 2 of the 3 wells.
Histology of Bone Fragments and Immunhistochemical Analysis of Co-Cultures
Hematoxylin & Eosin (H & E) staining of bone fragment tissue sections at time 0 revealed typical cancellous bone architecture consisting of a lattice of ossified bone spicules (with associated osteocytes, and osteoblasts and osteoclasts) throughout the marrow compartment (Fig. 3a). Marrow cells including hematopoietic (red and white blood cell progenitors) and stromal cells (with numerous adipocytes) were present within the lattice of bone spicules. Post co-culture immunohistochemical staining of bone fragments at 72 h with anti-cytokeratin antibody revealed infiltration of MDA-MB-231-fLuc cells into the marrow compartment (Fig. 3a) and onto ossified bone surfaces (Fig. 3b).
Figure 3.
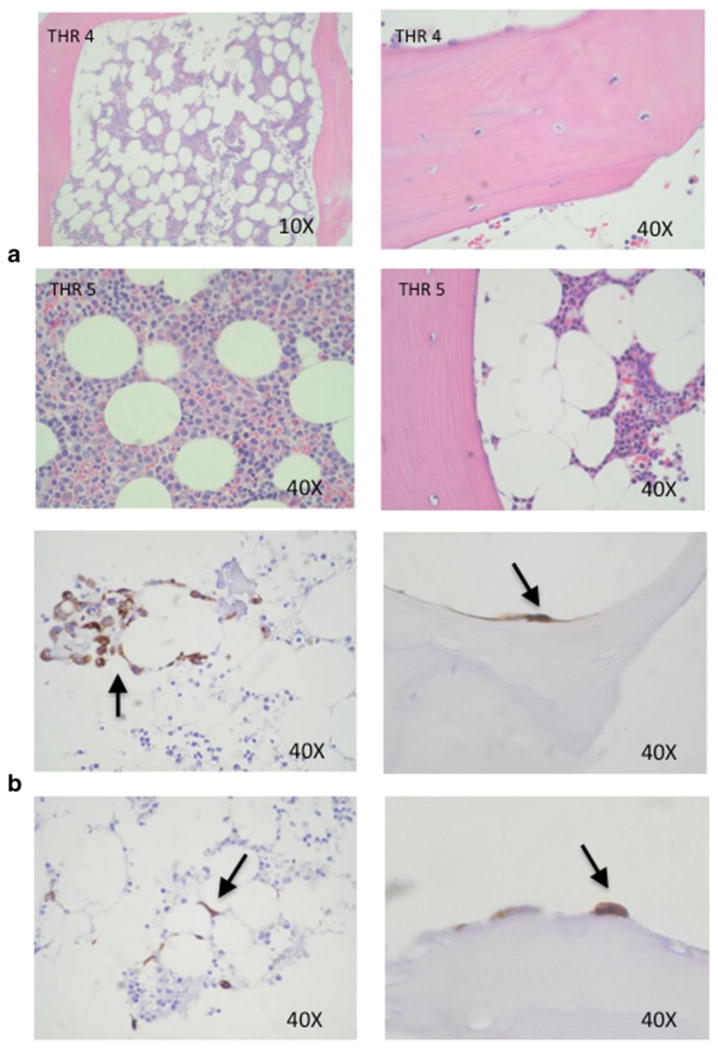
Breast cancer cell colonization of bone tissue. (a) H & E stained histological sections of human bone tissue specimens before culture. (b) Immunohistochemical staining of bone fragments after co-culture with MDA-MB-231-fLuc cells for 72 h with cytokeratin antibody reveals breast cancer cell colonization of the marrow compartment (left panels), and attachment to ossified bone surfaces (right panel).
Viability of Bone Marrow Compartment
Average viability observed for marrow cells flushed from bone fragments originating from 3 separate patients specimens were 72.3% (time 0), 69.5% (24h), 69.6% (48h), and 63.9% (72h).
