Abstract
In rats with persistent pain, spinal group I metabotropic glutamate receptor (mGluR) activity has been shown to be pronociceptive, whereas spinal group II/III activity is antinociceptive. In brain, group I mGluR activity produces positive feedback effects on glutamate release, whereas group II/III activity produces negative feedback effects. It is unknown whether the nociceptive vs. antinociceptive effects of spinal group I vs. group II/III mGluR activity depend on differential regulation of spinal glutamate release. Here we used behavioural nociceptive testing and in vivo microdialysis to assess the effect of intrathecal treatment with group I mGluR antagonists [cyclopropan[b] chromen-1a-carboxylate,(CPCCOEt), 2-methyl-6-(phenylethynyl) pyridine (MPEP)] or groups II [aminopyrrolidine-2R,4R-dicarboxylate (APDC)] and III [L-2-amino-4-phosphonobutyrate (L-AP4)] mGluR agonists or vehicle, on nociception and noxious stimulus-induced increases in glutamate release in the spinal cord dorsal horn (SCDH) of rats with a chronic constriction injury (CCI) of the sciatic nerve or hind paw injection of complete Freund’s adjuvant (CFA). None of the treatments significantly influenced basal spinal glutamate concentrations in either CCI or CFA rats. In CCI rats, formalin-induced nociception and increases in spinal glutamate concentrations were significantly attenuated by pretreatment with CPCCOEt, MPEP, APDC or L-AP4. In CFA rats, capsaicin-induced increases in nociception and spinal glutamate concentrations were significantly attenuated by pretreatment with CPCCOEt, MPEP or APDC, but not L-AP4. This study demonstrates that group I antagonists and group II/III mGluR agonists attenuated the enhanced nociception and noxious stimulus-induced glutamate release in SCDH of CCI and/or CFA rats in vivo, and suggests a possible mechanism for their antihyperalgesic effects.
Keywords: in vivo microdialysis, excitatory amino acids, chronic constriction injury, complete Freund’s adjuvant, formalin, capsaicin
Introduction
Endogenous excitatory amino acids, such as glutamate and aspartate, activate the ionotropic glutamate receptors (iGluRs), NMDA, AMPA and kainic acid, as well as metabotropic glutamate receptors (mGluRs) (Hollmann and Heinemann, 1994). Unlike iGluRs, which gate cation channels, mGluRs are coupled by G proteins to various intracellular messengers. Eight subtypes of mGluRs are divided into 3 groups based on sequence homology, signal transduction mechanisms and ligand selectivites. Group I includes mGluR1 & 5 which stimulate phospholipase C (PLC), leading to protein kinase C (PKC) activation, phosphoinositide (PI) hydrolysis and intracellular Ca2+ mobilization. Group II (mGluR2 & 3) and III (mGluR4 & 6–8) are negatively coupled to adenylate cyclase (Hollmann and Heinemann, 1994; Pin and Duvoisin, 1995).
A growing body of in vivo evidence indicates that mGluRs participate significantly in the modulation of glutamate release in various brain regions. The administration selective group II agonists produce reductions in basal (Cozzi et al., 1997)or stimulated (Battaglia et al., 1997) glutamate levels in the caudate and striatum, respectively. The group III mGluR agonists reduce glutamate release in the nucleus accumbens (Xi et al., 2003) and hippocampus (Martin et al., 2007). Conversely, local application of the group I agonists DHPG (Moroni et al., 1998) or CHPG (Pintor et al., 2000) increases, while a group I antagonist, MPEP (Thomas et al., 2001) decreases glutamate levels in the parietal cortex or striatum in vivo. While it is known mGluRs regulate glutamate neurotransmission in brain, their role in spinal glutamate release has received comparatively little investigation. A recent study (Lorrain et al., 2002) found i.t. administration of DHPG produces an enhanced spinal release of glutamate at doses which caused nociceptive behaviours, and both effects were blocked by pretreatment with the mGluR5 antagonist MPEP. DHPG also increases the frequency of evoked and spontaneous or miniature excitatory post-synaptic potentials (EPSPs) (reflective of enhanced glutamate release) in the spinal cord substantia gelatinosa (Park et al., 2004) and spinal trigeminal subnucleus oralis Song et al., 2009). Selective mGluR1 antagonists also attenuate the elevated spinal glutamate levels observed in rats following spinal cord injury (Mills et al., 2000).
Using in vivo microdialysis, we have previously reported that noxious stimulus-induced glutamate release in SCDH is enhanced in neuropathic rats (Coderre et al., 2005), and others have shown that there are enhanced levels of glutamate in the SCDH of rats with hind paw inflammation (Sluka KA, Westlund, 1992; Yang et al., 1996). We and others have also shown that group I mGluR antagonists and group II and III mGluR agonists reduce allodynia and/or hyperalgesia in animal models of neuropathic (Fisher et al., 2002; Simmons et al., 2002; Zhu et al., 2004) and inflammatory (Sharpe et al., 2002; Zhang et al., 2002; Zhu et al., 2004) pain. These findings combined with the above results, indicating that group I mGluRs generally enhance glutamate release, while group II and II mGluRs generally decrease glutamate release, suggest that group I mGluRs may be pronociceptive by enhancing the spinal release of glutamate, and group II and III mGluRs may be antinociceptive by suppressing the spinal release of glutamate. The purpose of the present study is to assess the role of mGluRs in the regulation of both nociception and noxious stimulus-induced glutamate release in SCDH in rats with persistent pain. Thus, we examined whether group I mGluR antagonists, or group II and III mGluR agonists, are able to reduce the enhanced nociception induced by formalin or capsaicin and the noxious stimulus-induced glutamate release in the SCDH of neuropathic rats or rats with hind paw inflammation.
Materials and Methods
Subjects
The present studies employed male Long Evans hooded rats (250–300 grams, Charles River, PQ). Rats were housed in groups of 3–4, with food and water available ad libitum, on a 12:12 hour light:dark cycle. All surgical and testing procedures were approved by the Animal Care Committee at McGill University, and conformed to the ethical guidelines of the Society for Neuroscience.
Chronic Constriction Injury (CCI)
Prior to surgery, rats were anesthetized with brief halothane (3.0%) anesthesia. Chronic constriction injury (CCI) was induced to the left sciatic nerve according to the method of Bennett and Xie (1988). Under sodium pentobarbital anesthesia (60 mg/kg, i.p.) supplemented with isofluorane anesthetic (2% in 95% O2, 5% CO2) when necessary, the sciatic nerve was exposed by blunt dissection at mid-thigh level, and the adhering tissue was dissected for an approximately 10 mm section of the nerve. Four ligatures (4.0 chromic gut, Ethicon) were loosely tied around the nerve at 2 mm spacings, to produce a loose constriction of the nerve. Wounds were closed with one or two sutures to the dissected muscle, followed by tissue glue to the skin, and treatment with Furacin topical antibacterial ointment (0.2% nitrofurazone). Sham rats received the same surgery expect that the sciatic nerve was only exposed and had no ligatures placed on it.
