Abstract
Movements that involve simultaneous coordination of muscles of the right and left lower limbs form a large part of our daily activities (e.g., standing, rising from a chair). This study used functional magnetic resonance imaging (fMRI) to determine which brain areas are used to control coordinated lower limb movements, specifically comparing regions that are activated during bilateral exertions to those performed unilaterally. Plantarflexor exertions were produced at a target force level of 15% of the participants’ maximum voluntary contraction, in three conditions, with their right (dominant) foot, with their left foot and with both feet simultaneously. A voxel-wise analysis determined which regions were active in the bilateral, but not in the unilateral conditions. In addition, a regions of interest (ROI) approach was used to determine differences in the percent signal change (PSC) between the conditions within motor areas. The voxel-wise analysis showed a large number of regions (cortical, subcortical and cerebellar) that were active during the bilateral condition, but not during either unilateral condition. The ROI analysis showed several motor regions with higher activation in the bilateral condition than unilateral conditions; further, the magnitude of bilateral PSC was more than the sum of the two unilateral conditions in several of these regions. We postulate that the greater levels of activation during bilateral exertions may arise from interhemispheric inhibition, as well as from the greater need for motor coordination (e.g., synchronizing the two limbs to activate together) and visual processing (e.g., monitoring of two visual stimuli).
Keywords: fMRI, Motor Control, Lower-Limb
Introduction
Movements that involve simultaneous coordination of muscles of the right and left lower limbs form a large part of our daily activities (e.g., standing, rising from a chair). With the advent of neuroimaging techniques, such as functional magnetic resonance imaging (fMRI), it is now possible to identify which brain areas are associated with the recruitment of coordinated motor tasks with the lower limbs, and associated actions that accompany this muscle activation.
While functional neuroimaging has been utilized to examine the brain areas during static (isometric) (Newton et al. 2008) or dynamic movements of a single leg (Ciccarelli et al. 2005; Debaere et al. 2001; Mehta et al. 2012; Trinastic et al. 2010), no previous studies have attempted to determine the additional brain areas required to coordinate simultaneous bilateral leg movements.
A review by Swinnen and Wenderoth (2004) showed that the supplementary motor area (SMA), dorsal premotor cortex (PMd) and cerebellum are commonly involved in the control of upper-extremity bimanual tasks. However, bilateral control may differ between the upper and lower extremities, particularly since the legs do not exhibit the same degree of side-dominance, nor fine motor skill as the arms (Kapreli et al. 2006).
The overall goal of this study was to determine which brain areas are used to control coordinated lower limb movements, specifically comparing regions that are activated during bilateral exertions to those performed unilaterally. Knowledge regarding the neural mechanisms involved in coordinated movements can help inform rehabilitation strategies in populations with difficulties with motor control. For example, there has been interest in the use of bilateral movements for rehabilitation purposes in patients with hemiparesis because it is thought that the extra neural activity that is required for coordinating bilateral movement could lead to greater neuroplasticity (Whitall et al. 2011). Specifically we used fMRI to determine the brain areas that are associated with the control of plantarflexion movements and compared regions that were activated during unilateral exertions to those performed bilaterally. We hypothesized that brain areas, specifically the SMA, would demonstrate activation due to its known role in the coordination of bilateral movements. We also hypothesized that greater activity would be observed in the PMd and the cerebellum, due to their known role in the control of coordinated movement patterns. It was further hypothesized that additional brain activation during a bilateral task would be beyond the sum required to activate each individual foot separately, as there would be unique areas of activation necessary to control the bilateral exertions.
Methods
Participants
A total of 11 individuals with normal or corrected to normal vision volunteered to participate in this study (4 males and 7 females, 19–34 years in age). Participants were screened to ensure they were free of any health conditions that would affect their performance in this study, and to ensure that there were no contraindications for MRI scanning. All participants self-reported being right hand dominant and were right foot dominant according to the revised Waterloo Footedness Questionnaire (Elias et al. 1998). All participants provided written and verbal informed consent prior to participation, and the local University and Hospital Ethical Review Boards evaluated all methods in this study.
Procedure
Force Recording Apparatus
Participants produced plantarflexion exertions against a custom made apparatus that measured the participant’s effort relative to their maximum voluntary contraction (MVC) (Figure 1). The device consisted of two 100cc syringes that were mounted on an adjustable base secured to the end of the patient table in the MRI suite via rails on the bottom of the platform. The position and angle of the syringes were adjusted such that the participant pushed on the plunger of the syringe (40 mm diameter) with the ball of each foot. Each of the syringes connected to a pressure transducer, located in the MRI control room, by a 1/4-inch (6.35 mm) diameter PVC hose. A standard bicycle pump was used to increase the air pressure within the chamber of the syringes from the MRI control room. The participants lay in a supine position while performing the task with their knees bolstered such that their knees were at 90°, while their hips were set at 30°, with the ankle joint at 90°. Participants were restrained by a Velcro strap around their hips and around the distal end of their legs, just superior to the ankle joint. Pressure within the chamber of each syringe was set to a baseline level (minimal pressure to overcome) between 8–10 PSI (55–78 kPa), which allowed 3–4 cm of movement when the participant pushed on the syringe with maximum effort levels. This pressure was sampled from the pressure transducers using custom LabVIEW software at a rate of 60 Hz.
Figure 1.
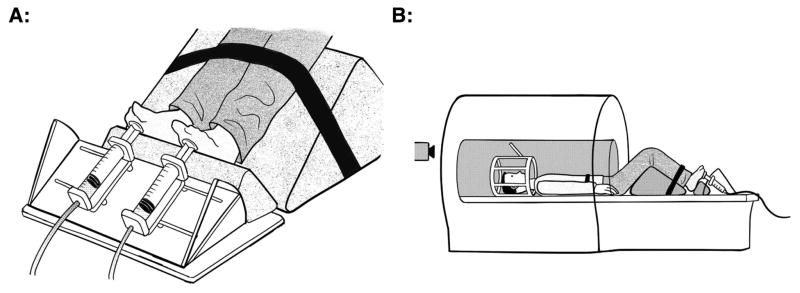
Drawings illustrating the custom apparatus that was used to measure the force of plantar flexion exertions. The device is shown in panel A, while the setup of the device within the MRI is shown in panel B.