MILLIPLEX® Analysis
Analysis of supernatants demonstrated the presence of bone-associated growth factors, cytokines and chemokines in cultures of bone alone, but at dramatically lower levels in most cultures of breast cells alone. In many cases the levels of these factors were dramatically higher when bones were co-cultured with breast cancer cells, as illustrated in Figure 4. Comparison of a panel of factors in supernatants collected from intact vs. marrow-depleted bone fragments revealed dramatic reductions in the levels of many cytokines, chemokines and growth factors as shown in Table 1.
Figure 4.

Protein biomarker profiles of co-culture supernatants from THR specimen 6. Profiles of MDA-MB-231-fLuc breast cancer cell culture (BrC), bone tissue culture (bone), and breast/bone co-culture (BrC/bone) supernatants at 24 h as characterized using MILLIPLEX® MAP Panels. MILLIPLEX® analysis demonstrates the ability to assay high numbers of biomarkers in a single supernatant, and detect bone-associated factors including osteocalcin, interleukin-6 (IL-6), tartrate-resistant acid phosphatase 5 (TRAP 5), osteopontin (OPN), and osteoprotegerin (OPG). (b) Profiles of control supernatants (media, FBS, and bone wax only), and supernatants from bone tissue cultures (without breast cells) at 24 and 48h. These results illustrate the minimal contribution of serum to the biomarker profiles. Each bar represents a biomarker value for 1 supernatant/bone fragment/well.
Discussion
Breast cancer metastasis
Most breast cancers originate from the epithelial cells lining the ductal-alveolar structures of the mammary gland in a series of steps characterized by localized proliferation. The metastatic process unfolds when breast cells invade the surrounding stroma, gain access to blood or lymphatic vessels, and survive transit through the circulation to extravasate into distant organs to seed micro-metastatic colonies. The most common site of distant breast cancer metastatic spread is bone [1-2], and the presence of rare disseminated tumor cells (DTCs) in bone marrow aspirates at initial diagnosis is associated with poor prognosis [21-26]. Micro-metastatic cells have the capacity to remain dormant for many years, and harbor varying potential for generating clinically detectable metastatic disease responsible for most breast cancer-associated mortality [27-29]. Clinically detectable bone metastasis involves extensive breast cancer cell colonization of the skeleton through complex interactions with bone cells. These interactions include the release of breast cancer cell factors that stimulate osteoclasts to degrade mineralized bone matrix, leading to the release of factors that stimulate breast cancer cell growth in what has been termed the “vicious cycle of metastasis” [30], and fueling malignant growth along with the widespread destruction of bone tissue, disruption of mineral homeostasis, pain, and fractures.
Bone Tissue Co-Culture Model
The co-culture of viable bone tissues with cancer cells offers a model system for characterizing and targeting these interactions within the 3-dimensional architecture of the native bone microenvironment, and others have demonstrated the utility of this approach using mouse bones. Schiller et al. developed a model in which whole neonatal mouse femurs were co-cultured with a variety of mouse and human cancer cell lines, demonstrating bone tissue viability and illustrating synergistic paracrine interactions between intact bone and tumor cells with enzyme-linked immunosorbent assay (ELISA) [31]. Curtin et al. developed a roller tube model system to co-culture mouse calvarial bones with breast and prostate cancer cell lines to demonstrate cancer cell colonization of the endosteal bone surfaces and subsequent promotion of osteoclast activity resulting in bone resorption [32]. Others have cultured mouse bones harvested from breast cancer xenograft models to examine cytokine production of mouse bones colonized by breast cancer cells [17, 33]. These mouse studies demonstrate the feasibility of bone co-culture models to simulate the microenvironment and study dynamic cell interactions within the bone metastatic niche. However, Kuperwasser et al. demonstrated that human breast cancer cells preferentially home to human bone fragments implanted in mice, suggesting species-specific osteotropism and reinforcing the need to study these interactions in human bone tissues [5].
