Abstract
Background
Electronic medical records and insurance claims data from the Geisinger Health System were examined to assess the real-world healthcare costs of being overweight or obese at different glycemic stages, including normal glycemia, pre-diabetes (PreD), and type 2 diabetes (T2D).
Methods
The medical history of the sample subjects was segmented into different glycemic stages via diagnosis codes, glycosylated hemoglobin A1c or fasting plasma glucose laboratory results, and use of antidiabetic drugs. Healthcare resource utilization captured by the claims and associated costs (in 2013 values) were examined for each glycemic stage. The association between costs and body mass index (BMI) was estimated by regressions, and adjusted for sociodemographics. We predicted the adjusted incremental annual costs associated with high BMI, relative to normal BMI (18.5–24.9 kg/m2).
Results
We identified 48,344 adults in normal glycemic stage, 3,085 in the PreD stage, and 9,526 in the T2D stage (mean age 46, 58, and 60 years, respectively; mean BMI 29, 32, and 33 kg/m2, respectively). The adjusted incremental annual costs associated with high BMI relative to normal BMI ranged from $336 for overweight (25–29.9 kg/m2) to $1,850 for class III obesity (≥40 kg/m2) during normal glycemic stage; were only significant for class III ($2,434) during the PreD stage; and ranged from $1,139 for overweight to $4,649 for class III during the T2D stage (all p < 0.05).
Conclusions
Positive associations between healthcare costs and BMI levels were observed within each glycemic stage. Management of body weight is important in reducing the overall healthcare costs, especially for subjects with PreD or T2D.
Key Points for Decision Makers
| Based on a regional US claims dataset, positive associations between total healthcare costs and BMI levels were observed within each glycemic stage. Overweight and obese subjects (BMI ≥25 kg/m2) had higher costs than those with normal BMI in both the normal glycemic stage and the type 2 diabetes (T2D) stage, while during the pre-diabetes (PreD) stage, subjects with extreme obesity (BMI ≥40 kg/m2) had higher costs. |
| The costs of being overweight and obese, relative to normal BMI (18.5–24.9 kg/m2), were much higher among subjects with T2D than those with normal glycemic levels. Extreme obesity had a noticeable impact on healthcare costs within each glycemic stage. |
| Targeted weight-control programs aimed at people with PreD, or at-risk normal glycemic subjects, as well as those with frank T2D, should be able to generate a significant return on investment by effectively reducing the economic burden of overweight and obesity in the US. |
Introduction
Obesity, a disease classified as body mass index (BMI) ≥30 kg/m2, has become a major public health problem in the US, affecting over one-third (35.7 %) of the population [1]. Being overweight or obese is a risk factor for numerous costly co-morbidities, such as metabolic diseases, cardiovascular diseases, and certain types of cancers [2]. Annual spending related to obesity and its associated comorbidities is estimated to be $315.8 billion in 2010, accounting for 27.5 % of US healthcare expenditures [3]. Costs related to being overweight or obese are projected to reach $861 billion in 2030 [4].
Obesity is strongly associated with pre-diabetes (PreD) and type 2 diabetes (T2D). Diabetes, a chronic metabolic disorder characterized by hyperglycemia, affected approximately 9 % (29 million) of the US population in 2012 [5]. In addition, 37 % (81 million) of Americans aged 20 years and older had PreD [6]. Diabetes was responsible for direct medical costs of $176 billion in 2012 [7]. T2D comprises approximately 90–95 % of all diabetes cases [6]. Obesity is one of the most common risk factors for T2D, and T2D is one of the most common obesity-related co-morbidity [8]. The risk of developing T2D has been reported to increase by 6.7-fold compared with normal BMI for male obese patients, and 12.4-fold for female obese patients [2]. In fact, management of body weight is also a key factor in the successful management of T2D [9]. More than 60 % of patients with T2D are obese [10] and approximately 57 % of the total costs of T2D are attributable to obesity [11]. The impact of obesity on T2D extends to patients with PreD. Higher BMI is associated with a higher risk of developing PreD and accelerates the progression from PreD to T2D [12]. Obesity at least partially explains the increased costs in patients with PreD compared with subjects with normal glycemia [13].
The assessment of the economic burden of overweight and obesity given a person’s diabetes status could be an informative and useful tool in the decision-making process on cost-effective strategies for the prevention and management of obesity, as considered by various healthcare providers and policy makers, but the quantitative data are scarce. The objective of this study was to help fill this gap in the literature and evaluate the healthcare costs in the US of being overweight or obese at different glycemic stages, including the normal glycemic stage (i.e. euglycemia), PreD, and T2D.
Methods
Data Source
Data were obtained from the MedMining® database, which contains electronic medical records (EMRs) from the Geisinger Health System, an integrated health system serving northern Pennsylvania with 880+ multispecialty physician group practices, 5 hospital campuses, 72 primary and specialty clinic sites, and a health plan. Health records, which have been kept in electronic form at the Geisinger Health System since 1996, cover over 4 million subjects and contain information on demographic characteristics (age, sex, and race/ethnicity), encounter details from inpatient, outpatient, and office-based settings (including diagnostic and procedure codes), medication orders, vital signs, laboratory results, and actual costs incurred by the Geisinger Health System for those encounters.
Claims-level data are available for the subset of patients who are enrolled in the Geisinger Health Plan (GHP) and are linked to the EMR data. The GHP claims data include the claims records for all eligible GHP members for all services (including medical services and prescription fills). The GHP enrollees are covered by various types of insurance (46 % are covered by a health maintenance organization (HMO), 34 % by a preferred provider organization (PPO), 11 % by a Medicare HMO, 3 % by a Medicare PPO, and 6 % by other types).
Study Design
A cross-sectional study design was used to examine the association between BMI and healthcare costs during each glycemic stage. The analyses were conducted separately for normal glycemia, PreD, and T2D stages. One individual could have multiple glycemic stages over time due to transitions between stages, and thus could be included in multiple analyses.
