Abstract
Background and aims
Cardiovascular co-morbidities are prevalent after stroke, with heart disease, hypertension and impaired glucose tolerance present in the majority of cases. Exercise has the potential to mediate cardiovascular risk factors commonly present in people with stroke. This single-blinded randomized controlled trial compared the effects of high versus low intensity exercise on fitness, cardiovascular risk factors, and cardiac function after stroke.
Methods
Fifty participants (age 50–80y, >1y post-stroke) were randomized to a high-intensity Aerobic Exercise (AE) or low-intensity non-aerobic Balance/Flexibility (BF) program (6 months, 3 60-minute sessions/week). Outcomes assessed by VO2peak (primary outcome), arterial stiffness, ambulatory capacity, hemodynamics and cardiac function using echocardiography, and lipid, glucose and homocysteine levels. Assessors were blinded to group allocation.
Results
Twenty-three (92%) of 25 AE group participants (withdrawals unrelated to the intervention) and all BF group participants completed the program. One BF group participant experienced 2 non-injurious falls during class. No other adverse events occurred. There were no changes in VO2peak in either group (AE 16.9±7 to 17.4±7 ml•kg−1•min−1 vs. BF 16.9±6 to 16.6±5 ml•kg−1•min−1, P=0.45), but AE group demonstrated greater improvement in right atrial emptying fraction (AE 30±22 to 37±22% vs. BF 35±20 to 31±20%, P=0.04). Both groups demonstrated improvements in lipid profiles, glucose and homocysteine levels, and ambulatory capacity (P<0.04).
Conclusions
This was the first study to examine the effects of aerobic exercise after stroke on cardiovascular hemodynamics. High-intensity exercise improved right-sided function and early myocardial relaxation. Low-intensity exercise may also benefit plasma lipid, glucose and inflammatory markers, and ambulatory capacity. This study is an important step towards understanding mechanisms by which exercise may reduce cardiovascular risk and function.
Clinical Trial Registration Information
http://www.clinicaltrials.gov. Unique identifier: NCT01189045
Keywords: Stroke, Rehabilitation, Clinical trial, Risk factors, Sonography, Exercise
Introduction
After stroke, the 5- and 10-year rates for recurrent events are 26% and 40%, respectively (1). Cardiovascular co-morbidities are highly prevalent, with 75% with heart disease, 84% with hypertension (2), and 80% with impaired glucose tolerance and type 2 diabetes mellitus (3). Exercise and increased physical activity levels are potentially effective strategies in mediating cardiovascular risk. Low aerobic capacity is associated with indices of poor cardiovascular health, including mortality (4), inflammatory processes, and left ventricular dysfunction (5), and exercise interventions in older adults and high-risk groups are effective in improving lipoprotein levels (6,7), glucose control (8), and arterial stiffness (9).
For individuals with stroke, there are unique challenges to implementing exercise programs, particularly in elevating heart rate (HR) in the presence of neuromotor impairments. The ability to achieve high heart rates during exercise was associated with leg impairment and cognitive function after stroke (10). While post-stroke exercise interventions can improve aerobic capacity and walking (11), measuring these outcomes are dependent on performing a physical task (e.g. walking, pedaling). The effects of post-stroke exercise training on cardiovascular risk factors are not clear, as some studies have demonstrated improved insulin sensitivity (3), and lower systolic blood pressure (BP) (12) and resting HR (13), while others have reported no changes in BP, HR (14,15), or lipid profiles (15). Moreover, no previous study has focused on the effects of post-stroke exercise on measures of arterial stiffness or cardiac structure and function, yet hemodynamic changes may represent early measurable improvement in cardiovascular function. By directly assessing cardiovascular function and risk factors, we may better understand the physiological mechanisms underlying post-stroke exercise, and potential impact on future event risk.
Aims and Hypotheses
This study aimed to compare the effects of two 6-month stroke exercise programs (low and high intensity) on fitness and cardiovascular risk factors, including novel measures of cardiac hemodynamics and structure. Based on results of previous stroke exercise trials, it was hypothesized that greater improvements would be observed in aerobic capacity and ambulatory capacity (11,16), lipid profiles and glucose tolerance (3,12) following aerobic exercise (high intensity), relative to balance and flexibility exercises (low intensity). No previous exercise intervention studies have included novel outcomes related to arterial stiffness, hemodynamics and cardiac function in individuals with stroke, but extrapolating from previously established associations between endurance training and exercise capacity with cardiac function, it was anticipated that higher intensity exercise would have greater benefit to left ventricular function (17,18) relative to lower intensity training.
Methods
This was a prospective single-blind randomized controlled trial with concealed allocation and intention-to-treat analysis. The study was approved by the University of British Columbia Clinical Research Ethics Board in May 2010. All participants provided informed written consent.