Protocol
All participants in this study attended two sessions. In the first session the participants practiced the task that they would perform in the MRI. During the practice session electromyography (EMG) was recorded from several lower limb muscles, to ensure there were no mirror movements during unilateral tasks and that the prime mover for the task was limited to the soleus muscle.
The task required the participant to produce a plantarflexion exertion against resistance to match a target force level presented on a screen. Maximal voluntary contraction (MVC) for each limb was obtained unilaterally, over a 5s trial. Three MVC trials were carried out for each foot and the highest level of pressure achieved across the three trials with that foot was used for the MVC. Separate MVCs were obtained in the practice session and at the fMRI scanning session.
Three primary conditions were analyzed: 1) right foot (R15-ONLY) at 15% of the participant’s MVC, 2) left foot (L15-ONLY) at 15% MVC, 3) with both feet simultaneously (BILAT15) with each foot at 15% MVC. All exertions were near isometric lasting for 5 seconds. Additional unilateral contractions were assessed at 30% MVC (R30-ONLY and L30-ONLY); these conditions were utilized to compare the brain activation between unilateral exertions to bilateral exertions when the same overall level of force (15% MVC per foot during the bilateral condition or 30% MVC unilaterally) was being produced by the motor system. The conditions were presented in a random order. Participants were cued to time exertions by a back-projected image in the MRI, where two adjacent solid bars would appear on a screen with the left bar representing the left foot, and right bar representing the right foot. Participants had to match a moving bar, which indicated their current force level, to the target bar. Participants were instructed to try to achieve the target force level as quickly as possible and to stop producing force as quickly as possible once the bars disappeared. In between exertions, the participants were asked to fixate on a small cross at the centre of the screen. Two seconds prior to each exertion the cross would double in size to indicate an upcoming trial. Between each exertion the participant rested between 10 and 16 s; the length of rest periods was random. There were a total of 4 exertions in each condition during each run of exertions. There were an additional number of unilateral conditions that were recorded during the same sessions at different force levels, which were not analyzed for this study.
fMRI data acquisition
All MRI data was acquired with a Philips Gyroscan Intera 3.0 T scanner (Philips, Best, the Netherlands), which was equipped with a 16-channel head-coil. Four functional runs were carried out where T2*-weighted echo-planar images (EPI) were acquired (matrix size = 128 x 128, pixel size = 1.9 x 1.9 mm, TR=2000 ms, TE = 3.7 ms, Flip Angle = 90°). Each functional run acquired a volume consisting of 36 axial slices of 3 mm thickness, with a gap thickness of 1 mm. Each functional run lasted a total of 572 s (286 TRs). A high-resolution T1-weighted anatomical scan was also acquired for each participant (170 axial slices, matrix size =256x256, voxel size = 1.0 x 1.0 x 1.0 mm, TE = 5ms, TR = 24ms, Flip Angle = 40°). In the MRI the participants lie in a supine position with their lower limbs in the same position as the practice trials.
To minimize head movement during fMRI data acquisition, participants’ lower limbs were restrained with a Velcro strap, just proximal to the ankle joints, and around the hips. Additionally participants’ heads were held in place by a memory foam pillow within the head coil.
Data Processing
Behavioral Data
Pressure data from the force recording apparatus from the fMRI session were filtered at 6Hz with a dual pass second order Butterworth filter to eliminate any high frequency noise. The mean magnitude of the pressure was extracted from the filtered pressure signal for the middle 2s of each 5s exertion. The mean magnitude was recorded relative to the participants’ maximum effort (%MVC). These values were analyzed with a two-factor repeated measures ANOVA to determine whether there were any differences in the forces produced by the left or right foot, or between the laterality conditions (forces produced unilaterally or bilaterally).
fMRI Analysis
The fMRI data was analyzed using an event-related paradigm. All fMRI data were analyzed using the open-source software Analysis of Functional NeuroImaging (AFNI) (Cox 1996). Functional data for each participant were first spatially aligned to remove head motion artifacts, then data from the three runs were co-registered to the same coordinate system and concatenated into a single series of volumes for data analysis. None of the participants had head motion that exceeded 3 mm in any direction. The skull was stripped from the anatomical image, which was then aligned and registered to the concatenated functional data. The functional data were then analyzed with a General Linear Model (GLM) to produce impulse response functions (IRFs) on a voxel-wise level, for each participant. The regression matrix for the GLM consisted of seven vectors, containing boxcar functions representing the timing of the stimuli to produce an exertion in each condition (R15-ONLY, L15-ONLY, R30-ONLY, L30-ONLY and BILAT15 and two other unilateral conditions that were not examined in this study), with each block in the boxcar function having a duration of 5s. An additional six vectors were included in the regression matrix that described the participant’s head motion (3-translation vectors and 3-rotation vectors). These vectors were included as regressors of no-interest to help account for variability induced by head motion.
The results of the GLM analysis provided baseline coefficients for each functional run, which defined the variation in baseline activity during the resting periods, for each participant. The percent signal change (PSC) was estimated on a voxel-wise level across the whole brain, for each participant, by dividing the regression coefficient for the four conditions by the average of the baseline coefficients and multiplying by 100. The PSC value was calculated on a voxel-wise basis, for each participant.
All data was transformed to Talairach-Tournoux space (Talairach and Tournoux 1988) by the AFNI software package using an automated process. The transformation was determined by the software package and applied to both the processed functional images and the anatomical image. A Gaussian blur with a kernel width of 4 mm at full-width-half-maximum was then applied to each of the processed functional images containing the PSC values, to account for anatomical variability and allow for the application of statistical tests between participants.