Sample Selection
The base study population, from MedMining EMRs and claims data between January 2004 and May 2013, was required to meet all of the following inclusion criteria: (1) ≥2 years’ enrollment in the GHP; (2) non-null BMI (or weight and height) value; (3) age ≥18 years; and (4) no diagnosis codes of type 1 diabetes (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]: 250.x1, 250.x3) or secondary diabetes (ICD-9-CM: 249.xx). The base study population contained 153,561 subjects. Due to data size restriction, we could obtain a total sample of up to 100,000 subjects from the MedMining dataset. We first selected all subjects with potential PreD or T2D stages (Part I sample) from the base study population, i.e. all patients with glycemic laboratory tests (fasting plasma glucose [FPG] or glycosylated hemoglobin [HbA1c]), T2D diagnosis (ICD-9-CM: 250.x0, 250.x2), or use of antidiabetic medications. There were 43,639 subjects in the Part I sample. The remaining subjects in the base study population, not meeting any of the Part I sample criteria, are assumed to have normal glycemic status. We randomly selected a 51 % sample of these subjects (Part II sample) up to our subject number limit.
Subjects (both Part I and Part II samples) were excluded from the study if, during the longest continuous enrollment in the GHP plan and up until any pregnancy (the study period), they had (1) <2 years of data; (2) evidence of underweight (BMI <18.5 kg/m2); (3) conditions associated with unintentional weight change (malignancy, human immunodeficiency virus, cachexia, anorexia, abnormal weight gain or loss, feeding difficulties, gastrointestinal disorders, inflammatory bowel diseases, pancreatitis, and nutritional deficiencies with the exception of vitamin deficiency); or (4) bariatric surgery since the dramatic weight reduction and high costs associated with the surgery may bias the association between BMI and costs.
Glycemic Stage Identification
Data between January 2004 and May 2013 were examined to identify the glycemic stages for each subject, as shown in Fig. 1. T2D, being the end-stage of the glycemic continuum, was first identified by at least one diagnosis code of 250.x0 or 250.x2, or at least two laboratory results of HbA1c ≥6.5 % and/or FPG ≥126 mg/dL, or any use of non-metformin antidiabetic medications. Before the occurrence of T2D, or if T2D was not found throughout the entire data, PreD was searched and defined as at least one laboratory result of HbA1c 5.7–6.4 % and/or FPG 100–126 mg/dL. Last, subjects were considered to be in the normal glycemic stage (without a preceding history of PreD or T2D) if they had ≥2 years of continuous enrollment without laboratory results beyond normal glycemic level and without use of antidiabetic medications. The laboratory result cutoffs to define PreD and T2D follow the American Diabetes Association recommendations [9].
Fig. 1.
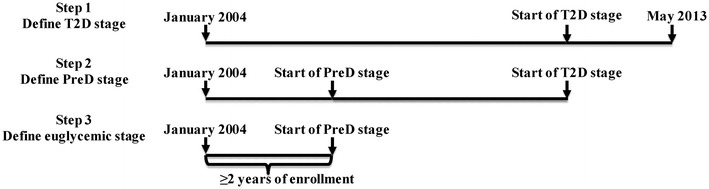
Glycemic staging. PreD pre-diabetes, T2D type 2 diabetes
For each glycemic stage, the data during the study period were analyzed and referred to as the follow-up period. In addition to the sample selection criteria above, subjects were required to have ≥1 year of follow-up period with at least one BMI value.
Study Measures
Patient demographics were measured at the start of the follow-up period during a glycemic stage, and included age, sex, race, smoking, employment status, insurance type, and year to start follow-up (from 2004 to 2011). The mean BMI values during a follow-up period were examined and categorized into five levels according to the WHO classification: 18.5 ≤ BMI < 25 kg/m2 (normal BMI), 25 ≤ BMI < 30 kg/m2 (overweight), 30 ≤ BMI < 35 kg/m2 (class I obesity), 35 ≤ BMI < 40 kg/m2 (class II obesity), and BMI ≥40 kg/m2 (class III obesity) [14].
The healthcare resource utilization in the follow-up period during a glycemic stage, as recorded by the GHP claims data, was classified by type of service, including inpatient hospitalizations, outpatient services, and outpatient pharmacy prescriptions. The number of total services received and length of inpatient stays were examined. To measure healthcare costs, unit costs from external sources [15–17] were assigned to GHP claims as financial amounts were not released by Geisinger. Inpatient stays recorded in the GHP claims data were matched to costs reported in the Healthcare Cost and Utilization Project (HCUP) survey (2010 data) according to diagnosis code, region, and length of stay [17]. Health services in outpatient settings (including physician services [18], clinical laboratory services [19], ambulance services [20], and the use of durable medical equipment [21] associated with physician office, emergency room, hospital outpatient, and other outpatient visits) were mapped to costs reported in the Centers for Medicare & Medicaid Services (CMS) 2012 fee-for-service fee schedules according to Healthcare Common Procedure Coding System (HCPCS) procedure codes [16]. Pharmacy costs were estimated based on average wholesale price from Truven Health Analytics’ Red Book™ [15]. Costs were adjusted to 2013 dollars using the US Medical Consumer Price Index [22]. Due to the variable duration of the follow-up period, utilization and cost measures were standardized as annual utilization and costs.
Statistical Analysis
The analyses were conducted separately for each glycemic stage. Descriptive statistics of all study measures were reported and stratified by BMI categories. The association between the BMI categories and the costs (total healthcare costs, inpatient costs, outpatient costs, and pharmacy costs) was assessed by multivariable regressions, adjusting for demographics. The regressions were generalized linear regression models with log-link and gamma distribution, which can estimate the adjusted ratios of costs associated with high BMI categories (from overweight to class III obesity) versus normal BMI level. The scaled deviance was used to assess the goodness of fit of the models [23]. The adjusted incremental total costs were also estimated for each of the high BMI categories relative to normal BMI level, such that adjusted costs were predicted by assuming all sample subjects were in one BMI level and the average change in adjusted costs between BMI levels was calculated [24]. In all the regression analyses, subjects in the Part II sample were assigned a uniform sampling weight representing their true proportion in the base population. Specifically, each subject in the Part II sample was considered to represent 1.96 subjects (i.e. inverse of the 51 % sampling fraction). Analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC, USA).