Participants
Eligible participants were 50–80 years old, >1 year post-stroke, completed stroke rehabilitation, living in the community, and able to walk 5 meters independently with or without assistive devices. Exclusion criteria included: stroke from aneurysm, tumor, or infection, presence of cardiovascular abnormalities, pacemaker, serious musculoskeletal or other conditions that would preclude participation in exercise. Two study coordinators performed eligibility screening and enrollment.
Randomization
Participants were randomly allocated into a 6-month high-intensity Aerobic Exercise (AE) or low-intensity Balance and Flexibility (BF) program. Participants were stratified according to age (< and ≥65 years old) and 6-Minute Walk Test (6MWT) distance (< and ≥300 meters). An independent person not involved in enrollment, screening or outcome assessment performed the randomization using a computer-generated 1:1 allocation sequence and permuted block sizes of 2 or 4. Blinded outcome assessors were used.
Interventions
All classes took place 3 times/week for 60 minutes in a multi-purpose space at a research facility with 3 instructors for 12–13 participants. Exercise duration, type, and intensity, and occurrence of adverse events were recorded. HR monitors (Polar Electro Inc, Lake Success NY; Impact Sports Technologies, USA) or ratings of perceived exertion (RPE, 6–20 scale) (19) were used to monitor intensity. To minimize risk of contamination, the groups exercised at different times of the day.
Aerobic Exercise (AE) Program
Each session consisted of a 10-minute warm up, 30–40 minute aerobic component and 10-minute cool down. Training intensity was prescribed using %HR reserve = [(peak HR-resting HR) x % + resting HR], and peak HR was determined from the exercise test. RPE was maintained between 11–14. Intensity progressed from 40% to 70–80% HRR by increasing 10% HRR every 4 weeks (20). If needed, sessions were divided into 10-minute bouts and increased by 5 minutes/week to 30 minutes of continuous exercise. Training modalities included brisk level and inclined overground walking, upright and recumbent cycle ergometry, and non-traditional forms of exercise utilizing functional movements, such as marching-on-the-spot, repeated sit-to-stand, and step-ups onto platform steppers (20). To ensure that sufficient aerobic stimulus was provided, target HR zones were adjusted midway through the intervention to use the highest values of the following methods: %HRR based on exercise test results, %HRR based on age-predicted maximum HR, or % of age-predicted maximum HR.
Balance and Flexibility (BF) Program
The BF program was designed to be non-aerobic in nature, with intensity <40% HRR. Stretching, weight bearing, postural awareness and balance exercises were performed. Participants progressed through increasingly challenging activities, but to minimize aerobic stimulus, any increase in cardiovascular effort was not sustained.
Assessments
Participant characteristics were recorded. The presence or past history of chronic conditions (such as high blood pressure, diabetes, heart or respiratory disease, arthritis, bladder and bower conditions) was collected via self-report. The National Institutes of Health Stroke Scale (21) characterized stroke severity (maximum 42). The Chedoke-McMaster Stroke Assessment (22) described limb motor impairment (maximum 14).
The following measures were performed before and after the intervention.
Primary outcome
Aerobic capacity
A graded maximal exercise test performed on a leg cycle ergometer (Excalibur, Lode Medical Technology, Groningen NL) was used to evaluate VO2peak (20). Breath-to-breath gas exchange was measured (ParvoMedics, Sandy UT), with continuous 12-lead electrocardiogram (CardioCard, Nasiff Associates, New York NY) monitoring. Tests were terminated according to published guidelines (23), or when pedaling fell below 45 revolutions/minute.
Secondary outcomes
Arterial stiffness
Pulse pressure, indicative of the cushioning capacity of the arterial system to minimize pulsatility, is an independent predictor of adverse cardiovascular outcomes (24). Larger values indicate greater systemic stiffness. After 10 minutes quiet supine rest, two readings of brachial BP of the non-paretic side was measured (Dinamap, GE Healthcare, Buckinghamshire UK) and averaged. If values differed >5 mmHg, 2 more readings were done and the average taken across 4 readings (25).
Ambulatory capacity
The 6MWT was designed to reflect the typical demands of everyday functional mobility (26). Standardized instructions (26) were given to walk as far as possible in 6 minutes over a 30-meter square course, and distance covered was measured. The same gait aids were used at both time points.
Hemodynamics and cardiac function
Comprehensive 2D, Doppler, and tissue Doppler echocardiography assessments (iE33 Echocardiography System, Philips, Andover MA) were performed by experienced sonographers. Left ventricular ejection fraction was assessed using Simpson’s biplane method of discs from apical 2- and 4-chamber long-axis views (27). Left and right atrial emptying fractions, global indices of left and right atrial function, were calculated as [Maximal – minimal volume indexed to body surface area (BSA)]/[Maximal volume indexed to BSA]. LA volumes were determined by the standard criteria using biplane area-length method (27), and right atrial volume measurements were obtained from apical 4-chamber views using the area-length method, indexed for BSA (right atrial volume=0.85 x A2/L) (28). Maximal right atrial volume was recorded immediately before atrio-ventricular valve opening, while minimal right atrial volume was determined at the onset of valve closure.