Voxel-wise fMRI Analysis
A voxel-wise fMRI analysis was completed with a two factor mixed model ANOVA, with the fixed factor being the exertion condition and the participants as a random factor. Because we were primarily interested in areas that were uniquely activated when both feet were moved at the same time, a conjunction analysis was carried out to identify clusters that were significantly active during the bilateral condition, but were not active during either unilateral condition (BILAT15 ∩ ¬(R15-ONLY ∪ L15-ONLY)). A voxel was considered to be significantly active if it achieved a t-value of 5.05, which corresponded to a p-value of 0.0005. A clustering algorithm was applied to the conjunction map in order to find clusters that consisted of more than 200 voxels. The anatomical location associated with the centre of mass of each cluster was identified with the use of an automated online Talairach atlas (Lancaster et al. 2000). The average PSC values within each cluster, in each of the conditions were then determined and compared with a repeated measured ANOVA. Post-hoc comparisons of Bonferroni corrected means were then used to determine differences in the magnitude of activation between each condition. Alpha was set to 0.01 for these comparisons.
A similar conjunction analysis was used to determine areas of the brain that were uniquely active in the bilateral condition compared the unilateral exertions at 30% MVC (BILAT15 ∩ ¬(R30-ONLY ∪ L30-ONLY)). Again, voxels were considered to be significantly active if the analysis achieved a t-value of 5.05, which corresponded to a p-value of 0.0005. A clustering algorithm was used to identify clusters greater than 200 voxels. An online Talairach atlas (Lancaster et al. 2000) was used to identify the brain region associated with the centre of mass of each cluster. The mean PSC value in each cluster was identified in the BILAT-15, R30-ONLY and L30-ONLY conditions and as compared between conditions with a repeated measures ANOVA. Post-hoc comparisons were carried out to determine significant differences between conditions by comparing Bonferroni corrected means. An alpha value of 0.01 was used for these comparisons. This secondary analysis was conducted to highlight the magnitude of the differences in brain activity in the bilateral condition at 15%, compared to unilateral conditions where the participants produced the same net level of force.
Regions of Interest fMRI Analysis
Given the motor nature of the task, a regions of interest analysis was carried out using standard templates that represent human cortical motor areas (Mayka et al. 2006). The average PSC values in each of laterality conditions (R15-ONLY, L15-ONLY, BILAT15) were identified in 6 ROIs for the right and left hemisphere for a total of 12 ROIs. The following cortical ROIs were identified a priori and analyzed for the right and left hemisphere: primary motor cortex (M1), primary sensory cortex (S1), supplementary motor area (SMA), pre-supplementary motor area (pSMA), dorsal pre-motor cortex (PMd), and ventral pre-motor cortex (PMv). A repeated measures ANOVA was carried out to determine any differences in the mean PSC between conditions for each ROI. The individual ANOVAs were considered significant at an alpha level of 0.01. To compare the brain activation between unilateral exertions to bilateral exertions at the same overall level of force, separate ANOVAs were carried out to determine the differences between BILAT15 and R30-ONLY or L30-ONLY. A post-hoc analysis of Bonferroni corrected pairwise comparisons was carried out on the results of significant ANOVAs.
Results
Behavioural Results
Analysis of the electromyography data obtained during the practice session showed that there were no mirror movements during the unilateral exertions (no muscle activation on the contralateral limb). It was also observed that the task was isolated to the soleus muscle, with a small burst of activity in the tibialis anterior muscle at the end of each exertion, to lift the foot off the pedal.
The raw and normalized mean pressure level generated by the participants in each of the conditions is shown in Table 1. All the participants were able to match the target pressure level within 1% MVC error with the right foot and within 2% MVC error with the left foot, whether they were performing the task unilaterally or bilaterally. Although there was no significant difference in the absolute maximal effort force produced between the left and right foot (t(10) = −0.92, p = 0.382), the forces produced during L15-ONLY were 13% lower than R15-ONLY (F(1,10) = 5.66, p = 0.039). There was a significant effect on the applied force between the bilateral and unilateral exertions (F(1,10) = 6.561, p = 0.028); post-hoc analysis showed the participants produced the same amount of force with their left foot between L15-ONLY and BILAT15, while with the right foot a 1% higher level of force was produced in R-15-ONLY compared to BILAT15. This small difference between conditions was not considered to be meaningful.
Table 1.
Mean (N = 11) raw and scaled pressures produced by the participants in each condition.
| L15-ONLY | R15-ONLY | BILAT15 - Left | BILAT15 - Right | L30 - ONLY | R30 -ONLY | |
|---|---|---|---|---|---|---|
| Raw Force (kPa) | 11.5 | 14.4 | 11.1 | 13.9 | 23.4 | 28.1 |
| SD (kPa) | 2.4 | 5.4 | 2.3 | 4.7 | 4.7 | 7.6 |
| Scaled Force (% MVC) | 14.0 | 16.1 | 13.5 | 15.6 | 28.2 | 30.3 |
| SD (% MVC) | 1.3 | 3.4 | 2.7 | 3.7 | 1.8 | 2.97 |
The right foot also produced slightly higher forces than the left foot when the target was set to 30% MVC (t(10) = −2.36, p = 0.016). In terms of absolute difference, the force produced by the left foot was only 2.1 kPa or 6.8% lower than the force produced by the right foot. This small difference was not considered to be meaningful, despite being statistically significant.
Voxel wise Results
Illustrations of the conjunction analysis showing clusters that were significantly activated in the BILAT-15 condition compared to the R15-ONLY and L15-ONLY conditions are shown in Figure 2. A total of 22 clusters that were greater than 200 voxels in size were identified were identified as being uniquely active in the BILAT15 condition. Details regarding these clusters are given in Table 2. The post-hoc analysis of the average PSC values in each cluster revealed that the average PSC values were greater in the BILAT15 condition compared to the unilateral conditions. There were no differences observed between the R15-ONLY and L15-ONLY conditions.