We have developed a co-culture model using human bone tissue fragments isolated from total hip replacement surgery specimens and have established proof-of-principle for using optical imaging approaches to monitor the dynamics of luciferase-positive human breast cancer cells (MDA-MB-231-fLuc) co-cultured with them. Serial bioluminescence imaging (BLI) was performed to quantify breast cancer cell growth, track breast cell migration toward bones, and monitor breast cell colonization of bone tissue over time. We observed consistent patterns of breast cancer cell behavior indicating that novel interventions that disrupt this behavior could be readily studied in this model. MDA-MB-231-fLuc cell proliferation was reproducibly increased by the presence of bone fragments, and depletion of the marrow compartment resulted in reduced breast cell proliferation. MDA-MB-231-fLuc cell migration toward bone fragments was also observed with BLI, and breast cancer cell colonization of bone tissues was confirmed with immunohistochemical staining, which revealed cytokeratin-positive breast cancer cells within the marrow and ossified compartments. Interestingly, the MDA-MB-231-fLuc cells were often seen nestled specifically between the adipose cells of the marrow, resembling patterns reported for metastatic ovarian cancer cells within the omentum [34].
The breast cancer cell behaviors observed with BLI suggested mediation by paracrine interactions. To determine if we could detect bone-associated factors in our co-culture supernatants we used previously-established MILLIPLEX® panels for bone, cytokine, cancer, metastasis, and angiogenesis markers. This broad interrogation revealed the presence of bone-associated factors, including osteocalcin (secreted by osteoblasts), osteopontin (OPN, synthesized by osteoblasts and osteocytes), tartrate-resistant acid phosphatase 5 (TRAP 5, produced by osteoclasts), and interleukin-6 (IL-6, produced by osteoblasts). Interestingly, we detected high levels of osteoprotegerin (OPG) in supernatants from breast cells cultured in the absence of bone. OPG is a member of the tumor necrosis factor receptor (TNFR) superfamily, secreted by osteoblasts to inhibit osteoclast formation. Along with RANK (receptor activator of nuclear-kβ), and RANKL (RANK ligand), which regulate osteoclast activation and bone turnover, OPG is also produced by the mammary gland to regulate calcium release from the skeleton during lactation [35-36], and its expression has been reported in breast tissues and breast cancer cell lines, including MDA-MB-231 cells [37-39].
Analysis of supernatants revealed the up-regulation of bone-associated factors in the presence of breast cancer cells, suggesting active cross-talk during co-culture. Depletion of the marrow compartment resulted in reduced breast cell proliferation, in keeping with dramatic reductions in the levels of cytokines, chemokines, and growth factors in the co-culture supernatants, as detected by MILLIPLEX® analysis. Because there is considerable overlap between the constellation of cytokines, chemokines and growth factors that mediate cancer progression and wound healing, it is possible that factors released in response to the surgical removal of bone tissues could mediate some of the observed responses. However, we observed differential growth responses with serial BLI through 96 h of culture, even when tissue culture media were replaced at 24 h intervals, suggesting the active production and release of factors during culture. Our preliminary secretome analysis establishes proof-of-principle for future mechanistic studies of paracrine interactions in our co-culture model.
Study Limitations
Thus far we have studied cancer cell/bone interactions during relatively short time periods for up to 96 h, and our co-culture model has not yet been defined and optimized for extended bone viability. In keeping with other reports, we observed a decline in marrow viability after 48 hours as revealed by trypan blue exclusion measurement and appearance in histologic section [31]. An important next step will involve the systematic optimization of conditions for long-term culture. In addition, although blood vessels are present throughout the bone tissue, potentially enabling angiocrine interactions [40], the lack of a functioning circulatory system prevents the study of breast cell extravasation with this model.