Doppler and tissue Doppler imaging were used to quantify diastolic function with early (E) and late (A) transmitral inflow velocities, and lateral mitral and tricuspid annular velocities (e′). Annular velocities are surrogate measures of ventricular relaxation (29) and correlate with VO2peak (30). The addition of mitral annular velocity to standard clinical data provides incremental predictive power for the endpoint of cardiac mortality (31), and tricuspid annular velocity is associated with mortality and adverse outcomes among individuals with heart failure (32). Echocardiographic analyses were performed according to published guidelines (27,33).
Lipid, glucose and inflammatory biomarker levels
Following a 12-hour fast, plasma levels of total, high- (HDL) and low-density lipoprotein (LDL) cholesterol, triglycerides, glucose and homocysteine were collected on-site by a staff phlebotomist, and samples were analyzed by the hospital’s laboratory services.
Statistical analysis
The study sample size was determined as n=24 per group, based on a 10% change in aerobic capacity from 16.6 to 18.8 ml•kg−1•min−1(14) (two-tailed type I error 0.05; type II error 90%; SD 3).
Descriptive statistics were performed for all participant characteristics for each group. Intention-to-treat analysis was performed. Independent t-tests and chi-square tests were performed to determine group differences in baseline characteristics. Repeated-measures analyses of variance were used to determine time and group-time interaction effects. Due to known age-associated changes in cardiovascular function (34) and sex differences in cardiovascular disease risk (35), secondary analyses were performed with age and sex as covariates. Effect sizes were calculated and defined as small 0.2, medium 0.5 and large 0.8 (36). Statistical Package for the Social Sciences (Version 17.0, Chicago IL) was used with a significance level of P<0.05.
Results
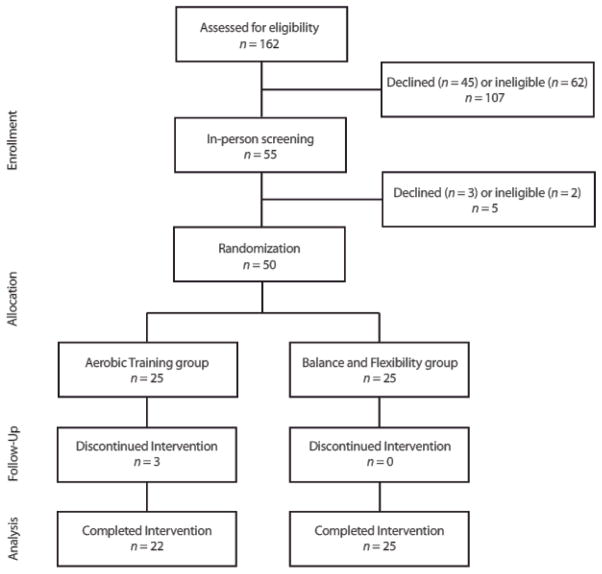
The Figure describes the participant flow through the study. Recruitment took place July to October 2010. Fifty participants were randomized. The exercise programs were conducted October 2010 to April 2011. Baseline characteristics were similar between groups (Table 1). While National Institutes of Health Stroke Scale scores suggest that participants presented with mild severity of stroke, 6MWT distance was 60±28.6% (37) and VO2peak was 53.7±138% of normative values (23). Class attendance was also similar, at 81.4% and 80% for the BF and AE groups, respectively. Exercise HR in the BF group was <16.8% HRR throughout the program, while AE group participants progressed to 58.1% HRR and RPE 14.2 by the end of the intervention.
Figure.
CONSORT diagram
Table 1.