Persistent inflammation
Persistent inflammation was induced by injecting 100 μl of complete Freund’s adjuvant (CFA) (heat-killed and dried Mycobacterium tuberculosis, 0.5 mg/ml, suspended in 85% paraffin oil and 15% mannide monooleate) (Sigma, Oakville, ON, Canada) subcutaneously into the dorsal surface of the left hind paw, while the rats were briefly anesthetized with isofluorane (2% in 95% O2, 5% CO2). Sham rats received a 100 μl injection of the CFA vehicle.
Sustained Nociceptive Behaviours
Sustained nociceptive behaviours were measured in CCI or sham rats given a 50 μl intraplantar hind paw injection of 1.0% formalin, and in CFA or sham rats given a 30 μl intraplantar hind paw injection of 0.03% capsaicin. All behavioral observation were made while each rat freely moving in an observation chamber (30 × 30 × 30 cm) fitted with an acrylic floor under which was placed a mirror to allow an unobstructed view of the animal’s paws. Each rat was first placed in the chamber for a 30–40 minute habituation period, the last five minutes of which were used to obtain pre-drug, baseline measurements. Each rat was then anesthetized lightly using isofluorane for an intrathecal injection of either an mGluR agent or vehicle, and then returned to the chamber. Post-injection measurements began 20 minutes after injection, when rats had fully recovered from the anesthesia. Sustained nociceptive behaviors were recorded for one minute every five minutes for the duration of the post-drug observation period, which was 35 minutes for the CFA/capsaicin experiments and 60 minutes for the CCI/formalin experiments. Behavior was rated using a portable computer which calculated, over a one minute period, the time spent in 4 behavioral categories: 0 = normal weight bearing on the injected paw; 1 = limping during locomotion, cupping or resting the paw lightly on the floor (guarding); 2 = elevation of the injected paw; and 3 = licking, biting or grooming the injected paw. A nociceptive score was calculated from the weighted sum of time measures of the four categories, divided by the total time for each time period (i.e., 60 sec within each 5 min period). Nociceptive scores were thus obtained every five minutes for either 35 (after capsaicin) or 60 minutes (after formalin).
Microdialysis and intrathecal (i.t.) catheter
Rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and a microdialysis (hollow) fiber was implanted according to the method described by Sluka & Westlund (1998). After shaving the fur and sterilizing the skin on the back, the T13 thoracic vertebra was exposed, and a small hole was made on each side of the vertebra at the posterior pedicle. A single dialysis fiber (200 μm outer diameter, 45,000 mol. wt. cut-off, Hospal AN69-HF) was passed transversely through the spinal cord. The dialysis fiber was coated with epoxy except for a 2 mm active zone, which was positioned within the grey matter of the spinal cord (approximately lamina III–VI). Each end of the fiber was connected to PE-20 tubing with Super glue® and fixed to the vertebral bone with the dental cement (Lang Dental Mfg Co, Wheeling, IL). The inlet and outlet tubing was tunneled under the skin and externalized at the back of the neck.
During the same surgery, some rats were also implanted with a chronic indwelling i.t. catheter, which was inserted between the L5 and L-6 vertebrae during a lumbar puncture according to the methods of Pogatzki et al. (2000). The lumbar puncture was performed using a 23 gauge needle, and PE-32 polyurethane tubing was pushed 3 cm through the needle to the lumbar enlargement, before the needle was removed. The tubing was then anchored to the back muscles using 3-0 silk sutures. This procedure was performed following placement of the microdialysis catheter, while the rats were still anesthetized as described above, and the i.t. catheter was placed only in CCI rats between 7 and 14 days post-CCI surgery or 1 day after CFA injection. Only rats with normal behavior and no paralysis of hind limbs after the surgery were included in the microdialysis studies.
In vivo Microdialysis
After recovery from surgery, rats with microdialysis catheters were placed in a Raturn® Interactive System (Bioanalytic Systems, Inc. West Lafayette, IN) with a tethering system which allowed tubing from the microdialysis catheter to be connected to a syringe pump on one end and to a refrigerated fraction collector on the other. The artificial CSF contained (in mM) 154.7 Na+, 2.9 K+, 1.1Ca2+, 0.82 Mg2+, 132.49 Cl−, and was bubbled with 95% oxygen and 5% CO2 at the start of the experiment to adjust the pH to 7.4. After a 1 hr stabilization period, microdialysis samples were collected every 5 min at a flow rate of 5 μl/min. For both CCI and CFA rats, 3 basal samples were collected, as well as 6 samples after i.t. drug treatment with mGluR agents or vehicle. For CCI rats, an additional 12 samples were collected following s.c. injection of 50 μl of 1.0% formalin into the ipsilateral plantar hind paw. For CFA rats, an additional 6 samples were collected following s.c. injection of 30 μl of 0.03% capsaicin into the ipsilateral plantar hind paw. Samples were stored at −80°C until assayed for glutamate by HPLC. Following the collection of samples, a solution of methylene blue was administered for 10 min through the filament. Staining in the tissue indicated the location of the permeable portion of the fiber.
At the end of the experiment, rats were killed with an overdose of sodium pentobarbital (200 mg/kg, i.p.) and the lumbar spinal cord was removed. The tissue was post-fixed with formaldehyde, cryoprotected in a 30% sucrose solution and sectioned (50 μm). The sections were slide mounted and cover-slipped, and the location of the dye mark was determined when viewing with a light microscope. An investigator who was blind to the experimental condition determined the spinal cord level of the dialysis fiber in the dorsal horn. Samples were included in the analysis only if the microdialysis catheter was in the dorsal horn (i.e., the gray matter of the spinal cord above the central canal).
HPLC Assay
Glutamate content in each sample was determined using reverse phase HPLC with fluorescent detection as described by Böttcher et al (2003). In brief, pre-column derivatization of amino acids in samples were carried out in the autoinjector of the HPLC system (Waters Alliance 2690). Samples of 10 μl microdialysis perfusate were incubated with 10 μl of derivatizing reagent consisting of o-phthalaldehyde (incomplete reagent) and 2-mercaptoethanol 0.4% (v/v) (both Sigma, St. Louis, MO) for 1.5 minutes and then injected into HPLC system. The system was fitted with a C18 ODS Supelco column (15 cm × 4.6 mm, 5 μm, Supelco, Bellefonte, PA). The derivatized samples were eluted with a mobile phase at a flow rate of 1 ml/min. The mobile consisted of 70 mM sodium acetate pH-6.95, 1.5 % of tetrahydrofuran and 11 % methanol. An isocratic elution was maintained for 8.5 min to get clear separation of glutamate. The temperature of column heater and sample chamber was kept at 37°C and 5°C, respectively. The eluted derivatives were detected with a fluorescent detector (Waters 474) at excitation and emission wavelength 330nm and 450 nm, respectively. Peak area was used to calculate the concentration of each analyte, and quantification of glutamate was determined from a standard curve derived using external standards. The data were expressed in percentage of baseline concentration for each animal for ease of calculation, and since this is a standard method of presentation.