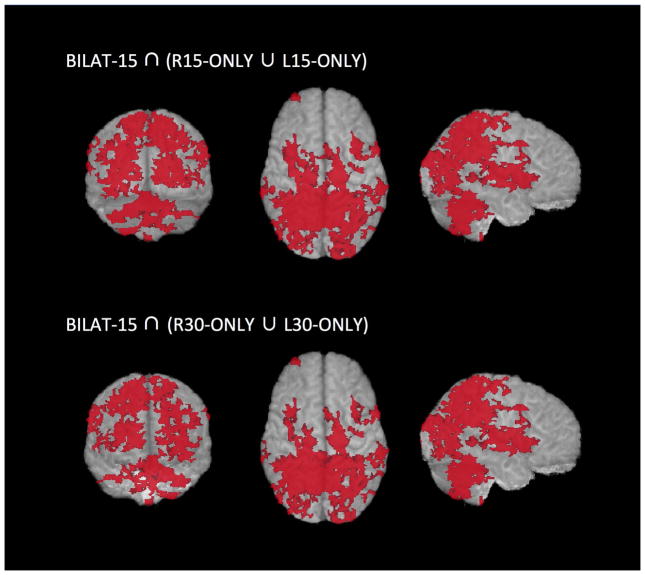
Figure 2.
Glass-brain representations of the results of the conjunction analysis (BILAT-15 ∩ ¬(R15-ONLY ∪ L15-ONLY)). Clusters that are uniquely activated in the bilateral condition are shown when compared to the unilateral exertions at 15% MVC in the top row, and compared to the unilateral exertions at 30% MVC in the bottom row. Clusters greater than 200 voxels in size are shown.
Table 2.
Clusters showing unique activation in the BILAT condition, compared to the unilateral conditions at 15% MVC. Clusters of more than 200mm3. Coordinates are listed in MNI space, and indicate the centre of mass for each cluster. All of the clusters listed had greater average PSC values in the BILAT condition, compared to each of the unilateral conditions at 15% MVC. There were no differences detected in average PSC values within these clusters between the L15-ONLY and R15-ONLY conditions.
| Cluster # | Voxels | CoM Coordinates
|
Description | BA | ||
|---|---|---|---|---|---|---|
| MNI X (mm) | MNI Y (mm) | MNI Z (mm) | ||||
| 1 | 28722 | −6 | −55 | 43 | L. Precuneus | 7 |
| 2 | 17870 | −6 | −53 | −36 | L. Nodule | |
| 3 | 5608 | −27 | −9 | 8 | L. Lentiform Nuc. / Putamen | |
| 4 | 4085 | 23 | −84 | 13 | R. Middle Occipital Gyrus | 17 |
| 5 | 2944 | 15 | −19 | 9 | R. Thalamus (VPL) | |
| 6 | 2165 | 58 | −36 | 32 | R. Inferior Parietal Lobule | 40 |
| 7 | 1772 | 37 | 8 | 6 | R. Insula | 13 |
| 8 | 843 | −40 | −55 | −15 | L. Fusiform Gyrus | 37 |
| 9 | 680 | 6 | 2 | 43 | R. Cingulate Gyrus | 24 |
| 10 | 564 | 10 | −68 | 1 | R. Lingual Gyrus | 18 |
| 11 | 560 | 54 | 6 | 28 | R. Inferior Frontal Gyrus | 44 |
| 12 | 559 | −62 | −36 | 38 | L. Inferior Parietal Lobule | 40 |
| 13 | 424 | −21 | −89 | −11 | L. Middle Occipital Gyrus | 18 |
| 14 | 412 | −19 | −26 | 63 | L. Precentral Gyrus | 4a/6 |
| 15 | 389 | −14 | −77 | −13 | L. Lingual Gyrus | 18 |
| 16 | 349 | 45 | −77 | 4 | R. Middle Occipital Gyrus | 19 |
| 17 | 337 | 19 | −21 | 44 | R. Cingulate Gyrus | 24 |
| 18 | 331 | 14 | −14 | 61 | R. Medial Frontal Gyrus | 6 |
| 19 | 294 | −47 | −81 | 22 | Middle Temporal Gyrus | 39 |
| 20 | 294 | −10 | −21 | 71 | L. Precentral Gyrus | 6 |
| 21 | 218 | −64 | −35 | 16 | L. Superior Temporal Gyrus | 42 |
| 22 | 210 | 9 | −32 | 36 | R. Cingulate Gyrus | 31 |
ROI Results
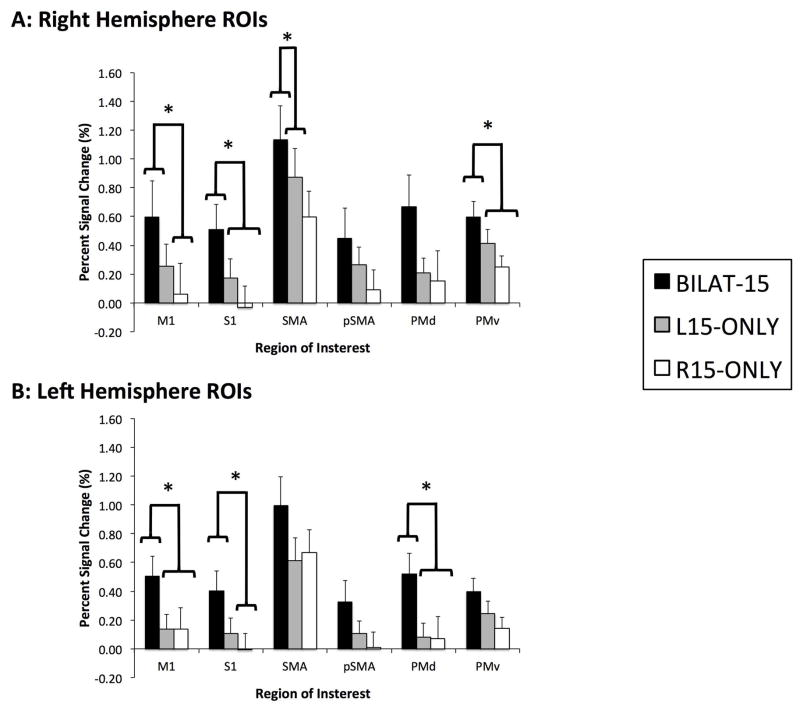
The mean PSC value within each ROI for each condition is shown in Figure 3, with the results of the ANOVAs carried out to determine differences between conditions being shown in Table 3. In all 12 ROIs, the mean BILAT15 PSC was at least 30% greater than each of the unilateral conditions, and in 8 of the ROIs, the mean BILAT15 PSC was more than 25% greater than the sum of the two unilateral conditions (R15-ONLY or L15-ONLY). A total of 7 of the 12 ROIs reached significance between the conditions (p<0.01). In four of the ROIs (M1 and PMd in the left hemisphere and S1 and PMv in the right), the post-hoc comparison of Bonferonni corrected means showed that the average PSC value during BILAT15 was greater than the PSC values during either R15-ONLY or L15-ONLY. In two ROIs (M1 in the right hemisphere and S1 in the left hemisphere), the post-hoc analysis showed that the average PSC value during BILAT15 was greater than R15-ONLY. Lastly, post-hoc analysis showed that the right SMA had greater activation during BILAT15 compared to L-ONLY. Due to the presence of sphericity in the differences between conditions, the difference in the right SMA is not a statistically meaningful difference.