Baseline characteristics
| Aerobic Exercise n=25 |
Balance and Flexibility n=25 |
|
|---|---|---|
| Age, y | 65.9±6.4 (51–76) | 66.9±7.8 (55–80) |
| Men/Women | 14 (56)/11 (44) | 15 (60)/10 (40) |
| Type | ||
| Lacunar/Ischemic/Hemorrhagic/Unknown | 3 (12)/7 (28)/9 (36)/6 (24) | 4 (16)/12 (48)/7 (28)/2 (8) |
| Location | ||
| Cortical/Subcortical/Brainstem/Unknown | 4 (16)/7 (28)/5 (20)/9 (36) | 6 (24)/7 (28)/9 (36)/3 (12) |
| Limbs affected, R/L/Bilateral | 10 (40)/15 (60)/0 (0) | 8 (32)/16 (64)/1 (4) |
| Years post-stroke | 4.3±2.9 (1.3–9.8) | 4.0±3.0 (1.1–11.2) |
| # chronic conditions | 4.0±2.1 (1–11) | 4.0±2.7 (1–14) |
| Medications | ||
| B-blockers | 11 (44) | 8 (32) |
| Calcium-channel blockers | 8 (32) | 9 (36) |
| Other antihypertensives | 15 (62) | 19 (76) |
| Lipid-lowering agents | 14 (56) | 18 (72) |
| Anti-platelets | 9 (36) | 18 (76) |
| Diuretics | 9 (36) | 5 (20) |
| Diabetes, None/Type 2 | 18 (72)/7 (28) | 20 (80)/5 (20) |
| Smoking status | ||
| Never/Former/Current | 15 (60)/10 (40)/0 (0) | 10 (40)/13 (52)/2 (8) |
| Body mass index, kg/m2 | 28.0±3.5 (21–35) | 27.1±5.7 (16–37) |
| Blood pressure, mmHg | ||
| Systolic | 123.1±11.3 (104–144) | 120.8±13.4 (90–144) |
| Diastolic | 68.8±5.1 (63–90) | 65.6±8.2 (45–81) |
| National Institutes of Health Stroke Scale | 2.0±2.6 (0–10) | 1.0±1.5 (0–6) |
| Chedoke-McMaster Stroke Assessment | ||
| Upper limb | 11.0±4.3 (2–14) | 12.0±3.1 (3–14) |
| Lower limb | 11.7±3.0 (3–15) | 11.8±2.1 (7–14) |
| No gait aids/Cane/Walker | 13 (52)/10 (40)/2 (8) | 17 (68)/5 (20)/3 (12) |
Values are Mean±SD (min-max) or n (%)
Dropouts and adverse events
Three AE group participants discontinued the study for reasons unrelated to the training: pacemaker insertion (unrelated to study), hip fracture from fall at home, and episodes of angina (upon questioning, it was found that previous episodes of angina were not disclosed). These participants tended to have more chronic conditions compared to those who completed the AE program (6.0 vs. 3.7). There were no dropouts in the BF group.
Twenty participants experienced 38 falls (n=11 AE group, n=9 BF group). This rate of 3.8 falls/person•year is aligned with the 1.4–5.0 falls/person•year previously reported for community-dwelling stroke survivors (38). There was no group difference in fall rate (negative binomial regression P=0.24). Most incidents (n=36, 95%) occurred outside of the exercise classes, where injuries were minor (cuts, bruises, scrapes, n=6, 16%) to none (n=29, 76%). Three subjects (8%) required medical attention; 1 individual was ultimately withdrawn from the study (described above). Only 1 participant experienced any falls during exercise classes (2 non-injurious falls occurred during separate BF classes while performing standing balance activities), but completed the program without further incident.
Post-program changes
The 3 participants who discontinued the study were deemed cases missing completely at random, thus complete-case analyses were used to examine post-program effects (n=22 AE group, n=25 BF group). Specific data points were missing for 6% of data due to inability to schedule appointments (n=6), collect VO2 data (n=2: unable to tolerate sitting on cycle ergometer, gas calibration issues), and analyze select echocardiographic variables (n=7). For these cases, the baseline score was carried forward to represent the post-training value.
Tables 2, 3 and 4 displays pre- and post-program results. There were no changes in VO2peak in either group (time-group interaction effect P=0.45) (Table 2), but right atrial emptying fraction increased in the AE group and decreased in the BF group (time-group interaction effect P=0.04) (Table 3). Both groups improved in 6MWT distance (time effect P=0.02) (Table 2), and reduced total, LDL cholesterol and triglyceride levels, total: HDL cholesterol ratio, glucose and homocysteine levels (time effect all P<0.03) (Table 4). After controlling for age and sex, time-group interaction effects were significant for right atrial emptying fraction (P=0.04), lateral tricuspid annular e′ (P=0.02), and triglyceride levels (P=0.04).
Table 2.
Changes in aerobic capacity, arterial stiffness, and ambulatory capacity
| Aerobic Exercise
|
Balance and Flexibility
|
P values
|
Effect size | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Post | n | Baseline | Post | Time | Time* Group | ||
| VO2peak, ml•kg−1•min−1 | 21 | 16.9±7.1 | 17.4±7.0 | 25 | 16.9±6.1 | 16.6±5.3 | 0.85 | 0.45 | 0.23 |
| Pulse pressure, mmHg | 22 | 53.8±10.2 | 54.3±11.0 | 25 | 55.2±11.3 | 58.2±13.1 | 0.21 | 0.38 | −0.27 |
| 6MWT distance, m | 22 | 278.2±128.5 | 298.1±134.2 | 25 | 322.2±142.4 | 331.5±149.2 | 0.02* | 0.39 | 0.26 |
Values are Mean±SD
Abbreviation: 6MWT – 6-Minute Walk Test
Table 3.