Drugs
Drugs used included the mGluR agents aminopyrrolidine-2R,4R-dicarboxylate (APDC), L-2-amino-4-phosphonobutyrate (L-AP4), cyclopropan[b] chromen-1a-carboxylate (CPCCOEt), 2-Methyl-6-(phenylethynyl) pyridine (MPEP), all obtained from Tocris Bioscience (Ellisville, MO) and mixed in a vehicle of dilute NaOH in distilled water. For both behavioural and in vivo microdialysis studies, rats were given i.t. injections of either CPCCOEt (500 nmoles), MPEP (100 nmoles), APDC (500 nmoles), L-AP4 (500 nmoles) or vehicle (20 μl) 20 min prior to hind paw formalin or capsaicin. The above doses were chosen based on established ED50’s for central administration and in vivo ED50/in vitro IC50 ratios that ensure the dosages are selective for their respective mGluR targets (Soliman et al. 2005). Other drugs included formalin diluted to 1.0% in 0.9% saline, and capsaicin (obtained from Sigma, St. Louis, MO) at 0.03% dissolved in 10% ethanol and 5% Tween 80 in 0.9% saline.
Statistical Analyses
The effects of i.t. treatment with mGluR agents on sustained nociceptive behaviours or noxious stimulus-evoked release of glutamate in CCI and CFA rats was assessed with two way ANOVA followed by Fisher’s least squared difference post hoc comparisons between groups, or Dunnett’s post hoc t-tests for pre- and post-injection comparisons within groups.
Results
Effects on mGluR agents on formalin-induced nociception in sham-treated and CCI rats
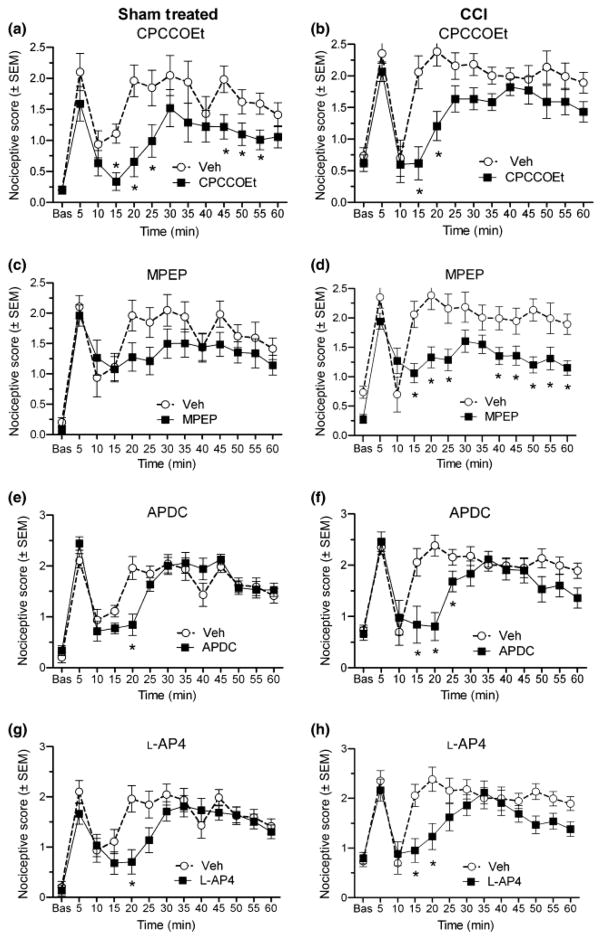
Sustained nociceptive responses to formalin were enhanced in CCI rats as compared to sham-treated rats with scores (other than the 10 min inter-phase score) ranging between ~2.0–2.5 for CCI rats, as compared to ~1.1–2.0 for sham-treated rats (Fig. 1). This difference was confirmed by a significant group effect determined by ANOVA (F(1,11) = 4.68, p < 0.05). Drug treatment with CPCCOEt significantly reduced nociceptive scores between 15–25 and 45–55 min post-formalin for sham-treated rats (Fig. 1a), and between 15 and 20 min after formalin for CCI rats (Fig. 1b) (p < 0.05, Fisher’s LSD post hoc test). Drug treatment with MPEP reduced did not affect formalin-induced nociceptive scores at any time point for sham-treated rats (Fig. 1c), but significantly reduced nociceptive scores between 15–25, 40–60 min after formalin for CCI rats (Fig. 1d) (p < 0.05, Fisher’s LSD post hoc test). Drug treatment with APDC significantly reduced nociceptive scores at 20 min post-formalin for sham-treated rats (Fig. 1e), and between 15 and 20 min after formalin for CCI rats (Fig. 1f) (p < 0.05, Fisher’s LSD post hoc test). Drug treatment with L-AP-4 reduced nociceptive scores at 20 min post-formalin for sham-treated (Fig. 1g) and between 15–20 min post-formalin for CCI rats (Fig 1h) (p < 0.05, Fisher’s LSD post hoc test).
Fig. 1.
Effects of antagonists of mGluR1 (A,B) and mGluR5 (C,D), and agonists of group II (E,F) and group III (G,H) mGluRs on nociceptive responses (nociceptive score +/− standard error of the mean (SEM)) to formalin in both sham (left column) and CCI (right column) rats. The mGluR agents reduce formalin-induced nociception to varying degrees in both sham and CCI rats. Asterisks indicate the time points where nociceptive scores are significantly reduced by the drug as compared to vehicle (*p < 0.05, Fisher’s LSD post hoc).
Effects on mGluR agents on capsaicin-induced nociception in sham-treated and CFA rats
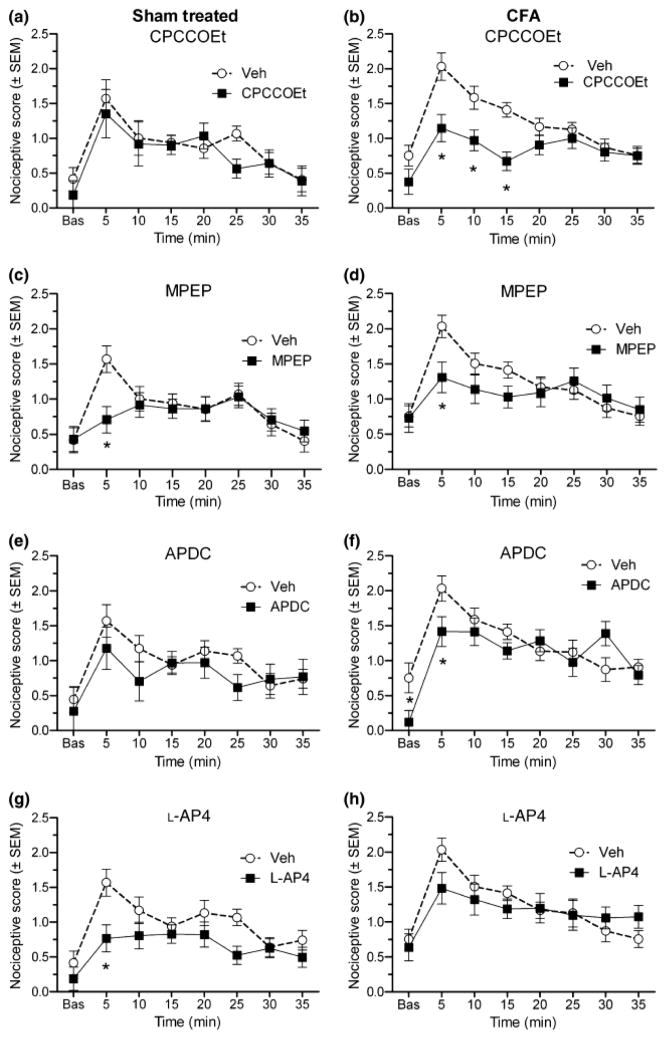
Sustained nociceptive responses to capsaicin were enhanced in CFA rats as compared to sham-treated rats with scores ranging between 0.75–2.0 for CFA rats, as compared to 0.37–1.6 for sham-treated rats (Fig. 2). This difference was confirmed by a significant group effect determined by ANOVA (F(1,13) = 5.49, p < 0.05). Drug treatment with CPCCOEt did not significantly affect capsaicin-induced nociceptive scores at any time point for sham-treated rats (Fig. 2a), but significantly reduced nociceptive scores between 5 and 15 min after capsaicin for CFA rats (Fig,. 2b) (p < 0.05, Fisher’s LSD post hoc test). Drug treatment with MPEP significantly reduced nociceptive scores at 5 min post-capsaicin for both sham-treated rats (Fig 2c) and CFA rats (Fig. 2d) (p < 0.05, Fisher’s LSD post hoc test). Drug treatment with APDC did not significantly affect capsaicin-induced nociception in sham-treated rats (Fig. 2e), but significantly reduced nociceptive scores at 5 min after capsaicin for CFA rats (Fig. 2f) (p < 0.05, Fisher’s LSD post hoc test), as well as reducing baseline behaviours in CFA rats. Drug treatment with L-AP4 significantly reduced nociceptive scores at 5 min post-capsaicin for sham-treated rats (Fig. 2g), but did not affect capsaicin-induced nociception in CFA rats (Fig. 2h) (p < 0.05, Fisher’s LSD post hoc test).