Figure 3.
Mean (n = 11) average PSC value in each ROI, in the BILAT15, L15-ONLY and R15-ONLY conditions. ROIs with a significant difference (p < 0.01) between conditions are indicated with an asterisk (*). Results of the post-hoc analysis between conditions (Bonferroni corrected means) are indicated with the braces. M1 = Primary Motor Cortex, S1 = Primary Sensory Cortex, SMA = Supplementary Motor Area, pSMA = Pre-Supplementary Motor Area, PMd = Dorsal Premotor Cortex and PMv = Ventral Premotor Cortex.
Table 3.
Clusters showing unique activation in the BILAT condition, compared to the unilateral conditions at 30% MVC. Clusters of more than 200mm3 are listed. Coordinates are listed in T-T space, and indicate the centre of mass for each cluster. All of the clusters listed had greater average PSC values in the BILAT condition, compared to each of the unilateral conditions at 15% MVC. There were no differences detected in average PSC values within these clusters between the L30-ONLY and R30-ONLY conditions.
| Cluster # | Voxels | CoM Coordinates
|
Description | BA | ||
|---|---|---|---|---|---|---|
| MNI X (mm) | MNI Y (mm) | MNI Z (mm) | ||||
| 1 | 19563 | −5 | −51 | 43 | L. Precuneus | 7 |
| 2 | 12492 | −9 | −55 | −37 | L. Nodule | |
| 3 | 2983 | 23 | −83 | 14 | R. Middle Occipital Gyrus | 17 |
| 4 | 2479 | 15 | −18 | 8 | R. Thalamus (VPL) | |
| 5 | 1814 | −26 | −17 | 6 | L. Lentiform Nuc. / Putamen | |
| 6 | 1751 | 60 | −37 | 33 | R. inferior Parietal Lobule | 40 |
| 7 | 835 | −40 | −55 | −15 | L. Fusiform Gyrus | 37 |
| 8 | 807 | −35 | 10 | 6 | L. Insula | 13 |
| 9 | 699 | 33 | 11 | 7 | R. Insula | 13 |
| 10 | 564 | −66 | −68 | 1 | R. Lingual Gyrus | 18 |
| 11 | 493 | 7 | 2 | 43 | R. Cingulate Gyrus | 24/32 |
| 12 | 489 | −28 | −80 | −9 | L. Lingual Gyrus | 18 |
| 13 | 477 | 55 | 6 | 28 | R. Inferior Frontal Gyrus | 9 |
| 14 | 382 | −21 | −89 | 11 | L. Middle Occipital Gyrus | 18 |
| 15 | 348 | 51 | 3 | 3 | R. Superior Temporal Gyrus | 22 |
| 16 | 332 | −18 | −27 | 63 | L. Precentral Gyrus | 4 |
| 17 | 327 | 19 | −21 | 44 | R. Cingulate Gyrus | 24/31 |
| 18 | 319 | −65 | −37 | 35 | L. Inferior Parietal Lobule | 40 |
| 19 | 302 | 46 | −76 | 6 | R. Middle Occipital Gyrus | 19 |
| 20 | 294 | −47 | −81 | 22 | L. Middle Temporal Gyrus | 39 |
| 21 | 293 | −14 | −74 | −11 | L. Lingual Gyrus | 18 |
| 22 | 262 | 14 | −37 | −40 | R. Cerebellar Tonsil | |
| 23 | 227 | −19 | −96 | 24 | L. Cuneus | 19 |
| 24 | 206 | 15 | −13 | 60 | R. Medial Frontal Gyrus | 6 |
Effect of Greater Unilateral Forces
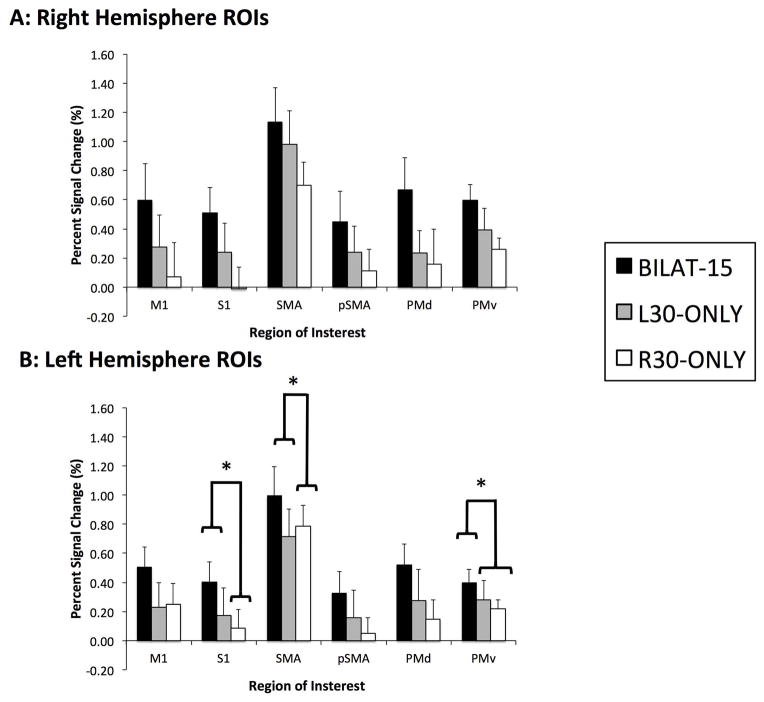
To determine whether the greater level of activation that was observed in the bilateral condition was simply due to the motor system producing a greater net level of force, a separate analysis was conducted to compare the level of brain activity between BILAT15 and when the force was produced unilaterally at 30% MVC (R30-ONLY, L30-ONLY). The plots illustrating the mean percent signal change within each of the ROIs is shown in Figure 4. Despite performing a unilateral force at twice the magnitude of each foot in the bilateral condition (BILAT15) PSC values were greater in several ROIs during the bilateral condition. In the right hemisphere, PSC values of M1, S1, SMA and PMv were significantly greater during BILAT15 compared to R30-ONLY. The reverse observation was found in the right PMv where the PSC value was greater during BILAT15 compared to either R30-ONLY or L30-ONLY. In the left hemisphere the average PSC value in each ROI was always greatest during BILAT15, compared to R30-ONLY and L30-ONLY, however this difference in average PSC was not found to be statistically significant in any of the ROIs analyzed.
Figure 4.