Changes in hemodynamics and cardiac function
| Aerobic Exercise
|
Balance and Flexibility
|
P values
|
Effect size | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Post | n | Baseline | Post | Time | Time* Group | ||
| Left ventricular ejection fraction, % | 20 | 61.3±3.6 | 61.5±4.0 | 24 | 60.0±4.7 | 60.2±6.0 | 0.63 | 0.97 | 0.01 |
| Transmitral inflow E, cm/s | 20 | 68.7±19.1 | 70.2±21.4 | 24 | 70.8±20.3 | 75.6±16.3 | 0.26 | 0.56 | −0.18 |
| Transmitral inflow A, cm/s | 20 | 76.9±22.8 | 81.1±23.3 | 23 | 79.4±22.5 | 81.5±25.3 | 0.09 | 0.55 | 0.19 |
| Lateral mitral annulus e′, cm/s | 19 | 8.2±3.0 | 8.1±2.4 | 24 | 8.9±2.4 | 8.8±1.9 | 0.83 | 0.91 | −0.04 |
| Right atrial emptying fraction, % | 20 | 30.2±21.6 | 37.4±22.0 | 24 | 34.8±19.8 | 30.5±20.1 | 0.58 | 0.04* | 0.67 |
| Lateral tricuspid annulus e′, cm/s | 20 | 9.2±2.7 | 10.2±2.6 | 22 | 11.2±3.3 | 10.7±4.1 | 0.46 | 0.09 | 0.56 |
Values are Mean±SD
Table 4.
Changes in blood test results
| Aerobic Exercise
|
Balance and Flexibility
|
P values
|
Effect size | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Post | n | Baseline | Post | Time | Time* Group | ||
| Total cholesterol, mmol/L | 22 | 4.5±0.9 | 4.4±0.9 | 25 | 4.3±0.9 | 4.0±0.9 | 0.002* | 0.12 | 0.48 |
| LDL cholesterol, mmol/L | 22 | 2.5±0.8 | 2.4±0.8 | 25 | 2.3±0.7 | 2.1±0.7 | 0.02* | 0.34 | 0.30 |
| HDL cholesterol, mmol/L | 22 | 1.4±0.4 | 1.4±0.3 | 25 | 1.3±0.3 | 1.3±0.4 | 0.96 | 0.53 | −0.19 |
| Total:HDL cholesterol | 22 | 3.4±0.8 | 3.3±0.8 | 25 | 3.4±1.0 | 3.2±1.0 | 0.03* | 0.20 | 0.40 |
| Triglycerides, mmol/L | 22 | 1.4±0.4 | 1.4±0.4 | 25 | 1.6±0.8 | 1.3±0.5 | 0.02* | 0.06 | 0.59 |
| Fasting glucose, mmol/L | 22 | 5.3±1.2 | 5.1±1.0 | 25 | 5.4±1.4 | 5.0±0.9 | 0.004* | 0.29 | 0.33 |
| Homocysteine, μmol/L | 22 | 13.4±4.9 | 10.8±3.7 | 25 | 13.9±5.4 | 11.2±4.7 | 0.000* | 0.94 | 0.02 |
Values are Mean±SD
Abbreviations: LDL – Low-density lipoprotein; HDL – High-density lipoprotein
Co-interventions that may have influenced the study results included: Midodrine therapy for management of orthostatic hypotension (n=1 AE group), and attendance at diabetes lifestyle management sessions (no changes in medications) (n=1 BF group). With these cases removed, post-hoc analyses revealed no changes in study results except changes in triglyceride levels and total: HDL cholesterol ratio were no longer significant for time effect (both P=0.07).
Contrary to our hypotheses, there were no changes in VO2peak in either group. We noted 7 AE group participants who were unable to progress in intensity (31.5% HRR, RPE 14.5), whereas the remaining 15 participants achieved 78.6% HRR and RPE 14.0.
Discussion
This study did not demonstrate hypothesized differences in the primary outcome, VO2peak, either between or within the intervention groups. There were also unanticipated improvements across both groups in several secondary outcomes (lipid, glucose and homocysteine levels, and ambulatory capacity). Results from this study do, however, provide the first echocardiographic evidence of improved cardiac function following exercise post-stroke. High-intensity aerobic exercise was effective in improving right atrial emptying fraction, a global index of right atrial function, and after controlling for age and sex, lateral tricuspid annular e′, indicative of early myocardial relaxation. Right-sided cardiac function, particularly right ventricular function, is emerging as an important prognostic indicator in cardiovascular disease (39,40). These observed changes in right atrial function and ventricular relaxation may represent the earliest measurable improvement in subclinical cardiac function in this population.
While aerobic training is typically associated with left ventricular adaptation, findings in studies involving older adults, particularly those with underlying cardiovascular disease, have been equivocal. Post-exercise improvements in left ventricular end-systolic and -diastolic volume, ejection fraction have been reported for older adults with chronic heart failure (41), and reduced left ventricular mass observed among those with mild to moderate hypertension (42). In contrast, no changes were observed in left ventricular diastolic function in a study involving adults with untreated mild hypertension (43). Interestingly, a study involving individuals with neuromotor impairment (incomplete spinal cord injury) demonstrated that robotic treadmill training increased ejection fraction and E/A ratio, and lowered left ventricular end-systolic and -diastolic volumes (44). However, while younger subjects (mean age 51y) free from underlying cardiovascular co-morbidities were included (44), mean age in the current study was 65, and most had cardiovascular co-morbidities. Age-related changes in left-sided cardiac function (34) is further advanced in the presence of multiple cardiovascular risk factors and as such, reverse remodeling may take longer than the right side, given differences in chamber wall thickness and compliance. Thus, right-sided chambers may be more readily modifiable, and reversal of remodeling detectable earlier. To our knowledge, this is the first study to report exercise-induced changes in right-sided function in a clinical population.