Conclusions
The proximal femur is a common site of breast cancer metastasis [41], and total hip replacement surgeries constitute a valuable, renewable source of non-diseased tissues from this region for modeling in co-culture. These specimens harbor both the marrow and ossified compartments necessary to study mechanisms underlying micro-metastasis, as well as clinically detectable metastatic disease. The specimens in this study originated from both male and female patients of various ages, both of which may contribute to the variability observed across specimens. However, BLI has revealed highly reproducible patterns of breast cell/bone tissue interactions within these cultures, consistently demonstrating enhanced breast cell proliferation in the presence vs. absence of bone, and attenuation of signal following bone marrow depletion. Because of the accelerated time course of interactions, and the immediate quantitation afforded by BLI, this approach enables the direct, rapid study of dynamic processes within a simulated metastatic niche. This approach circumvents the prolonged time course associated with in vivo metastasis models, offering the potential for high throughput perturbation of specific targets to identify and evaluate therapeutic interventions to prevent and treat bone metastasis.
Figure 5.

Protein biomarker levels attenuated by bone marrow depletion. Three THR specimens (9, 10 and 11) were used for the comparison of biomarker levels in supernatants from cultures with intact (blue bars) vs. marrow-depleted (red bars) bone fragments at 24h using MILLIPLEX® analysis. Results are shown for 8 biomarkers including macrophage migration inhibitory factor (MIF), sFAS, monocyte-specific chemokine 3 (MCP3), hepatocyte growth factor (HGF), fibroglast growth factor 2 (FGF2), neuron specific enolase (NSE), YKL-40 and TNF-related weak inducer of apoptosis (TWEAK) demonstrating 2-fold or greater attenuation following marrow depletion. Each bar represents the average of duplicate analyses of a given supernatant/bone fragment/well for specimens THRs 9 and 10 and three of the analytes measured for THR 11 (MIF, sFAS and MCP3). The remaining analytes measured for THR 11 samples were single measurements.
Acknowledgments
This study was funded by grants from the Alternative Research & Development Foundation, 107588, and the National Institute of Health, 1U54CA136465-04S1. We gratefully acknowledge Chona Enrile for tissue processing and slide preparation, Edward Gilbert, H.T. (ASCP), QIHC for immunohistochemistry, and Nancy Bellagamba for facilitating the collection of THR specimens.
Footnotes
Conflict of Interest. Dr. Contag is a founder and consultant for Xenogen Corporation (now Caliper Life Sciences/PerkinElmer, Inc.).
References
- 1.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein RH, Weinberg RA, Rosenblatt M. Of mice and (wo)men: mouse models of breast cancer metastasis to bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:431–436. doi: 10.1002/jbmr.68. [DOI] [PubMed] [Google Scholar]
- 4.Kim IS, Baek SH. Mouse models for breast cancer metastasis. Biochemical and biophysical research communications. 2010;394:443–447. doi: 10.1016/j.bbrc.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 5.Kuperwasser C, Dessain S, Bierbaum BE, et al. A mouse model of human breast cancer metastasis to human bone. Cancer research. 2005;65:6130–6138. doi: 10.1158/0008-5472.CAN-04-1408. [DOI] [PubMed] [Google Scholar]