Results
Study Sample and Characteristics
The final study sample, as shown in Fig. 2, contained 48,344 subjects (representing 81,983 subjects after weighting) in the normal glycemic stage, 3,085 in the PreD stage, and 9,526 in the T2D stage. Individual characteristics in each glycemic stage, stratified by BMI category, are described in Table 1. The mean BMI was 29.4, 32.4, and 33.2 kg/m2 during the normal glycemic, PreD, and T2D stages, respectively. The mean duration of follow-up was 5.2, 3.1, and 4.6 years, respectively. The duration was stable across BMI categories within each glycemic stage.
Fig. 2.
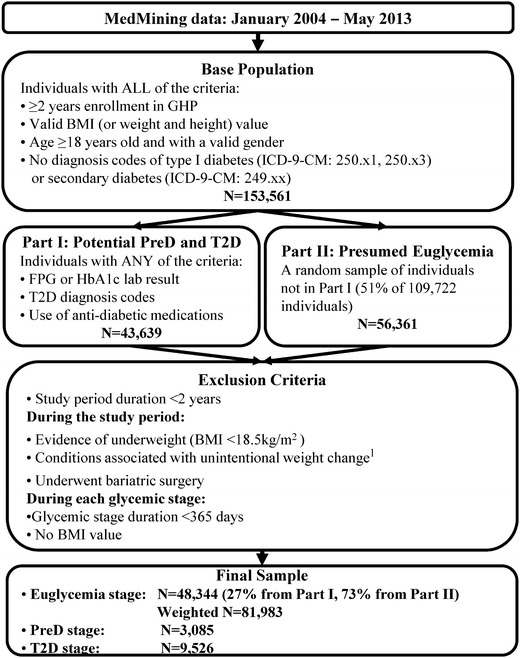
Sample selection. 1 Conditions associated with unintentional weight change include malignancy, human immunodeficiency virus, cachexia, anorexia, abnormal weight gain or loss, feeding difficulties, gastrointestinal disorders, inflammatory bowel diseases, pancreatitis, and nutritional deficiencies (except for vitamin deficiency). BMI body mass index, FPG fasting plasma glucose, GHP Geisinger Health Plan, HbA1c glycosylated hemoglobin, ICD-9-CM International Classification of Diseases, Ninth Revision, Clinical Modification, PreD pre-diabetes, T2D type 2 diabetes
Table 1.
Demographics by BMI in each glycemic stage
| All | 18.5 ≤ BMI <25 | 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | 35 ≤ BMI < 40 | 40 ≤ BMI | |
|---|---|---|---|---|---|---|
| Normal glycemic stage | ||||||
| No. of subjects (%) | 48,344 (100) | 11,655 (24) | 17,674 (37) | 11,243 (23) | 4,862 (10) | 2,910 (6) |
| BMI [mean (SD)] | 29.4 (6.1) | 22.8 (1.5) | 27.4 (1.4) | 32.2 (1.4) | 37.1 (1.4) | 44.8 (4.7) |
| Follow-up duration [years; mean (SD)] | 5.2 (2.3) | 5.1 (2.3) | 5.3 (2.4) | 5.3 (2.3) | 5.1 (2.3) | 4.9 (2.2) |
| Stage starting since 2004 (%) | 59.6 | 59.6 | 57.4 | 60.2 | 60.9 | 59.8 |
| Age [mean (SD)] | 46.2 (15.3) | 43.4 (16.2) | 47.5 (15.5) | 47.5 (14.5) | 46 (14.1) | 44.2 (13) |
| Male (%) | 47.9 | 34.8 | 55.0 | 54.7 | 45.4 | 36.0 |
| White (%) | 97.7 | 96.8 | 97.6 | 98.2 | 98.5 | 98.3 |
| Current smoker (%) | 21.2 | 24.9 | 21.0 | 20.3 | 17.6 | 16.6 |
| Full-time employment (%) | 60.7 | 57.7 | 59.9 | 62.0 | 63.9 | 67.7 |
| Covered by HMO plans (%) | 82.9 | 81.6 | 83.1 | 83.5 | 83.9 | 82.3 |
| PreD stage | ||||||
| No. of subjects (%) | 3,085 (100) | 309 (10) | 963 (31) | 925 (30) | 498 (16) | 390 (13) |
| BMI [mean (SD)] | 32.4 (6.9) | 23.2 (1.4) | 27.6 (1.4) | 32.3 (1.4) | 37.3 (1.5) | 45.8 (5.3) |
| Follow-up duration [years; mean (SD)] | 3.1 (1.8) | 3.0 (1.7) | 3.3 (1.8) | 3.1 (1.7) | 3.1 (1.7) | 3.0 (1.8) |
| Stage starting since 2004 (%) | 8.2 | 5.5 | 7.4 | 9.7 | 7.0 | 10.5 |
| Age [mean (SD)] | 57.6 (13.7) | 62.1 (15.4) | 60.3 (13.4) | 58.4 (13.0) | 53.6 (12.5) | 50.2 (12.2) |
| Male (%) | 47.7 | 41.1 | 55.8 | 50.7 | 44.4 | 30.3 |
| White (%) | 97.3 | 95.1 | 96.9 | 97.4 | 98.4 | 98.2 |
| Current smoker (%) | 17.5 | 25.6 | 19.8 | 14.1 | 15.5 | 15.9 |
| Full-time employment (%) | 48.7 | 40.8 | 43.3 | 46.1 | 57.6 | 63.3 |
| Covered by HMO plans (%) | 87.3 | 83.8 | 89.1 | 89.0 | 83.7 | 86.4 |
| T2D stage | ||||||
| No. of subjects (%) | 9,526 (100) | 776 (8) | 2,672 (28) | 2,874 (30) | 1,810 (19) | 1,394 (15) |
| BMI [mean (SD)] | 33.2 (6.9) | 23.2 (1.4) | 27.7 (1.4) | 32.4 (1.4) | 37.2 (1.4) | 45.6 (5.2) |
| Follow-up duration [years; mean (SD)] | 4.6 (2.3) | 4.5 (2.3) | 4.7 (2.3) | 4.7 (2.3) | 4.6 (2.3) | 4.3 (2.2) |
| Stage starting since 2004 (%) | 32.5 | 32.3 | 33.8 | 33.2 | 32.6 | 28.5 |
| Age [mean (SD)] | 59.8 (13.2) | 64.8 (14.8) | 63.7 (12.7) | 60.2 (12.6) | 56.6 (12.1) | 53.2 (12.3) |
| Male (%) | 51.0 | 42.8 | 54.5 | 54.8 | 50.9 | 41.0 |
| White (%) | 97.7 | 95.1 | 97.2 | 97.9 | 98.4 | 98.6 |
| Current smoker (%) | 14.6 | 17.4 | 14.6 | 14.4 | 13.8 | 14.6 |
| Full-time employment (%) | 39.0 | 28.0 | 31.4 | 38.7 | 45.4 | 52.2 |
| Covered by HMO plans (%) | 85.7 | 85.4 | 87.4 | 87.0 | 85.0 | 81.1 |
The study measures were created for the analysis in each glycemic stage separately. Patient demographics were measured at the start of the glycemic stage and included age, sex, race (White, Black, and other), smoking status (never smoked, former smoker, current smoker, and other), employment status (full-time employed, not employed, and other), insurance type (HMO plans or other), and year with the start of the glycemic stage (from 2004 to 2011)