Fig. 2.
Effects of antagonists of mGluR1 (A,B) and mGluR5 (C,D), and agonists of group II (E,F) and group III (G,H) mGluRs on nociceptive responses (nociceptive score +/− standard error of the mean (SEM)) to capsaicin in both sham (left column) and CFA (right column) rats. The mGluR agents reduce capsaicin-induced nociception to varying degrees in both sham and CFA rats. Asterisks indicate the time points where nociceptive scores are significantly reduced by the drug as compared to vehicle (*p < 0.05, Fisher’s LSD post hoc).
Effects on mGluR agents on formalin-induced enhancement of glutamate concentration in CCI rats
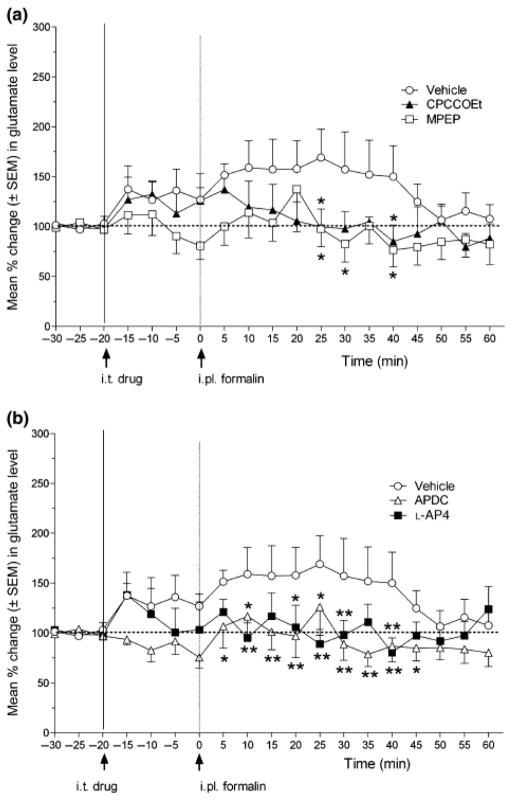
The concentration of glutamate in the microdialysis samples of CCI rats showed a sustained increase after 1.0% formalin injection for about 40 min following the formalin injection (Fig. 3). Although neither CPCCOEt nor MPEP significantly affected basal glutamate concentrations, CPCCOEt significantly reduced formalin-induced increases in glutamate concentrations at 25 and 40 minutes post-formalin. MPEP significantly reduced the formalin-induced increases in glutamate at 25, 30 and 40 min post-formalin (Fig. 3a). Furthermore, although neither APDC nor L-AP4 significantly affected basal glutamate concentrations, APDC significantly reduced the formalin-induced increases in glutamate between 5 and 45 min post-formalin, and L-AP4 significantly reduced the formalin-induced increases in glutamate at 10, 20, 25, 30 and 40 min post-formalin (Fig. 3b).
Fig. 3.
Effect of group I mGluR antagonists (A) and group II and III mGluR agonists on the increase in spinal glutamate release induced by noxious hind paw stimulation (1.0% formalin injection) in CCI rats. The line graph shows post-drug/vehicle treatment, and post-formalin injection time points for groups of rats in (A) treated with CPCCOEt (N=6), MPEP (N=7) or vehicle (N=10) (arrows indicate timing of i.t. drug/vehicle and i.pl. formalin injections at −20 and 0 min, respectively), or (B) treated with L-AP4 (N=6), APDC (N=9) or vehicle (same group as shown in A). ANOVA revealed a non-significant main effect of drug (F(4, 33) = 1.20, P > 0.05), but a significant main effect of time (F(18, 594) = 3.24, P < 0.0001) and a significant drug X time interaction (F(72, 594) = 1.98, P < 0.05). Post hoc comparisons (Fisher’s) indicated that 1.0% formalin injection produced significant increases in glutamate release up to 40 min post-formalin. Furthermore, CPCCOEt and MPEP reduced glutamate levels at the 25, 40 and 25, 30 and 40 min time points after formalin, respectively (comparison to vehicle †p < 0.05). following hind paw injection of formalin. In addition, L-AP4 reduced glutamate levels at the 10, 20, 25, 30 and 40 min time points after formalin, while APDC reduced glutamate levels at from 5 to 40 min after formalin (comparison to vehicle †p < 0.05, ** p < 0.01).