Unexpectedly, improvements in plasma lipid, glucose and homocysteine levels were observed across the entire study cohort. Given the degree to which aerobic capacity is compromised after stroke (45), activities in the BF program, although lower in intensity, may have provided sufficient cardiovascular challenge. An earlier study also demonstrated that both low- and moderate-intensity exercise was effective in reducing triglyceride levels among individuals with stroke (12). Moreover, there is some evidence that gentle, low-intensity physical activity interventions (46,47) may have benefit to cardiovascular risk factors, but no study has compared these interventions to aerobic exercise. It is also possible that these improvements were a product of increased non-exercise physical activity (48), an important consideration given that leisure-time physical activity is lowest in stroke compared to other chronic health groups (49). Thus, even at low intensities, community exercise programs may have important health benefits for this compromised and highly sedentary group.
That we did not observe between-group changes in VO2peak may be attributed to neurological sequelae that may confound exercise tests results (10) and variability in training intensities. Indeed, nearly 1/3 of the participants in this study were unable to progress in exercise intensity. The ability to achieve the necessary exercise targets is a realistic and ongoing challenge when working with individuals with stroke, given the presence of neurological impairments and broad range of functional abilities, but should not preclude these individuals from participating in exercise.
Limitations
Given the aim of this study was to examine the potential effects of post-stroke exercise on cardiovascular risk factors, a large number of outcomes were considered, including novel outcomes in cardiac function and hemodynamics not previously examined in this population. We did not adjust for multiple comparisons in the analyses of treatment effects. The sample size was powered based on anticipated change in VO2peak, and not on the parameters in which significant changes were observed. To confirm that these benefits were not due to type 1 error, future research using these measures as primary outcomes may be warranted. Additionally, since a no-intervention arm was not included, we cannot conclude that the cardiovascular changes demonstrated by the entire cohort (lipid profiles, glucose and homocysteine levels) were not part of the natural course of stroke recovery. This is unlikely, however, especially since study participants were in the chronic stages of stroke, as health (1) and function (50) tend to worsen, not improve, in the long-term.
Conclusions
This study contributes novel findings regarding improved right-sided cardiac function with progressive aerobic exercise after stroke. It appears that even lower intensity exercise may benefit lipid profiles, glucose and homocysteine levels and ambulatory capacity in this compromised, at-risk group, possibly by increasing non-exercise physical activity levels.
Acknowledgments
Funding
Study funded by the Vancouver Foundation/Carl and Elsie Halterman Research Fund and the Canadian Institutes of Health Research (CIHR) (MOP-111183). AT was supported by CIHR (MFE-98550) and the Michael Smith Foundation for Health Research (MSFHR) (ST-PDF-03003(11-1)CLIN), JJE is supported by CIHR (MSH-63617) and the MSFHR, AVK is supported by CIHR, Heart and Stroke Foundation of British Columbia and Yukon, Christopher and Dana Reeve Foundation and the Rick Hansen Institute, KMM is supported by CIHR Institute of Aging.
Footnotes
Conflict of interest
None declared
Contributor Information
Ada Tang, University of British Columbia, Faculty of Medicine, Department of Physical Therapy; Vancouver Coastal Health. Address: GF Strong Rehabilitation Research Lab, 4255 Laurel Street, Vancouver BC Canada V5Z 2G9.
Janice J Eng, University of British Columbia, Faculty of Medicine, Department of Physical Therapy; Vancouver Coastal Health; International Collaboration on Repair Discoveries. Department of Physical Therapy, University of British Columbia, 212-2177 Wesbrook Mall, Vancouver BC Canada V6T 1Z3, Tel (604) 714-4105, Fax (604) 714-4168.
Andrei V Krassioukov, University of British Columbia, Faculty of Medicine, Division of Physical Medicine and Rehabilitation; Vancouver Coastal Health; International Collaboration on Repair Discoveries. Address: Blusson Spinal Cord Centre, 818 West 10th Avenue, Vancouver BC Canada V5Z 1M9.
Kenneth M Madden, University of British Columbia, Faculty of Medicine, Division of Geriatric Medicine; Vancouver Coastal Health. Address: Diamond Health Care Centre, 2775 Laurel Street, 9th floor, Vancouver BC Canada V5Z 1M9.
Azam Mohammadi, University of British Columbia, Faculty of Medicine, Division of Cardiology; Vancouver Coastal Health. Address; Diamond Health Care Centre, 2775 Laurel Street, 9th floor, Vancouver BC Canada V5Z 1M9.