BMI body mass index, HMO health maintenance organization, PreD pre-diabetes, SD standard deviation, T2D type 2 diabetes
Subjects were approximately 10 years younger at the start of follow-up during the normal glycemic stage than those progressing to the PreD or T2D stages (46.2 vs. 57.6 or 59.8 years of age). The age of subjects in the normal glycemic stage remained approximately the same regardless of their BMI. However, the age of subjects in the PreD and T2D stages declined as the BMI increased.
Descriptive Analysis of Healthcare Resource Utilization and Costs
Table 2 presents the annualized healthcare resource utilization and costs in each glycemic stage. During the normal glycemic stage, one hospitalization occurred per ten subjects per year, with an average stay of 1.9 days. Subjects during the normal glycemic stage averaged 9.1 outpatient visits and 11.8 prescription fills per year. The use of inpatient services was stable across BMI categories but the use of outpatient services and pharmacy fills increased with BMI. The healthcare utilization translates to an average $4,467 in healthcare costs (median $2,022) per person per year during the normal glycemic stage. The mean healthcare costs were divided equally among inpatient, outpatient, and pharmacy across BMI categories.
Table 2.
Annualized healthcare utilization and costs in each glycemic stage
| All | 18.5 ≤ BMI <25 | 25 ≤ BMI <30 | 30 ≤ BMI <35 | 35 ≤ BMI <40 | 40 ≤ BMI | |
|---|---|---|---|---|---|---|
| Normal glycemic stage | ||||||
| No. of patients | 48,344 | 11,655 | 17,674 | 11,243 | 4,862 | 2,910 |
| No. of hospitalizations [mean (SD)] | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.3) |
| Length of stay (days) if hospitalized [mean (SD)] | 1.9 (3.2) | 2.1 (3.8) | 1.9 (3.3) | 1.8 (2.7) | 1.7 (2.4) | 2.1 (4.4) |
| No. of outpatient visits [mean (SD)] | 9.1 (8.3) | 8.3 (7.9) | 9 (8.3) | 9.6 (8.5) | 10 (8.7) | 10.3 (8.9) |
| No. of pharmacy fills [mean (SD)] | 11.8 (15.3) | 9.8 (13.3) | 11.3 (14.9) | 12.9 (15.9) | 14.2 (17.1) | 15.5 (17.8) |
| Total healthcare costs [mean (SE); median] | 4,467 (39); 2,022 | 3,924 (80); 1,632 | 4,397 (64); 1,986 | 4,761 (78); 2,303 | 4,737 (105); 2,367 | 5,480 (203); 2,694 |
| Distribution of costs by setting (%) | ||||||
| Inpatient costs | 31.3 | 31.2 | 32.3 | 31.0 | 28.0 | 32.9 |
| Outpatient costs | 35.6 | 37.1 | 35.3 | 35.1 | 36.6 | 32.8 |
| Pharmacy costs | 33.1 | 31.7 | 32.4 | 33.9 | 35.4 | 34.3 |
| PreD stage | ||||||
| No. of patients | 3,085 | 309 | 963 | 925 | 498 | 390 |
| No. of hospitalizations [mean (SD)] | 0.2 (0.5) | 0.3 (0.6) | 0.2 (0.6) | 0.2 (0.5) | 0.1 (0.4) | 0.1 (0.3) |
| Length of stay (days) if hospitalized [mean (SD)] | 4.0 (6.8) | 5.5 (9.8) | 4.3 (7.6) | 3.8 (5.6) | 3.0 (5.0) | 2.7 (3.8) |
| No. of outpatient visits [mean (SD)] | 15.7 (12.2) | 16.3 (12.9) | 16 (12.3) | 15.8 (12.7) | 14.5 (11.2) | 15.5 (11.8) |
| No. of pharmacy fills [mean (SD)] | 24.3 (24.3) | 21.4 (22.6) | 24.0 (24.3) | 25.3 (24.4) | 24.8 (25.8) | 24.4 (23.1) |
| Total healthcare costs [mean (SE); median] | 9,342 (168); 4,608 | 11,498 (654); 5,045 | 9,923 (319); 4,774 | 9,328 (306); 4,639 | 7,655 (343); 4,498 | 8,386 (424); 4,217 |
| Distribution of costs by setting (%) | ||||||
| Inpatient costs | 37.5 | 53.2 | 43.2 | 34.5 | 27.6 | 23.0 |
| Outpatient costs | 30.9 | 24.9 | 27.8 | 34.7 | 33.2 | 34.0 |
| Pharmacy costs | 31.6 | 21.9 | 28.9 | 30.8 | 39.2 | 43.0 |
| T2D stage | ||||||
| No. of patients | 9,526 | 776 | 2,672 | 2,874 | 1,810 | 1,394 |
| No. of hospitalizations [mean (SD)] | 0.2 (0.5) | 0.3 (0.7) | 0.3 (0.6) | 0.2 (0.5) | 0.2 (0.5) | 0.2 (0.5) |
| Length of stay (days) if hospitalized [mean (SD)] | 3.8 (6.8) | 4.5 (7) | 4.1 (7.7) | 3.4 (6.1) | 3.5 (6.1) | 3.7 (7.2) |
| No. of outpatient visits [mean (SD)] | 18.6 (13) | 18.7 (13.6) | 18.8 (13.2) | 18.5 (12.5) | 18.4 (13) | 19 (13.1) |
| No. of pharmacy fills [mean (SD)] | 32.2 (27.3) | 27.8 (25.2) | 29.6 (25.8) | 32.2 (26.8) | 34.0 (28.2) | 37.7 (30.1) |
| Total healthcare costs [mean (SE); median] | 11,983 (122); 6,811 | 12,567 (451); 6,280 | 12,165 (235); 6,434 | 11,643 (217); 6,839 | 11,791 (277); 6,910 | 12,261 (328); 7,645 |
| Distribution of costs by setting (%) | ||||||
| Inpatient costs | 41.5 | 50.4 | 46.1 | 39.7 | 36.6 | 37.4 |
| Outpatient costs | 26.6 | 25.2 | 26.0 | 27.7 | 27.5 | 25.5 |
| Pharmacy costs | 31.8 | 24.4 | 28.0 | 32.6 | 35.9 | 37.1 |