Effects on mGluR agents on capsaicin-induced enhancement of glutamate concentration in CFA rats
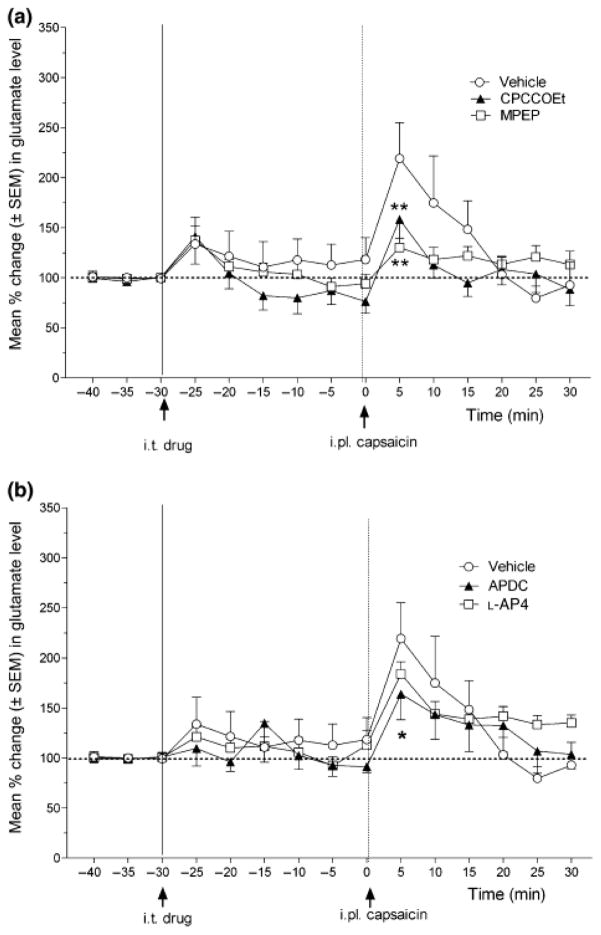
The concentration of glutamate in the microdialysis samples of CFA rats showed a sustained increase after 0.03% capsaicin injection for about 15 min following the capsaicin injection (Fig. 4). Although neither CPCCOEt nor MPEP significantly affected basal glutamate concentrations, both CPCCOEt and MPEP significantly reduced the capsaicin-induced increases in glutamate 5 min post-capsaicin (Fig. 4a). Furthermore, although neither APDC nor L-AP4 significantly affected basal glutamate concentrations, APDC (but not L-AP4) significantly reduced the capsaicin-induced increases in glutamate at 5 min post-formalin (Fig. 4b).
Fig. 4.
Effect of group I mGluR antagonists (A) and group II and III mGluR agonists on the increase in spinal glutamate release induced by noxious hind paw stimulation (0.03% capsaicin injection) in CFA rats. The line graph shows post-drug/vehicle treatment, and post-capsaicin injection time points for groups of rats in (A) treated with CPCCOEt (N=6), MPEP (N=9) or vehicle (N=6) (arrows indicate timing of i.t. drug/vehicle and i.pl. capsaicin injections at −20 and 0 min, respectively), or (B) treated with L-AP4 (N=6), APDC (N=6) or vehicle (same group as shown in A). ANOVA revealed a non-significant main effect of drug (F(4, 29) = 1.09, P > 0.05), but a significant main effect of time (F(14, 281) = 12.65, P < 0.0001) and a significant drug X time interaction (F(56, 281) = 1.46, P < 0.05). Post hoc comparisons (Fisher’s) indicated that 0.03% capsaicin injection produced significant increases in glutamate release up to 10 min post-capsaicin. Furthermore, CPCCOEt, MPEP and APDC reduced glutamate levels at the 5 min time points after capsaicin (comparison to vehicle *p < 0.05, ** p < 0.01) following hind paw injection of capsaicin, while L-AP4 was not effective.
Discussion
We had previously shown that while CCI rats do not have higher basal levels of spinal glutamate, they do have enhanced nociceptive responses to dilute (1.0%) formalin, as compared with shams (Coderre et al., 2005). We report here also that CCI rats show greater nociceptive reponses to formalin, a result that is consistent with enhanced formalin nociception in rats with spinal nerve ligation (LaBuda et al., 2001) or diabetic neuropathy (Cesena & Calcutt, 1999; Courteix et al., 1993). However, opposite effects have been found in CCI rats by Vissers et al. (2003) or SNL rats by Kaku et al. (2007), findings that at least for CCI rats may depend on hormonal effects (Vissers et al., 2003). Pilot studies demonstrated that CFA rats failed to exhibit significant elevations in spinal glutamate concentrations after intraplantar formalin injection (data not shown). Thus, we used formalin to stimulate spinal glutamate release in CCI rats, and capsaicin to stimulate spinal glutamate release in CFA rats. We show here that CFA rats exhibited a pronounced and persistent elevation of spinal glutamate in response to intraplantar capsaicin injections. This finding is consistent with previous studies showing that capsaicin-evoked glutamate release in spinal cord in vitro is enhanced in spinal slices taken from rats with chronic inflammation (Sasaki et al., 1998). We also find that nociceptive responses to capsaicin are enhanced in CFA rats, a finding that has been reported previously for both CFA and carrageenan-treated rats (Menendez et al., 2004), and is consistent with observations that CFA treatment leads to an enhanced response to capsaicin in non-peptidergic (IB4-positive) primary afferent nociceptors (Breese et al., 2005).
While none of the mGluR agents used affected the pre-noxious stimulus concentrations of spinal glutamate, most were effective at reducing noxious stimulus-induced increased in spinal glutamate, as well as the nociceptive responses to formalin and capsaicin. The mGluR1 antagonist CPCCOEt significantly reduced the nociceptive effects to formalin in both shams and CCI rats, but only reduced capsaicin-induced nociception in CFA rats. This is consistent with an earlier report that CPCCOEt did not affect dorsal horn neuron responses in naïve rats, but reduced enhanced dorsal horn neuronal activity in rats with knee inflammation (Li & Neugebauer, 2004). mGluR1 antagonists have also previously been found to reduce allodynia or hyperalgesia in rats with both nerve injury (Fisher et al., 2002; Kohara et al., 2007) or hind paw inflammation (Zhang et al., 2002; Kohara et al., 2007). The mGluR1 antagonist CPCCOEt also significantly reduced capsaicin-induced enhancement of spinal glutamate in CFA rats, and suppressed formalin-induced enhancement of spinal glutamate in CCI rats. The presence of mGluR1 on both myelinated and unmyelinated primary afferent neurons (Zhuo et al., 2001), and a spinal presynaptic localization of these receptors (Tang & Sim, 1999), suggests that mGluR1 may play a role in regulating spinal glutamate release. The present study provides the first evidence that an mGluR1 antagonist reduces noxious stimulus-induced glutamate release in CCI and CFA rats. mGluR1 is found throughout the dorsal and ventral spinal cord, with the exception of the superficial dorsal horn (Alvarez et al. 2000; Pitcher et al., 2007). Its highest concentration in lamina V (Pitcher et al., 2007), the termination zone of Aβ and C-fiber primary afferents, and the site of most wide dynamic range projection neurons (Todd and Ribeiro da Silva, 2005), is appropriate for a role in nociceptive processing.