- 6.Mollard S, Mousseau Y, Baaj Y, et al. How can grafted breast cancer models be optimized? Cancer Biol Ther. 2011;12:855–864. doi: 10.4161/cbt.12.10.18139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim PK, Bliss SA, Patel SA, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer research. 2011;71:1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 8.Oh HS, Moharita A, Potian JG, et al. Bone marrow stroma influences transforming growth factor-beta production in breast cancer cells to regulate c-myc activation of the preprotachykinin-I gene in breast cancer cells. Cancer research. 2004;64:6327–6336. doi: 10.1158/0008-5472.CAN-03-3122. [DOI] [PubMed] [Google Scholar]
- 9.Moharita AL, Taborga M, Corcoran KE, et al. SDF-1alpha regulation in breast cancer cells contacting bone marrow stroma is critical for normal hematopoiesis. Blood. 2006;108:3245–3252. doi: 10.1182/blood-2006-01-017459. [DOI] [PubMed] [Google Scholar]
- 10.Koro K, Parkin S, Pohorelic B, et al. Interactions between breast cancer cells and bone marrow derived cells in vitro define a role for osteopontin in affecting breast cancer cell migration. Breast cancer research and treatment. 2011;126:73–83. doi: 10.1007/s10549-010-0889-9. [DOI] [PubMed] [Google Scholar]
- 11.Pohorelic B, Singh R, Parkin S, et al. Role of Src in breast cancer cell migration and invasion in a breast cell/bone-derived cell microenvironment. Breast cancer research and treatment. 2012;133:201–214. doi: 10.1007/s10549-011-1753-2. [DOI] [PubMed] [Google Scholar]
- 12.Nicola MH, Bizon R, Machado JJ, et al. Breast cancer micrometastases: different interactions of carcinoma cells with normal and cancer patients' bone marrow stromata. Clinical & experimental metastasis. 2003;20:471–479. doi: 10.1023/a:1025462417256. [DOI] [PubMed] [Google Scholar]
- 13.Korah R, Boots M, Wieder R. Integrin alpha5beta1 promotes survival of growth-arrested breast cancer cells: an in vitro paradigm for breast cancer dormancy in bone marrow. Cancer research. 2004;64:4514–4522. doi: 10.1158/0008-5472.CAN-03-3853. [DOI] [PubMed] [Google Scholar]
- 14.Martin FT, Dwyer RM, Kelly J, et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT) Breast cancer research and treatment. 2010;124:317–326. doi: 10.1007/s10549-010-0734-1. [DOI] [PubMed] [Google Scholar]
- 15.Rajski M, Vogel B, Baty F, et al. Global gene expression analysis of the interaction between cancer cells and osteoblasts to predict bone metastasis in breast cancer. PloS one. 2012;7:e29743. doi: 10.1371/journal.pone.0029743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnan V, Shuman LA, Sosnoski DM, et al. Dynamic interaction between breast cancer cells and osteoblastic tissue: comparison of two- and three-dimensional cultures. J Cell Physiol. 2011;226:2150–2158. doi: 10.1002/jcp.22550. [DOI] [PubMed] [Google Scholar]
- 17.Bussard KM, Venzon DJ, Mastro AM. Osteoblasts are a major source of inflammatory cytokines in the tumor microenvironment of bone metastatic breast cancer. Journal of cellular biochemistry. 2010;111:1138–1148. doi: 10.1002/jcb.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weatherholt AM, Fuchs RK, Warden SJ. Specialized connective tissue: bone, the structural framework of the upper extremity. Journal of hand therapy : official journal of the American Society of Hand Therapists. 2012;25:123–131. doi: 10.1016/j.jht.2011.08.003. quiz 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne SH, Barak Y, Liang W, et al. CNOB/ChrR6, a new prodrug enzyme cancer chemotherapy. Mol Cancer Ther. 2009;8:333–341. doi: 10.1158/1535-7163.MCT-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun S, Pantel K, Muller P, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. The New England journal of medicine. 2000;342:525–533. doi: 10.1056/NEJM200002243420801. [DOI] [PubMed] [Google Scholar]
- 22.Coombes RC, Berger U, Mansi J, et al. Prognostic significance of micrometastases in bone marrow in patients with primary breast cancer. NCI monographs : a publication of the National Cancer Institute. 1986:51–53. [PubMed] [Google Scholar]