Mean of the annualized healthcare resource utilization and costs represented the average utilization and costs per person per year
BMI body mass index, PreD pre-diabetes, SD standard deviation, SE standard error, T2D type 2 diabetes
Healthcare resource utilization was much higher during the PreD stage than the normal glycemic stage. The annual healthcare costs were, on average, $9,342 (median $4,608). For the normal BMI subjects with PreD, inpatient costs accounted for the most costs (53.2 %) and pharmacy costs accounted for the least (21.9 %). The contribution of inpatient costs was lower at higher levels of BMI, while the contribution of pharmacy costs increased. For class III obesity in PreD subjects, healthcare costs came least from inpatient services (23.0 %) and most from pharmacy fills (43.0 %).
Subjects with T2D had higher healthcare resource utilization than those with PreD, especially in the number of pharmacy fills (mean 32.2), which increased with higher BMI. The annual healthcare costs were, on average, $11,983 (median $6,811). Inpatient costs were the largest component (50.4 %) for normal BMI, but had similar contribution as pharmacy costs (~37 %) for class III obesity.
Regression Analysis of Costs
With adjustment for demographics, the ratio of costs associated with each BMI category versus normal BMI is presented in Table 3. The cost ratios associated with all the covariates are presented in Table 4 Appendix. The regression models fit the data reasonably well, with scaled deviances close to 1 (Table 4 Appendix). During the normal glycemic stage, the ratio of annual total costs increased from 1.09 (95 % confidence interval [CI] 1.07–1.11) for overweight BMI to 1.51 (1.45–1.56) for class III obesity. The cost ratio also increased with BMI for all the cost components (all p < 0.05). During the PreD stage, only class III obesity was associated with higher total costs than normal BMI (1.28 [1.09–1.51]), and it also had higher outpatient costs (1.26 [1.10–1.44]) and pharmacy costs (1.48 [1.16–1.89]). Higher outpatient costs were also observed for class I obesity (1.22 [1.09–1.37]). During the T2D stage, all the BMI levels higher than normal BMI were associated with higher costs, and the cost ratio increased with BMI, from 1.11 (1.03–1.20) for overweight BMI to 1.46 (1.34–1.59) for class III obesity. The cost ratio of outpatient costs and pharmacy costs also increased with BMI. For inpatient costs, only class III obesity had statistically significant higher costs than normal BMI, with a cost ratio of 1.60 (1.27–2.01).
Table 3.
Cost ratio of annualized costs, relative to 18.5 ≤ BMI < 25, in each glycemic stage
| 25 ≤ BMI < 30 | 30 ≤ BMI < 35 | 35 ≤ BMI < 40 | 40 ≤ BMI | |
|---|---|---|---|---|
| Normal glycemic stage | ||||
| Total healthcare costs | 1.09 (1.07–1.11) | 1.25 (1.22–1.27) | 1.30 (1.26–1.34) | 1.51 (1.45–1.56) |
| Inpatient costs | 1.11 (1.06–1.16) | 1.35 (1.29–1.43) | 1.41 (1.32–1.51) | 2.06 (1.89–2.24) |
| Outpatient costs | 1.05 (1.03–1.06) | 1.15 (1.13–1.17) | 1.23 (1.20–1.26) | 1.28 (1.24–1.32) |
| Pharmacy costs | 1.16 (1.13–1.20) | 1.33 (1.29–1.38) | 1.36 (1.30–1.42) | 1.47 (1.40–1.56) |
| PreD stage | ||||
| Total healthcare costs | 1.09 (0.95–1.25) | 1.09 (0.95–1.25) | 1.04 (0.90–1.22) | 1.28 (1.09–1.51) |
| Inpatient costs | 1.23 (0.86–1.76) | 1.01 (0.71–1.45) | 0.89 (0.59–1.33) | 1.15 (0.75–1.75) |
| Outpatient costs | 1.09 (0.98–1.23) | 1.22 (1.09–1.37) | 1.09 (0.96–1.24) | 1.26 (1.10–1.44) |
| Pharmacy costs | 1.20 (0.98–1.46) | 1.19 (0.97–1.45) | 1.24 (1.00–1.56) | 1.48 (1.16–1.89) |
| T2D stage | ||||
| Total healthcare costs | 1.11 (1.03–1.20) | 1.17 (1.09–1.27) | 1.29 (1.19–1.40) | 1.46 (1.34–1.59) |
| Inpatient costs | 1.11 (0.91–1.37) | 1.09 (0.89–1.34) | 1.20 (0.96–1.49) | 1.60 (1.27–2.01) |
| Outpatient costs | 1.11 (1.04–1.18) | 1.17 (1.09–1.25) | 1.22 (1.14–1.31) | 1.27 (1.18–1.37) |
| Pharmacy costs | 1.14 (1.02–1.27) | 1.26 (1.13–1.40) | 1.40 (1.24–1.57) | 1.48 (1.31–1.67) |
Cost ratios were estimated from generalized linear regression models with log-link and gamma distribution, adjusted for age, sex, race, smoking, employment status, insurance type, and year to start follow-up. The parentheses contain the 95 % confidence intervals of the cost ratios
BMI body mass index, PreD pre-diabetes, T2D type 2 diabetes
Table 4.