The mGluR5 antagonist MPEP also significantly reduced the nociceptive effects of both formalin and capsaicin. However, MPEP reduced nociception to formalin only in CCI rats and not shams. Importantly, mGluR5 antagonists have been found to reduce allodynia or hyperalgesia in rats with both nerve injury (Fisher et al., 2002; Zhu et al., 2004) or hind paw inflammation (Zhang et al., 2002; Zhu et al., 2004) pain. Also, an mGluR5 antagonist inhibits the enhanced spontaneous and evoked EPSPs in rats with diabetic neuropathy, implicating mGluR5-mediated increases in glutamate release in these animals (Li et al., 2009). The mGluR5 antagonist MPEP also reduced both formalin-stimulated increases in spinal glutamate in CCI rats, as well as capsaicin-stimulated increases in spinal glutamate in CFA rats. mGluR5 has been specifically localized to the superficial SCDH (Valerio et al. 1997; Alvarez et al. 2000), the termination zone of both Aδ and C-fiber nociceptors (Todd and Ribeiro da Silva, 2005), which likely accounts for its prominent role in nociceptive processing. We have recently shown that there is an upregulation and increased trafficking of mGluR5 receptors to neuronal membranes in SCDH neurons of CFA-treated rats (Pitcher et al., 2007). While most mGluR5 is exhibited on post-synaptic spinal neurons, the receptors are also observed on the central terminals of primary afferent neurons (Vidnyanszky et al 1994), and therefore are positioned to play a role in the regulation of spinal glutamate release. We have previously shown that i.t. DHPG produces nociceptive behaviours that are reduced by glutamate release inhibitors, suggesting that DHPG may be acting at presynaptic group I mGluRs to enhance glutamate release (Lefebvre et al., 2000). Furthermore, since both nociceptive behaviours and enhanced spinal release of glutamate induced by i.t. DHPG are blocked by pretreatment with the mGluR5 antagonist MPEP (Lorrain et al. 2002), this suggested that presynaptic mGluR5 may play a prominent role in the effects of i.t. DHPG. The present study provides the first evidence that an mGluR5 antagonist reduces noxious stimulus-induced glutamate release in CCI and CFA rats.
The group II mGluR agonist APDC significantly reduced the nociceptive effects to formalin in shams, and to formalin in CCI rats or capsaicin in CFA rats, again with greater effects in the injured rats than shams. This is consistent with findings that group II agonists reduce allodynia or hyperalgesia in rats with nerve injury (Simmons et al., 2002, Fisher et al., 2002) or hind paw inflammation (Sharpe et al., 2002), although group II antagonists have also been found to reduce mechanical allodynia in rats with peripheral inflammation (Zhang et al., 2009). Furthermore, a group II selective mGluR agonist caused both inhibition and facilitation of evoked responses in spinal projection neurons of normal animals, but produced only inhibition following carrageenan-induced inflammation (Stanfa & Dickenson, 1998). APDC also significantly reduced the increased glutamate release induced by formalin in CCI rats or by capsaicin in CFA rats. mGluR2/3 are expressed moderately in lamina II–V (Tang & Sim, 1999; Boxall et al., 1998), and have been found on axon terminals (Petralia et al., 1996; Carlton et al., 2001). Their presynaptic locus would allow for inhibitory effects on glutamate release. Indeed, there is electrophysiological evidence that activation of presynaptic group II mGluRs inhibits spinal glutamatergic neurotransmission (Gerber et al., 2000). The present study provides the first neurochemical evidence that a group II mGluR agonist reduces noxious stimulus-induced spinal glutamate release in CCI or CFA rats. Importantly, spinal mGluR3 is upregulated in rats with peripheral inflammation (Boxall et al., 1998), raising the possibility that group II receptors are increased as a defense mechanism. In addition, L-Acetylcarnitine a drug used to treat neuropathic pain, has been found to upregulate mGluR2 in the SCDH, as well as reducing hyperalgesia in CCI rats (Chiechio et al., 2002)
The group III agonist L-AP4 also reduced formalin-induced nociception in both sham and CCI rats, but while it reduced nociceptive responses to capsaicin at one time point in shams, it did not affect capsaicin-induced nociception in CFA rats. These results were paralled by the findings that L-AP4 reduced the formalin-induced increase in spinal glutamate release in CCI rats, but did not affect the capsaicin-induced increase in glutamate release in CFA rats. These results are consistent with findings that L-AP4 reduces allodynia and hyperalgesia and evoked dorsal horn neuron responses in neuropathic rats (Fisher et al., 2002; Chen & Pan, 2005). There are no reports that L-AP4 reduces allodynia or hyperalgesia in rats with peripheral inflammation, although recently a selective mGluR8 agonist has been demonstrated to be anti-hyperalgesic in mice with carrageenan-injected hind paws (Marabese et al., 2007). Another group III agonist ACPT-1 and PHCCC, a positive allosteric modulator of mGluR4, reduced hyperalgesia in rats with carrageenan-induced inflammatory or in neuropathic rats (Goudet et al., 2008). All group III mGluR receptors have been found in SCDH except mGluR6 (Valerio et al., 1997), which is mostly found in the retina. The presence of mGluR4,7 and 8 either on presynaptic terminals of primary afferent neurons in the SCDH (Azkue et al., 2001; Ohishi et al., 1995) implicate them as playing a role in the regulation of spinal glutamate release. There is also electrophysiological evidence that activation of presynaptic group III mGluRs inhibits spinal glutamatergic neurotransmission (Gerber et al., 2000), effects that have been attributed to actions at either mGluR4 or mGluR7 (Gerber et al., 2000), although a selective mGluR8 agonist also produces a presynaptic inhibitory effect on glutamate transmission in neonatal rat spinal cord (Thomas et al., 2001). Recent evidence indicates that the inhibitory effects of group III mGluRs on spinal glutamate neurotransmission (assessed by inhibition of miniature EPSPs) is enhanced in neuropathic rats (Zhang et al., 2009). The present study provides the first neurochemical evidence that a group III mGluR agonist reduces noxious stimulus-induced spinal glutamate release in CCI rats.
In summary, group I mGluR antagonists and/or group II agonists attenuate nociception and the noxious stimulus-induced glutamate release in SCDH of CCI and/or CFA rats in vivo, and suggests a possible mechanism for their antihyperalgesic effects. The data are consistent with the hypothesis that group I mGluRs are pronociceptive in part by enhancing, and group II and III mGluRs are antinociceptive by inhibiting, glutamate release in the SCDH.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) to TJC. The authors wish to thank Dr. Kathleen Sluka for technical assistance in the development of the methodology for in vivo microdialysis.