Michael YC Tsang, University of British Columbia, Faculty of Medicine, Division of Cardiology; Vancouver Coastal Health. Address: Diamond Health Care Centre, 2775 Laurel Street, 9th floor, Vancouver BC Canada V5Z 1M9.
Teresa SM Tsang, University of British Columbia, Faculty of Medicine, Division of Cardiology; Vancouver Coastal Health. Address: Diamond Health Care Centre, 2775 Laurel Street, 9th floor, Vancouver BC Canada V5Z 1M9.
References
- 1.Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011 May;42(5):1489–1494. doi: 10.1161/STROKEAHA.110.602615. [DOI] [PubMed] [Google Scholar]
- 2.Roth EJ. Heart disease in patients with stroke: incidence, impact, and implications for rehabilitation. Part 1: classification and prevalence. Arch Phys Med Rehabil. 1993;74:752–760. doi: 10.1016/0003-9993(93)90038-c. [DOI] [PubMed] [Google Scholar]
- 3.Ivey FM, Ryan AS, Hafer-Macko CE, Goldberg AP, Macko RF. Treadmill aerobic training improves glucose tolerance and indices of insulin sensitivity in disabled stroke survivors. Stroke. 2007;38(10):2752–2758. doi: 10.1161/STROKEAHA.107.490391. [DOI] [PubMed] [Google Scholar]
- 4.Powell KE, Paluch AE, Blair SN. Physical activity for health: What kind? How much? How intense? On top of what? Annu Rev Public Health. 2011;32:349–365. doi: 10.1146/annurev-publhealth-031210-101151. [DOI] [PubMed] [Google Scholar]
- 5.Van de Veire NR, De Winter O, Philippe J, et al. Maximum oxygen uptake at peak exercise in elderly patients with coronary artery disease and preserved left ventricular function: the role of inflammation on top of tissue Doppler-derived systolic and diastolic function. Am Heart J. 2006 Aug;152(2):297.e1–297.e7. doi: 10.1016/j.ahj.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Kelley GA, Kelley KS, Tran ZV. Exercise, lipids, and lipoproteins in older adults: a meta-analysis. Prev Cardiol. 2005 Fall;8(4):206–214. doi: 10.1111/j.0197-3118.2005.03769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley GA, Kelley KS, Franklin B. Aerobic exercise and lipids and lipoproteins in patients with cardiovascular disease: a meta-analysis of randomized controlled trials. J Cardiopulm Rehabil. 2006 May-Jun;26(3):131–139. doi: 10.1097/00008483-200605000-00002. quiz 140–141, discussion 142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000 Sep 12;102(11):1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 10.Tang A, Eng JJ, Tsang TS, Krassioukov AV. Cognition and motor impairment correlates with exercise test performance after stroke. Med Sci Sports Exerc. 2013 Apr;45(4):622–627. doi: 10.1249/MSS.0b013e31827a0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brazzelli M, Saunders DH, Greig CA, Mead GE. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011 Nov;9:11, CD003316. doi: 10.1002/14651858.CD003316.pub4. [DOI] [PubMed] [Google Scholar]
- 12.Rimmer JH, Rauworth AE, Wang EC, Nicola TL, Hill B. A preliminary study to examine the effects of aerobic and therapeutic (nonaerobic) exercise on cardiorespiratory fitness and coronary risk reduction in stroke survivors. Arch Phys Med Rehabil. 2009 Mar;90(3):407–412. doi: 10.1016/j.apmr.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 13.Katz-Leurer M, Shochina M, Carmeli E, Friedlander Y. The influence of early aerobic training on the functional capacity in patients with cerebrovascular accident at the subacute stage. Arch Phys Med Rehabil. 2003;84:1609–1614. doi: 10.1053/s0003-9993(03)00344-7. [DOI] [PubMed] [Google Scholar]
- 14.Potempa K, Lopez M, Braun LT, Szidon JP, Fogg L, Tinknell T. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke. 1995;26:101–105. doi: 10.1161/01.str.26.1.101. [DOI] [PubMed] [Google Scholar]
- 15.Lennon O, Carey A, Gaffney N, Stephenson J. A pilot randomized controlled trial to evaluate the benefit of the cardiac rehabilitation paradigm for the non-acute ischaemic stroke population. Clin Rehabil. 2008;22:125–133. doi: 10.1177/0269215507081580. [DOI] [PubMed] [Google Scholar]
- 16.Pang MYC, Eng JJ, Dawson AS, Gylfadottir S. The use of aerobic exercise training in improving aerobic capacity in individuals with stroke: a meta-analysis. Clin Rehabil. 2006;20:97–111. doi: 10.1191/0269215506cr926oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seals DR, Hagberg JM, Spina RJ, Rogers MA, Schechtman KB, Ehsani AA. Enhanced left ventricular performance in endurance trained older men. Circulation. 1994 Jan;89(1):198–205. doi: 10.1161/01.cir.89.1.198. [DOI] [PubMed] [Google Scholar]