Results of regression on annualized costs
| Dependent variable | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annualized total healthcare costs | Annualized inpatient costs | Annualized outpatient costs | Annualized pharmacy costs | |||||||||||||
| Cost ratio | 95 % CI | Cost ratio | 95 % CI | Cost ratio | 95 % CI | Cost ratio | 95 % CI | |||||||||
| Normal glycemic stage | ||||||||||||||||
| Covariates | ||||||||||||||||
| BMI level | ||||||||||||||||
| 18.5 ≤ BMI < 25 | Reference | Reference | Reference | Reference | ||||||||||||
| 25 ≤ BMI < 30 | 1.09 | (1.07–1.11) | 1.11 | (1.06–1.16) | 1.05 | (1.03–1.06) | 1.16 | (1.13–1.20) | ||||||||
| 30 ≤ BMI < 35 | 1.25 | (1.22–1.27) | 1.35 | (1.29–1.43) | 1.15 | (1.13–1.17) | 1.33 | (1.29–1.38) | ||||||||
| 35 ≤ BMI < 40 | 1.30 | (1.26–1.34) | 1.41 | (1.32–1.51) | 1.23 | (1.20–1.26) | 1.36 | (1.30–1.42) | ||||||||
| 40 ≤ BMI | 1.51 | (1.45–1.56) | 2.06 | (1.89–2.24) | 1.28 | (1.24–1.32) | 1.47 | (1.40–1.56) | ||||||||
| Age group, years | ||||||||||||||||
| 18–44 | Reference | Reference | Reference | Reference | ||||||||||||
| 45–64 | 1.73 | (1.7–1.75) | 2.40 | (2.31–2.5) | 1.51 | (1.49–1.53) | 1.66 | (1.62–1.71) | ||||||||
| 65–74 | 2.93 | (2.84–3.02) | 7.25 | (6.73–7.81) | 2.24 | (2.18–2.3) | 1.83 | (1.75–1.92) | ||||||||
| 75–84 | 4.87 | (4.66–5.1) | 17.35 | (15.62–19.27) | 2.88 | (2.78–2.99) | 1.95 | (1.82–2.08) | ||||||||
| 85+ | 7.61 | (6.79–8.52) | 34.85 | (26.7–45.49) | 2.88 | (2.62–3.17) | 1.91 | (1.61–2.27) | ||||||||
| Male | 1.30 | (1.28–1.32) | 1.07 | (1.04–1.11) | 1.35 | (1.33–1.36) | 1.43 | (1.4–1.46) | ||||||||
| White race | 1.23 | (1.17–1.29) | 1.15 | (1.03–1.3) | 1.17 | (1.12–1.22) | 1.28 | (1.19–1.38) | ||||||||
| Smoking status | ||||||||||||||||
| Never smoked | Reference | Reference | Reference | Reference | ||||||||||||
| Former smoker | 1.29 | (1.27–1.32) | 1.43 | (1.36–1.50) | 1.20 | (1.18–1.23) | 1.32 | (1.28–1.36) | ||||||||
| Current smoker | 1.20 | (1.17–1.22) | 1.45 | (1.39–1.52) | 1.15 | (1.13–1.17) | 1.11 | (1.08–1.14) | ||||||||
| Other/unknown | 1.25 | (1.22–1.29) | 1.80 | (1.67–1.94) | 1.22 | (1.19–1.25) | 1.04 | (0.99–1.09) | ||||||||
| Employment status | ||||||||||||||||
| Full-time | Reference | Reference | Reference | Reference | ||||||||||||
| Not employed | 1.18 | (1.15–1.21) | 1.19 | (1.13–1.25) | 1.05 | (1.03–1.07) | 1.29 | (1.24–1.33) | ||||||||
| Other/unknown | 1.04 | (1.02–1.06) | 1.10 | (1.04–1.16) | 1.07 | (1.05–1.08) | 0.97 | (0.94–1) | ||||||||
| Covered by HMO insurance | 1.02 | (1–1.05) | 1.11 | (1.05–1.17) | 1.00 | (0.98–1.02) | 1.00 | (0.96–1.03) | ||||||||
| Stage starting year | ||||||||||||||||
| 2004 | Reference | Reference | Reference | Reference | ||||||||||||
| 2005 | 1.10 | (1.07–1.13) | 1.04 | (0.97–1.12) | 1.14 | (1.11–1.17) | 1.11 | (1.06–1.16) | ||||||||
| 2006 | 1.10 | (1.07–1.13) | 1.09 | (1.02–1.17) | 1.11 | (1.09–1.14) | 1.14 | (1.09–1.19) | ||||||||
| 2007 | 1.20 | (1.16–1.23) | 1.12 | (1.04–1.2) | 1.17 | (1.14–1.2) | 1.32 | (1.26–1.38) | ||||||||
| 2008 | 1.20 | (1.16–1.24) | 1.05 | (0.98–1.13) | 1.24 | (1.21–1.28) | 1.26 | (1.21–1.32) | ||||||||
| 2009 | 1.23 | (1.2–1.27) | 1.11 | (1.04–1.19) | 1.30 | (1.27–1.34) | 1.24 | (1.18–1.29) | ||||||||
| 2010 | 1.43 | (1.37–1.5) | 1.43 | (1.29–1.59) | 1.52 | (1.47–1.58) | 1.35 | (1.26–1.45) | ||||||||
| 2011 | 0.56 | (0.31–0.99) | 0.00 | (0.00–0.01) | 0.74 | (0.46–1.2) | 0.81 | (0.34–1.92) | ||||||||
| Goodness of fit | ||||||||||||||||