References
- Alvarez FJ, Villalba RM, Carr PA, Grandes P, Somohano PM. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. J Comp Neurol. 2000;422:464–487. doi: 10.1002/1096-9861(20000703)422:3<464::aid-cne11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Azkue JJ, Murga M, Fernandez-Capetillo O, Mateos JM, Elezgarai I, Benitez R, Osorio A, Diez J, Puente N, Bilbao A, Bidaurrazaga A, Kuhn R, Grandes P. Immunoreactivity for the group III metabotropic glutamate receptor subtype mGluR4a in the superficial laminae of the rat spinal dorsal horn. J Comp Neurol. 2001;430:448–457. doi: 10.1002/1096-9861(20010219)430:4<448::aid-cne1042>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Monn JA, Schoepp DD. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY354740 in rats. Neurosci Lett. 1997;229:161–164. doi: 10.1016/s0304-3940(97)00442-4. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Böttcher T, Goiny M, Bering J, Domhof S, Nau R, Ungerstedt U. Regional differences in glutamine synthetase inhibition by L-methionine sulfoximine: a microdialysis study in the rabbit brain. Exp Brain Res. 2003;50:194–200. doi: 10.1007/s00221-003-1401-0. [DOI] [PubMed] [Google Scholar]
- Boxall SJ, Berthele A, Laurie DJ, Sommer B, Zieglgansberger W, Urban L, Tolle TR. Enhanced expression of metabotropic glutamate receptor 3 messenger RNA in the rat spinal cord during ultraviolet irradiation induced peripheral inflammation. Neuroscience. 1998;82:591–602. doi: 10.1016/s0306-4522(97)00246-7. [DOI] [PubMed] [Google Scholar]
- Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115:37–49. doi: 10.1016/j.pain.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience. 2001;105:957–969. doi: 10.1016/s0306-4522(01)00238-x. [DOI] [PubMed] [Google Scholar]
- Ceseña RM, Calcutt NA. Gabapentin prevents hyperalgesia during the formalin test in diabetic rats. Neurosci Lett. 1999;262:101–104. doi: 10.1016/s0304-3940(99)00057-9. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Distinct roles of group III metabotropic receptors in control of nociception and dorsal horn neurons in normal and nerve-injured rats. J Pharmacol Exp Ther. 2005;312:120–126. doi: 10.1124/jpet.104.073817. [DOI] [PubMed] [Google Scholar]
- Chiechio S, Caricasole A, Barletta E, Storto M, Catania MV, Copani A, Vertechy M, Nicolai R, Calvani M, Melchiorri D, Nicoletti F. L-Acetylcarnitine induces analgesia by selectively up-regulating mGlu2 metabotropic glutamate receptors. Mol Pharmacol. 2002;61:989–996. doi: 10.1124/mol.61.5.989. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Kumar N, Lefebvre CD, Yu JS. Evidence that gabapentin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem. 2005;94:1131–1139. doi: 10.1111/j.1471-4159.2005.03263.x. [DOI] [PubMed] [Google Scholar]
- Courteix C, Eschalier A, Lavarenne J. Streptozocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain. 1993;53:81–88. doi: 10.1016/0304-3959(93)90059-X. [DOI] [PubMed] [Google Scholar]
- Cozzi A, Attucci S, Peruginelli F, Marinozzi M, Luneia R, Pellicciari R, Moroni F. Type 2 metabotropic glutamate (mGlu) receptors tonically inhibit transmitter release in rat caudate nucleus: in vivo studies with (2S,1′S,2′S,3′R)-2-(2′-carboxy-3′-phenylcyclo-propyl)glycine, a new potent and selective antagonist. Eur J Neurosci. 1997;9:1350–1355. doi: 10.1111/j.1460-9568.1997.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Fisher K, Lefebvre CD, Coderre TJ. The effects of intrathecal administration of selective metabotropic glutamate receptor compounds in a rat model of neuropathic pain. Pharmacol Biochem Behav. 2002;73:411–418. doi: 10.1016/s0091-3057(02)00832-8. [DOI] [PubMed] [Google Scholar]
- Gerber G, Zhong J, Youn D, Randic M. Group II and group III metabotropic glutamate receptor agonists depress synaptic transmission in the rat spinal cord dorsal horn. Neuroscience. 2000;100:393–406. doi: 10.1016/s0306-4522(00)00269-4. [DOI] [PubMed] [Google Scholar]
- Goudet C, Chapuy E, Alloui A, Acher F, Pin JP, Eschalier A. Group III metabotropic glutamate receptors inhibit hyperalgesia in animal models of inflammation and neuropathic pain. Pain. 2008;137:112–124. doi: 10.1016/j.pain.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann SF. Cloned glutamate receptors. Ann Rev of Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Kaku R, Yokoyama M, Kobayashi H, Matsuoka Y, Sato T, Mizobuchi S, Itano Y, Morita K. Altered response to formalin by L5 spinal nerve ligation in rats: a behavioral and molecular study. Anesth Analg. 2007;104:936–943. doi: 10.1213/01.ane.0000258762.22607.15. [DOI] [PubMed] [Google Scholar]
- Kohara A, Nagakura Y, Kiso T, Toya T, Watabiki T, Tamura S, Shitaka Y, Itahana H, Okada M. Antinociceptive profile of a selective metabotropic glutamate receptor 1 antagonist YM-230888 in chronic pain rodent models. Eur J Pharmacol. 2007;571:8–16. doi: 10.1016/j.ejphar.2007.05.030. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Donahue R, Fuchs PN. Enhanced formalin nociceptive responses following L5 nerve ligation in the rat reveals neuropathy-induced inflammatory hyperalgesia. Pain. 2001;94:59–63. doi: 10.1016/S0304-3959(01)00341-4. [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Fisher K, Cahill CM, Coderre TJ. Evidence that DHPG-induced nociception depends on glutamate release from primary afferent C-fibres. Neuroreport. 2000;11:1631–1635. doi: 10.1097/00001756-200006050-00007. [DOI] [PubMed] [Google Scholar]
- Li JQ, Chen SR, Chen H, Cai YQ, Pan HL. Regulation of increased glutamatergic input to spinal dorsal horn neurons by mGluR5 in diabetic neuropathic pain. J Neurochem. 2010;112:162–172. doi: 10.1111/j.1471-4159.2009.06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Differential roles of mGluR1 and mGluR5 in brief and prolonged nociceptive processing in central amygdala neurons. J Neurophysiol. 2004;91:13–24. doi: 10.1152/jn.00485.2003. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Correa L, Anderson J, Varney M. Activation of spinal group I metabotropic glutamate receptors in rats evokes local glutamate release and spontaneous nociceptive behaviors: effects of 2-methyl-6-(phenylethynyl)-pyridine pretreatment. Neurosci Lett. 2002;327:198–202. doi: 10.1016/s0304-3940(02)00393-2. [DOI] [PubMed] [Google Scholar]
- Marabese I, de Novellis V, Palazzo E, Scafuro MA, Vita D, Rossi F, Maione S. Effects of (S)-3,4-DCPG, an mGlu8 receptor agonist, on inflammatory and neuropathic pain in mice. Neuropharmacol. 2007;52:253–262. doi: 10.1016/j.neuropharm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Martín R, Torres M, Sánchez-Prieto J. mGluR7 inhibits glutamate release through a PKC-independent decrease in the activity of P/Q-type Ca2+ channels and by diminishing cAMP in hippocampal nerve terminals. Eur J Neurosci. 2007;26:312–322. doi: 10.1111/j.1460-9568.2007.05660.x. [DOI] [PubMed] [Google Scholar]