Mean (n = 11) average PSC value in each ROI, in the BILAT15, L30-ONLY and R30-ONLY conditions. ROIs with a significant difference (p < 0.01) between conditions are indicated with an asterisk (*). Results of the post-hoc analysis between conditions (Bonferroni corrected means) are indicated with the braces. M1 = Primary Motor Cortex, S1 = Primary Sensory Cortex, SMA = Supplementary Motor Area, pSMA = Pre-Supplementary Motor Area, PMd = Dorsal Premotor Cortex and PMv = Ventral Premotor Cortex.
The voxel wise conjunction analysis comparing the brain activity between the BILAT15 condition and R30-ONLY and L30-ONLY conditions revealed a total of 24 clusters with unique activation in the BILAT15 condition. These clusters are illustrated in the lower panel of Figure 2. The details regarding these clusters are shown in Table 3. A large number of these clusters appeared to be similar to the clusters that were identified as being uniquely activated in the BILAT15 condition compared to the unilateral exertions at 15% MVC. Post-hoc analysis revealed that the average PSC values were greater in all of the clusters in the BILAT15 condition compared to the R30-ONLY and L30-ONLY. The post-hoc analysis revealed no significant differences in activation between the R30-ONLY and L30-ONLY conditions that were identified as being a uniquely active in the BILAT15 condition.
Discussion
This study is the first to identify the regions of the brain that were activated during coordinated bilateral as compared to unilateral lower limb movement tasks. Overall it was found that coordinated bilateral exertions of the soleus muscles during plantarflexion led to greater activation in the brain, across a wide variety of regions, and substantially more activation than a simple summation of unilateral right and left hemisphere activation. The fact that this greater activation is evident during bilateral exertions even when the unilateral task is performed at twice the force level further confirms that substantially more brain activity is required in order to coordinate bilateral exertions.
Our study showed that the brain regions responsible for the control of bilateral lower-limb movements are distributed over cortical, cerebellar and subcortical regions. There are a number of mechanisms that may contribute to the greater activation in the bilateral condition. Using the voxel-wise analysis, we found greater activity on the left and right precentral gyrus during the bilateral task, which was consistent with the greater activity that was observed in the M1 regions in the ROI analysis. We postulate that interhemispheric inhibition (IHI) may contribute to the greater activity in the bilateral condition. The IHI phenomenon occurs during unilateral exertions, where the M1 of the contralateral hemisphere inhibits the ipsilateral M1 via trans-callosal pathways (Ferbert et al. 1992). This phenomenon is thought to exist in order to prevent the occurrence of mirror movements and to remove any existing inhibition from the contralateral M1 (Duque et al. 2007). Although IHI has primarily been described in upper-limb tasks, there is some evidence from studies using transcranial magnetic stimulation, that it also exists in lower-limb muscles (Stokic et al. 1997). Thus, it is plausible that IHI may explain our results, given that during IHI each M1 mutually inhibits the other. Thus, an overall greater level of brain activity would be required to overcome these inhibitory signals and maintain the target force level. Previous IHI studies have generally focussed on the M1 regions, yet it is possible that other interhemispheric interactions could explain the greater activity in other cortical regions. For example it has been shown that activity in the PMd can inhibit the contralateral M1 (Mochizuki et al. 2004), therefore the higher activity that was observed in the bilateral condition may offset some of the inhibition from other sites.