| Scaled deviance | 1.18 | 1.12 | 1.13 | 1.34 | ||||||||||||
| PreD stage | ||||||||||||||||
| Covariates | ||||||||||||||||
| BMI level | ||||||||||||||||
| 18.5 ≤ BMI < 25 | Reference | Reference | Reference | Reference | ||||||||||||
| 25 ≤ BMI < 30 | 1.09 | (0.95–1.25) | 1.23 | (0.86–1.76) | 1.09 | (0.98–1.23) | 1.20 | (0.98–1.46) | ||||||||
| 30 ≤ BMI < 35 | 1.09 | (0.95–1.25) | 1.01 | (0.71–1.45) | 1.22 | (1.09–1.37) | 1.19 | (0.97–1.45) | ||||||||
| 35 ≤ BMI < 40 | 1.04 | (0.9–1.22) | 0.89 | (0.59–1.33) | 1.09 | (0.96–1.24) | 1.24 | (1.00–1.56) | ||||||||
| 40 ≤ BMI | 1.28 | (1.09–1.51) | 1.15 | (0.75–1.75) | 1.26 | (1.10–1.44) | 1.48 | (1.16–1.89) | ||||||||
| Age group, years | ||||||||||||||||
| 18–44 | Reference | Reference | Reference | Reference | ||||||||||||
| 45–64 | 1.17 | (1.06–1.31) | 2.09 | (1.59–2.77) | 1.06 | (0.97–1.16) | 1.11 | (0.94–1.3) | ||||||||
| 65–74 | 1.64 | (1.4–1.91) | 3.94 | (2.61–5.94) | 1.32 | (1.16–1.5) | 1.43 | (1.14–1.79) | ||||||||
| 75–84 | 3.09 | (2.59–3.69) | 12.71 | (7.94–20.36) | 1.58 | (1.36–1.82) | 1.49 | (1.15–1.93) | ||||||||
| 85+ | 6.68 | (4.81–9.27) | 37.11 | (15.68–87.84) | 2.07 | (1.58–2.72) | 1.94 | (1.21–3.12) | ||||||||
| Male | 1.08 | (1–1.17) | 0.80 | (0.65–0.98) | 1.10 | (1.03–1.17) | 1.29 | (1.15–1.44) | ||||||||
| White race | 1.91 | (1.51–2.4) | 3.12 | (1.7–5.74) | 1.47 | (1.21–1.78) | 1.89 | (1.35–2.65) | ||||||||
| Smoking status | ||||||||||||||||
| Never smoked | Reference | Reference | Reference | Reference | ||||||||||||
| Former smoker | 1.25 | (1.15–1.37) | 1.41 | (1.12–1.76) | 1.08 | (1.01–1.16) | 1.36 | (1.2–1.55) | ||||||||
| Current smoker | 1.25 | (1.13–1.39) | 1.81 | (1.38–2.38) | 1.09 | (1–1.19) | 1.16 | (1–1.36) | ||||||||
| Other/unknown | 0.99 | (0.75–1.31) | 0.83 | (0.4–1.71) | 0.82 | (0.65–1.04) | 1.33 | (0.87–2.01) | ||||||||
| Employment status | ||||||||||||||||
| Full-time | Reference | Reference | Reference | Reference | ||||||||||||
| Not employed | 1.57 | (1.38–1.78) | 2.36 | (1.68–3.31) | 1.73 | (1.55–1.93) | 1.25 | (1.03–1.51) | ||||||||
| Other/unknown | 1.02 | (0.91–1.14) | 1.27 | (0.94–1.71) | 1.19 | (1.08–1.3) | 0.77 | (0.66–0.9) | ||||||||
| Covered by HMO insurance | 1.02 | (0.91–1.15) | 1.08 | (0.78–1.48) | 0.98 | (0.89–1.08) | 0.98 | (0.83–1.16) | ||||||||
| Stage starting year | ||||||||||||||||
| 2004 | Reference | Reference | Reference | Reference | ||||||||||||
| 2005 | 1.03 | (0.87–1.23) | 1.50 | (0.95–2.35) | 1.27 | (1.1–1.47) | 0.85 | (0.65–1.1) | ||||||||
| 2006 | 0.99 | (0.84–1.16) | 1.35 | (0.88–2.05) | 1.23 | (1.08–1.41) | 0.82 | (0.64–1.05) | ||||||||
| 2007 | 0.99 | (0.84–1.16) | 0.90 | (0.59–1.38) | 1.22 | (1.06–1.4) | 0.96 | (0.74–1.22) | ||||||||
| 2008 | 1.01 | (0.85–1.19) | 0.94 | (0.61–1.43) | 1.23 | (1.07–1.41) | 1.01 | (0.79–1.3) | ||||||||
| 2009 | 1.20 | (1.02–1.41) | 1.29 | (0.86–1.94) | 1.59 | (1.39–1.81) | 1.00 | (0.78–1.28) | ||||||||
| 2010 | 1.09 | (0.93–1.28) | 1.29 | (0.85–1.96) | 1.32 | (1.16–1.51) | 0.93 | (0.73–1.18) | ||||||||
| 2011 | 1.33 | (1.09–1.62) | 1.49 | (0.89–2.49) | 1.75 | (1.48–2.06) | 1.02 | (0.76–1.37) | ||||||||
| Goodness of fit | ||||||||||||||||
| Scaled deviance | 1.17 | 1.16 | 1.13 | 1.31 | ||||||||||||
| T2D stage | ||||||||||||||||
| Covariates | ||||||||||||||||
| BMI level | ||||||||||||||||
| 18.5 ≤ BMI < 25 | Reference | Reference | Reference | Reference | ||||||||||||