- Menéndez L, Lastra A, Hidalgo A, Baamonde A. The analgesic effect induced by capsaicin is enhanced in inflammatory states. Life Sci. 2004;74:3235–3244. doi: 10.1016/j.lfs.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Mills CD, Xu GY, Johnson KM, McAdoo DJ, Hulsebosch CE. AIDA reduces glutamate release and attenuates mechanical allodynia after spinal cord injury. Neuroreport. 2000;11:3067–3070. doi: 10.1097/00001756-200009280-00007. [DOI] [PubMed] [Google Scholar]
- Moroni F, Cozzi A, Lombardi G, Sourtcheva S, Leonardi P, Carfi M, Pellicciari R. Presynaptic mGlu1 type receptors potentiate transmitter output in the rat cortex. Eur J Pharmacol. 1998;347:189–195. doi: 10.1016/s0014-2999(98)00124-1. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Nomura S, Ding YQ, Shigemoto R, Wada E, Kinoshita A, Li JL, Neki A, Nakanishi S, Mizuno N. Presynaptic localization of a metabotropic glutamate receptor, mGluR7, in the primary afferent neurons: an immunohistochemical study in the rat. Neurosci Lett. 1995;202:85–88. doi: 10.1016/0304-3940(95)12207-9. [DOI] [PubMed] [Google Scholar]
- Park YK, Galik J, Ryu PD, Randic M. Activation of presynaptic group I metabotropic glutamate receptors enhances glutamate release in the rat spinal cord substantia gelatinosa. Neurosci Lett. 2004;361:220–224. doi: 10.1016/j.neulet.2003.12.075. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacol. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Pintor A, Pezzola A, Reggio R, Quarta D, Popoli P. The mGlu5 receptor agonist CHPG stimulates glutamate release: possible involvement of A2A receptors. Neuroreport. 2000;11:3611–3614. doi: 10.1097/00001756-200011090-00042. [DOI] [PubMed] [Google Scholar]
- Pitcher MH, Ribiero-da-Silva A, Coderre TJ. Effects of persistent peripheral inflammation on the ultrastructural localization of spinal cord dorsal horn group I metabotropic glutamate receptors. J Comp Neurol. 2007;505:412–423. doi: 10.1002/cne.21506. [DOI] [PubMed] [Google Scholar]
- Pogatzki EM, Zahn PK, Brennan TJ. Lumbar catheterization of the subarachnoid space with a 32-gauge polyurethane catheter in the rat. Eur J Pain. 2000;4:111–113. doi: 10.1053/eujp.1999.0157. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Tohda C, Kuraishi Y. Region-specific increase in glutamate release from dorsal horn of rats with adjuvant inflammation. Neuroreport. 1998;9:3219–3222. doi: 10.1097/00001756-199810050-00016. [DOI] [PubMed] [Google Scholar]
- Sharpe EF, Kingston AE, Lodge D, Monn JA, Headley PM. Systemic pre-treatment with a group II mGlu agonist, LY379268, reduces hyperalgesia in vivo. Br J Pharmacol. 2002;135:1255–1262. doi: 10.1038/sj.bjp.0704583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RMA, Webster AA, Kalra AB, Iyenger S. Group II mGluR receptor agonists are effective in persistent and neuropathic pain models in rats. Pharmacol Biochem Behav. 2002;73:419–427. doi: 10.1016/s0091-3057(02)00849-3. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Westlund KN. An experimental arthritis in rats: dorsal horn aspartate and glutamate increases. Neurosci Lett. 1992;145:141–144. doi: 10.1016/0304-3940(92)90006-s. [DOI] [PubMed] [Google Scholar]
- Soliman AC, Yu JSC, Coderre TJ. mGlu and NMDA receptor contributions to capsaicin-induced thermal and mechanical hypersensitivity. Neuropharmacology. 2005;48:325–332. doi: 10.1016/j.neuropharm.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Song JH, Park ES, Han SM, Han SR, Ahn DK, Youn DH. Signal transduction mechanisms underlying group I mGluR-mediated increase in frequency and amplitude of spontaneous EPSCs in the spinal trigeminal subnucleus oralis of the rat. Mol Pain. 2009;5:50. doi: 10.1186/1744-8069-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfa LC, Dickenson AH. Inflammation alters the effects of mGlu receptor agonists on spinal nociceptive neurones. Eur J Pharmacol. 1998;347:165–172. doi: 10.1016/s0014-2999(98)00098-3. [DOI] [PubMed] [Google Scholar]
- Tang FR, Sim MK. Pre- and/or post-synaptic localisation of metabotropic glutamate receptor 1alpha (mGluR1alpha) and 2/3 (mGluR2/3) in the rat spinal cord. Neurosci Res. 1999;34:73–78. doi: 10.1016/s0168-0102(99)00035-8. [DOI] [PubMed] [Google Scholar]
- Thomas LS, Jane DE, Gasparini F, Croucher MJ. Glutamate release inhibiting properties of the novel mGlu(5) receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP): complementary in vitro and in vivo evidence. Neuropharmacol. 2001;41:523–527. doi: 10.1016/s0028-3908(01)00091-0. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Ribeiro da Silva A. Molecular architecture of the dorsal horn. In: Hunt S, Koltzenburg M, editors. The Neurobiology of Pain. Oxford University Press; New York: 2005. [Google Scholar]
- Valerio A, Paterlini M, Boifava M, Memo M, Spano P. Metabotropic glutamate receptor mRNA expression in rat spinal cord. Neuroreport. 1997;8:2695–2699. doi: 10.1097/00001756-199708180-00012. [DOI] [PubMed] [Google Scholar]
- Vidnyánszky Z, Hamori J, Negyessy L, Ruegg D, Knopfel T, Kuhn R, Gorcs TJ. Cellular and subcellular localization of the mGluR5a metabotropic glutamate receptor in rat spinal cord. Neuro Report. 1994;6:209–213. doi: 10.1097/00001756-199412300-00053. [DOI] [PubMed] [Google Scholar]
- Vissers K, Adriaensen H, De Coster R, De Deyne C, Meert TF. A chronic-constriction injury of the sciatic nerve reduces bilaterally the responsiveness to formalin in rats: a behavioral and hormonal evaluation. Anesth Analg. 2003;97:520–525. doi: 10.1213/01.ANE.0000068886.23855.C4. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Shen H, Baker DA, Kalivas PW. Inhibition of non-vesicular glutamate release by group III metabotropic glutamate receptors in the nucleus accumbens. J Neurochem. 2003;87:1204–1212. doi: 10.1046/j.1471-4159.2003.02093.x. [DOI] [PubMed] [Google Scholar]
- Yang LC, Marsala M, Yaksh TL. Characterization of time course of spinal amino acids, citrulline and PGE2 release after carrageenan/kaolin-induced knee joint inflammation: a chronic microdialysis study. Pain. 1996;67:345–354. doi: 10.1016/0304-3959(96)03106-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lu Y, Chen Y, Westlund KN. Group I metabotropic glutamate receptor antagonists block secondary thermal hyperalgesia in rats with knee joint inflammation. J Pharmacol Exp Ther. 2002;300:149–156. doi: 10.1124/jpet.300.1.149. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhang J, Shi J, Feng Y, Sun ZS, Li H. Antinociceptive synergistic effect of spinal mGluR2/3 antagonist and glial cells inhibitor on peripheral inflammation-induced mechanical hypersensitivity. Brain Res Bull. 2009;79:219–223. doi: 10.1016/j.brainresbull.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Zhu CZ, Wilson SG, Mikusa JP, Wismer CT, Gauvin DM, Lynch JJ, 3rd, Wade CL, Decker MW, Honore P. Assessing the role of metabotropic glutamate receptor 5 in multiple nociceptive modalities. Eur J Pharmacol. 2004;506:107–118. doi: 10.1016/j.ejphar.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Zhou S, Komak S, Du J, Carlton SM. Metabotropic glutamate 1a receptors on peripheral primary afferent fibers: their role in nociception. Brain Res. 2001;913:18–26. doi: 10.1016/s0006-8993(01)02747-0. [DOI] [PubMed] [Google Scholar]