In the cortex there was also substantially greater activity in visuomotor processing areas such as the precuneus, cuneus and other occipital regions. This extra activity may be due to the higher level of visual attention that is required to pay attention to the feedback that is associated with two visual stimuli. There was also activity in frontal areas, such as the inferior frontal gyrus and the medial frontal gyrus, which likely indicates that the bilateral task was more difficult from a cognitive perspective. There is evidence that these areas are involved in motor decisions based on visual stimuli presented to the individual (Talati and Hirsch 2005). Similarly the insula showed significant activation in the bilateral condition. Previous research has shown that the insula is involved in the monitoring and regulation of sensory information regarding the limb (Karnath et al. 2005). The Talairach tool identified the activation of the insula to be close to the claustrum, which is a thin band of gray matter between the putamen and the internal capsule. This region has previously been associated with integrating information between different modalities (i.e., visual, sensory, motor systems) and in the control of functional timing of information of processing in the brain (Crick and Koch 2005).
The regions identified during bilateral lower limb exertions were similar to the regions active in bilateral control of upper limb movements (Swinnen and Wenderoth 2004) and are likely responsible for the monitoring of task related sensory feedback and making modifications to the motor output. This finding indicates that the same regions may be used to coordinate bilateral movements in the upper and lower limbs and may indicate that the SMA and PMd are indeed involved in the coordination of lower-limb movements. Of interest, Mehta et al (2012) utilized fMRI to assess brain activation during a more complex, rhythmical, alternating leg cycling task which would require coordination between the two limbs, multiple joints of the same limb, and flexor/extensor muscle groups. Despite the complex task, they also found significant activation in primary motor and sensory areas, as well as the SMA compared to a passive cycling condition, indicating that these brain areas may be responsible for processing the sensory information associated with this task.
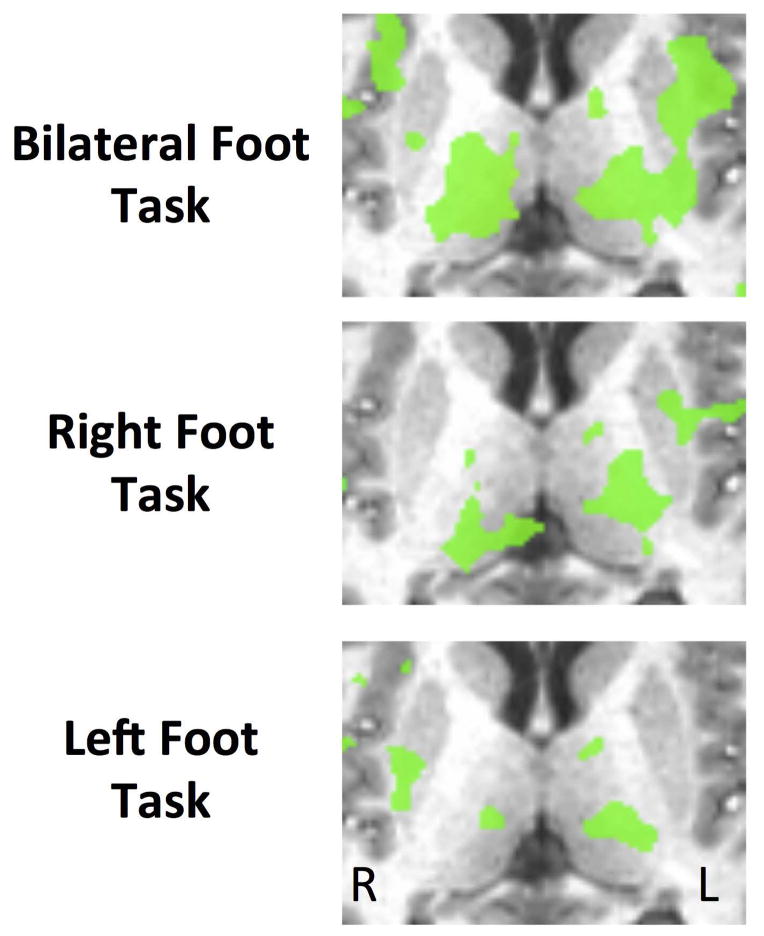
The thalamus was also identified in the bilateral condition. The differences in activation within the thalamus between conditions are shown in Figure 5. The extra activity in the thalamus is consistent with the large increase in activity that was observed in motor areas of the cortex, as extra activity in the direct motor pathway may decrease the level of inhibition in the thalamus and provide stimulation to cortical motor areas (DeLong and Wichmann 2007). It may also be expected that there is an increase in sensory signals originating from the lower limbs in the bilateral condition, as information from both limbs would need to be monitored. This extra sensory information may lead to an increase in activity within the thalamus via the dorsal column medial-lemniscus pathway, which has a synapse in the ventral posterolateral nucleus of the thalamus. Lastly, the thalamus may also be involved in the control of the saccade-type eye movements that were required to monitor the two visual feedback indicators in the bilateral condition (Tanaka and Kunimatsu 2011).
Figure 5.
Differences in areas of activation within the thalamus between the three conditions. Clusters of more than 200 mm3, that showed a level of significance of p < 0.001 are shown in green.
Finally, the greater neural activity that was observed during synchronous bilateral activation of lower-limb muscles lends support to the notion that bilateral tasks may stimulate greater neuroplasticity when compared to unilateral tasks. Studying individuals with stroke Whitall et al (2011) found that a bilateral upper limb rehabilitation protocol led to similar functional gains when compared to a unilateral protocol. However, the neural mechanisms underlying these changes were different. Only the bilateral protocol resulted in an increase in neural activity in contralesional premotor cortex, and this activity was correlated with improved functional performance. Our bilateral leg task also resulted in greater activation within the premotor cortex bilaterally. Further investigation would be required to determine the training effects of bilateral lower limb tasks versus unilateral tasks.
Limitations
Given the need to match the forces between the two limbs, it is possible that the processing of this visual feedback during the bilateral task contributed to some of the increased activation that was observed. While a single summated feedback bar might have simplified the task, we felt it was critical that the individual limb forces be specified to ensure the same forces between unilateral and bilateral tasks to control the motor output.