- 23.Cote RJ, Rosen PP, Lesser ML, et al. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1991;9:1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 24.Diel IJ, Kaufmann M, Costa SD, et al. Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. Journal of the National Cancer Institute. 1996;88:1652–1658. doi: 10.1093/jnci/88.22.1652. [DOI] [PubMed] [Google Scholar]
- 25.Gebauer G, Fehm T, Merkle E, et al. Epithelial cells in bone marrow of breast cancer patients at time of primary surgery: clinical outcome during long-term follow-up. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:3669–3674. doi: 10.1200/JCO.2001.19.16.3669. [DOI] [PubMed] [Google Scholar]
- 26.Krishnamurthy S, Cristofanilli M, Singh B, et al. Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer. 2010;116:3330–3337. doi: 10.1002/cncr.25145. [DOI] [PubMed] [Google Scholar]
- 27.Karrison TG, Ferguson DJ, Meier P. Dormancy of mammary carcinoma after mastectomy. Journal of the National Cancer Institute. 1999;91:80–85. doi: 10.1093/jnci/91.1.80. [DOI] [PubMed] [Google Scholar]
- 28.Willis L, Alarcon T, Elia G, et al. Breast cancer dormancy can be maintained by small numbers of micrometastases. Cancer research. 2010;70:4310–4317. doi: 10.1158/0008-5472.CAN-09-3144. [DOI] [PubMed] [Google Scholar]
- 29.Goss P, Chambers A. Does tumour dormancy offer a therapeutic target? Nature reviews cancer. 2010;10:871–877. doi: 10.1038/nrc2933. [DOI] [PubMed] [Google Scholar]
- 30.Guise TA, Mohammad KS, Clines G, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 31.Schiller KR, Zillhardt MR, Alley J, Borjesson DL, Beitz AJ, Mauro LJ. Secretion of MCP-1 and other paracrine factors in a novel tumor-bone coculture model. BMC Cancer. 2009;9:45. doi: 10.1186/1471-2407-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtin P, Youm H, Salih E. Three-dimensional cancer-bone metastasis model using ex-vivo co-cultures of live calvarial bones and cancer cells. Biomaterials. 2012;33:1065–1078. doi: 10.1016/j.biomaterials.2011.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sosnoski DM, Krishnan V, Kraemer WJ, et al. Changes in Cytokines of the Bone Microenvironment during Breast Cancer Metastasis. Int J Breast Cancer. 2012;2012:160265. doi: 10.1155/2012/160265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature medicine. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owen S, Ye L, Sanders AJ, Mason MD, Jiang WG. Expression profile of receptor activator of nuclear-kappaB (RANK), RANK ligand (RANKL) and osteoprotegerin (OPG) in breast cancer. Anticancer research. 2013;33:199–206. [PubMed] [Google Scholar]
- 36.Uemura H, Yasui T, Kiyokawa M, et al. Serum osteoprotegerin/osteoclastogenesis-inhibitory factor during pregnancy and lactation and the relationship with calcium-regulating hormones and bone turnover markers. J Endocrinol. 2002;174:353–359. doi: 10.1677/joe.0.1740353. [DOI] [PubMed] [Google Scholar]
- 37.Fata JE, Kong YY, Li J, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 38.Labovsky V, Vallone VB, Martinez LM, et al. Expression of osteoprotegerin, receptor activator of nuclear factor kappa-B ligand, tumor necrosis factor-related apoptosis-inducing ligand, stromal cell-derived factor-1 and their receptors in epithelial metastatic breast cancer cell lines. Cancer Cell Int. 2012;12:29. doi: 10.1186/1475-2867-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rachner TD, Benad P, Rauner M, et al. Osteoprotegerin production by breast cancer cells is suppressed by dexamethasone and confers resistance against TRAIL-induced apoptosis. Journal of cellular biochemistry. 2009;108:106–116. doi: 10.1002/jcb.22232. [DOI] [PubMed] [Google Scholar]
- 40.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riccio AI, Wodajo FM, Malawer M. Metastatic carcinoma of the long bones. Am Fam Physician. 2007;76:1489–1494. [PubMed] [Google Scholar]