- 18.Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. J Am Med Assoc. 2009 Jan 21;301(3):286–294. doi: 10.1001/jama.2008.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 20.Pang MYC, Eng JJ, Dawson AS, McKay HA, Harris JE. A community-based fitness and mobility exercise (FAME) program for older adults with chronic stroke: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:1667–1674. doi: 10.1111/j.1532-5415.2005.53521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 22.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 23.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 24.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107(22):2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 25.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005 Feb 8;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 26.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Resp Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 28.DePace NL, Ren JF, Kotler MN, Mintz GS, Kimbiris D, Kalman P. Two-dimensional echocardiographic determination of right atrial emptying volume: a noninvasive index in quantifying the degree of tricuspid regurgitation. Am J Cardiol. 1983 Sep 1;52(5):525–529. doi: 10.1016/0002-9149(83)90019-x. [DOI] [PubMed] [Google Scholar]
- 29.Tsang MY, Barnes ME, Tsang TS. Left atrial volume: clinical value revisited. Curr Cardiol Rep. 2012 Jun;14(3):374–380. doi: 10.1007/s11886-012-0268-8. [DOI] [PubMed] [Google Scholar]
- 30.Eroglu S, Sade LE, Aydinalp A, et al. Association between cardiac functional capacity and parameters of tissue Doppler imaging in patients with normal ejection fraction. Acta Cardiol. 2011;66(2):181–187. doi: 10.1080/ac.66.2.2071249. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Yip G, Yu CM, et al. Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function. J Am Coll Cardiol. 2005 Jan 18;45(2):272–277. doi: 10.1016/j.jacc.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 32.Meluzin J, Spinarová L, Hude P, et al. Prognostic importance of various echocardiographic right ventricular functional parameters in patients with symptomatic heart failure. J Am Soc Echocardiogr. 2005;18(5):435–444. doi: 10.1016/j.echo.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: A report from the Doppler quantification task force of the nomenclature and standards committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 34.Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002 Jan;7(1):29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- 35.Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J. 2010 Dec;18(12):598–602. doi: 10.1007/s12471-010-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum; 1988. [Google Scholar]
- 37.Enright PL, Sherrill DL. Reference equations for the Six-Minute Walk in healthy adults. Am J Resp Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 38.Weerdesteyn V, de Niet M, van Duijnhoven HJ, Geurts AC. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45(8):1195–1213. [PubMed] [Google Scholar]
- 39.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, Part II: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008 Apr 1;117(13):1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 40.Meyer P, Filippatos GS, Ahmed MI, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. 2010 Jan 19;121(2):252–258. doi: 10.1161/CIRCULATIONAHA.109.887570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA. 2000 Jun 21;283(23):3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 42.Turner MJ, Spina RJ, Kohrt WM, Ehsani AA. Effect of endurance exercise training on left ventricular size and remodeling in older adults with hypertension. J Gerontol A Biol Sci Med Sci. 2000 Apr;55(4):M245–51. doi: 10.1093/gerona/55.4.m245. [DOI] [PubMed] [Google Scholar]
- 43.Stewart KJ, Ouyang P, Bacher AC, Lima S, Shapiro EP. Exercise effects on cardiac size and left ventricular diastolic function: relationships to changes in fitness, fatness, blood pressure and insulin resistance. Heart. 2006;92(7):893–898. doi: 10.1136/hrt.2005.079962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turiel M, Sitia S, Cicala S, et al. Robotic treadmill training improves cardiovascular function in spinal cord injury patients. Int J Cardiol. 2011 Jun 16;149(3):323–329. doi: 10.1016/j.ijcard.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Rimmer JH, Wang E. Aerobic exercise training in stroke survivors. Top Stroke Rehabil. 2005;12(1):17–30. doi: 10.1310/L6HG-8X8N-QC9Q-HHM8. [DOI] [PubMed] [Google Scholar]
- 46.Anderson JG, Taylor AG. The metabolic syndrome and mind-body therapies: a systematic review. J Nutr Metab. 2011;2011:276419. doi: 10.1155/2011/276419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeh GY, Wang C, Wayne PM, Phillips R. Tai chi exercise for patients with cardiovascular conditions and risk factors: a systematic review. J Cardiopulm Rehabil Prev. 2009 May-Jun;29(3):152–160. doi: 10.1097/HCR.0b013e3181a33379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011 Jul;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 49.Ashe MC, Miller WC, Eng JJ, Noreau L Physical Activity and Chronic Conditions Research Team. Older adults, chronic disease and leisure-time physical activity. Gerontology. 2009;55(1):64–72. doi: 10.1159/000141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van de Port IGL, Kwakkel G, van Wijk I, Lindeman E. Susceptibility to deterioration of mobility long-term after stroke: a prospective cohort study. Stroke. 2006;37:167–171. doi: 10.1161/01.STR.0000195180.69904.f2. [DOI] [PubMed] [Google Scholar]