It is possible that the visual and cognitive processing required to process the visual feedback during the bilateral task is responsible for some of the increased activation that was observed. While we acknowledge that the bilateral task may be more challenging from a visual processing and cognitive standpoint, the observation of higher levels of activation in several motor areas with the ROI analysis suggests that additional motor resources are required to coordinate a bilateral task. Two of the clusters identified as being active during the bilateral task were similar to clusters that were associated with the processing of visual feedback regarding force production in an upper-limb task (Noble et al. 2013). These clusters included the cuneus and the precentral gyrus in the left hemisphere. It likely that these areas demonstrate higher levels of activation in the bilateral condition due to the processing of extra visual feedback. The remaining twenty clusters identified as being active in the BILAT-15 condition but not in the unilateral conditions were distributed throughout the brain. This finding illustrates the coordination of bilateral exertions in the lower-limb is clearly a complex task, and this cannot totally be attributed to the increased visual feedback. Future studies should investigate ways to collect bilateral efforts at with similar levels of effort in each limb without visual feedback. This will likely require extensive training for the participants. The role of visual feedback might also be considered via the study of regional brain activity when participants are presented with simulated visual feedback, but do not produce any force.
Future studies may choose to include a bilateral task that is performed at the higher force level (30% MVC) as it would give further insight into the control of bilateral lower-limb movements. In our study we decided not to include this condition as our pilot testing showed that bilateral exertions at higher force levels tended to cause excessive head movement.
It should also be noted that the BOLD signal can not conclusively show that the higher activation is due to the IHI phenomenon. Studies using transcranial magnetic stimulation to elicit motor evoked responses in the soleus muscle could be used to further investigate these findings.
Conclusion
This study found that producing forces bilaterally with the soleus muscle led to substantially more neural activation compared to unilateral exertions, even beyond the activation that would be expected from the sum of the activation due the unilateral conditions. Greater levels of activation were observed in cortical areas, subcortical areas and in the cerebellum to coordinate bilateral movements.
Acknowledgments
Funding for this project was provided by the Canadian Institutes of Health Research (CIHR CGR-86829). Dr. Janice Eng is a Michael Smith Foundation for Health Research Career Scientist. Dr. Lara Boyd holds a Canada Research Chair, in the Neurobiology of Motor Learning and is a Michael Smith Foundation for Health Research Career Scientist. Technical and programming support from Mr. Laurent Mingo and Mr. Ramin Sahebjavaher is acknowledged. Technical support and assistance in data collection from the staff at the University of British Columbia MRI Research Centre is also acknowledged.
References
- Ciccarelli O, Toosy AT, Marsden JF, Wheeler-Kingshott CM, Sahyoun C, Matthews PM, Miller DH, Thompson AJ. Identifying brain regions for integrative sensorimotor processing with ankle movements. Exp Brain Res. 2005;66:31–42. doi: 10.1007/s00221-005-2335-5. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crick FC, Koch C. What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci. 2005;360:1271–1279. doi: 10.1098/rstb.2005.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F, Swinnen SP, Beatse E, Sunaert S, Van Hecke P, Duysens J. Brain areas involved in interlimb coordination: a distributed network. Neuroimage. 2001;14:947–958. doi: 10.1006/nimg.2001.0892. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual Differences in movement-related interhemispheric inhibition. Journal of cognitive neuroscience. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Elias LJ, Bryden MP, Bulman-Fleming MB. Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia. 1998;36:37–43. doi: 10.1016/s0028-3932(97)00107-3. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Kapreli E, Athanasopoulos S, Papathanasiou M, Van Hecke P, Strimpakos N, Gouliamos A, Peeters R, Sunaert S. Lateralization of brain activity during lower limb joints movement. An fMRI study. Neuroimage. 2006;32:1709–1721. doi: 10.1016/j.neuroimage.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Baier B, Nagele T. Awareness of the functioning of one’s own limbs mediated by the insular cortex? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31:1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta JP, Verber MD, Wieser JA, Schmit BD, Schindler-Ivens SM. The Effect of Movement Rate and Complexity on Functional Magnetic Resonance Signal Change During Pedaling. Motor Control. 2012;16(2):158–75. doi: 10.1123/mcj.16.2.158. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. The Journal of physiology. 2004;561:331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble JW, Eng JJ, Boyd LA. Effect of Visual Feedback on Brain Activation During Motor Tasks: An fMRI Study. Motor Control. 2013;17(3):298–312. doi: 10.1123/mcj.17.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokic DS, McKay WB, Scott L, Sherwood AM, Dimitrijevic MR. Intracortical inhibition of lower limb motor-evoked potentials after paired transcranial magnetic stimulation. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1997;117:437–443. doi: 10.1007/s002210050238. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Wenderoth N. Two hands, one brain: cognitive neuroscience of bimanual skill. Trends Cogn Sci. 2004;8:18–25. doi: 10.1016/j.tics.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on “what,” “when,” and “where” related information: an fMRI study. Journal of cognitive neuroscience. 2005;17:981–993. doi: 10.1162/0898929054475226. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kunimatsu J. Contribution of the central thalamus to the generation of volitional saccades. The European journal of neuroscience. 2011;33:2046–2057. doi: 10.1111/j.1460-9568.2011.07699.x. [DOI] [PubMed] [Google Scholar]
- Trinastic JP, Kautz SA, McGregor K, Gregory C, Bowden M, Benjamin MB, Kurtzman M, Chang YL, Conway T, Crosson B. An fMRI study of the differences in brain activity during active ankle dorsiflexion and plantarflexion. Brain Imaging Behav. 2010;4:121–131. doi: 10.1007/s11682-010-9091-2. [DOI] [PubMed] [Google Scholar]
- Whitall J, Waller SM, Sorkin JD, Forrester LW, Macko RF, Hanley DF, Goldberg AP, Luft A. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single-blinded randomized controlled trial. Neurorehabilitation and Neural Repair. 2011;25:118–129. doi: 10.1177/1545968310380685. [DOI] [PMC free article] [PubMed] [Google Scholar]