| 25 ≤ BMI < 30 | 1.11 | (1.03–1.20) | 1.11 | (0.91–1.37) | 1.11 | (1.04–1.18) | 1.14 | (1.02–1.27) | ||||||||
| 30 ≤ BMI < 35 | 1.17 | (1.09–1.27) | 1.09 | (0.89–1.34) | 1.17 | (1.09–1.25) | 1.26 | (1.13–1.40) | ||||||||
| 35 ≤ BMI < 40 | 1.29 | (1.19–1.40) | 1.20 | (0.96–1.49) | 1.22 | (1.14–1.31) | 1.40 | (1.24–1.57) | ||||||||
| 40 ≤ BMI | 1.46 | (1.34–1.59) | 1.60 | (1.27–2.01) | 1.27 | (1.18–1.37) | 1.48 | (1.31–1.67) | ||||||||
| Age group, years | ||||||||||||||||
| 18–44 | reference | Reference | Reference | Reference | ||||||||||||
| 45–64 | 1.33 | (1.25–1.41) | 1.83 | (1.55–2.15) | 1.18 | (1.12–1.24) | 1.22 | (1.12–1.33) | ||||||||
| 65–74 | 1.73 | (1.6–1.87) | 3.37 | (2.74–4.15) | 1.46 | (1.37–1.56) | 1.12 | (1.01–1.25) | ||||||||
| 75–84 | 2.79 | (2.55–3.04) | 7.19 | (5.68–9.09) | 1.91 | (1.77–2.06) | 1.19 | (1.05–1.35) | ||||||||
| 85+ | 3.98 | (3.36–4.72) | 13.15 | (8.34–20.71) | 1.72 | (1.49–1.99) | 1.20 | (0.94–1.52) | ||||||||
| Male | 1.08 | (1.04–1.13) | 1.00 | (0.9–1.12) | 1.14 | (1.1–1.18) | 1.17 | (1.1–1.23) | ||||||||
| White race | 1.17 | (1.03–1.33) | 1.24 | (0.88–1.75) | 1.17 | (1.05–1.31) | 1.10 | (0.92–1.32) | ||||||||
| Smoking status | ||||||||||||||||
| Never smoked | Reference | Reference | Reference | Reference | ||||||||||||
| Former smoker | 1.21 | (1.16–1.27) | 1.47 | (1.3–1.65) | 1.16 | (1.12–1.21) | 1.08 | (1.01–1.15) | ||||||||
| Current smoker | 1.23 | (1.17–1.31) | 1.56 | (1.33–1.82) | 1.18 | (1.12–1.24) | 1.07 | (0.99–1.16) | ||||||||
| Other/unknown | 1.17 | (1.07–1.29) | 1.56 | (1.2–2.02) | 1.12 | (1.03–1.21) | 0.94 | (0.82–1.07) | ||||||||
| Employment status | ||||||||||||||||
| Full-time | Reference | Reference | Reference | Reference | ||||||||||||
| Not employed | 1.40 | (1.32–1.49) | 1.89 | (1.6–2.23) | 1.26 | (1.2–1.33) | 1.26 | (1.15–1.37) | ||||||||
| Other/unknown | 1.21 | (1.15–1.28) | 1.59 | (1.38–1.84) | 1.23 | (1.17–1.29) | 0.99 | (0.92–1.07) | ||||||||
| Covered by HMO insurance | 1.11 | (1.05–1.18) | 1.27 | (1.08–1.5) | 1.12 | (1.06–1.18) | 0.95 | (0.87–1.03) | ||||||||
| Stage starting year | ||||||||||||||||
| 2004 | Reference | Reference | Reference | Reference | ||||||||||||
| 2005 | 0.95 | (0.89–1.02) | 0.93 | (0.77–1.13) | 0.97 | (0.92–1.03) | 0.96 | (0.87–1.06) | ||||||||
| 2006 | 1.04 | (0.97–1.11) | 1.14 | (0.95–1.36) | 1.05 | (1–1.12) | 1.04 | (0.95–1.14) | ||||||||
| 2007 | 1.02 | (0.95–1.09) | 0.87 | (0.72–1.04) | 1.21 | (1.14–1.29) | 1.08 | (0.98–1.19) | ||||||||
| 2008 | 1.12 | (1.04–1.19) | 1.09 | (0.91–1.31) | 1.19 | (1.12–1.25) | 1.17 | (1.06–1.28) | ||||||||
| 2009 | 1.07 | (1–1.14) | 0.97 | (0.82–1.15) | 1.23 | (1.17–1.3) | 1.05 | (0.96–1.15) | ||||||||
| 2010 | 1.14 | (1.05–1.23) | 1.06 | (0.86–1.31) | 1.46 | (1.37–1.56) | 1.05 | (0.94–1.17) | ||||||||
| 2011 | 1.32 | (1.15–1.51) | 1.14 | (0.79–1.64) | 1.66 | (1.48–1.86) | 1.15 | (0.95–1.4) | ||||||||
| Goodness of fit | ||||||||||||||||
| Scaled deviance | 1.14 | 1.16 | 1.11 | 1.25 | ||||||||||||
Generalized linear regression models with log-link and gamma distribution were conducted
Cost ratio was the exponential of estimated coefficient
CI confidence interval, BMI body mass index, HMO health maintenance organization, PreD pre-diabetes, T2D type 2 diabetes
The adjusted cost ratios were translated to incremental costs, as illustrated in Fig. 3. For subjects with normal glycemia, the incremental annual total costs associated with overweight or obesity relative to normal BMI ranged from $336 for overweight to $1,850 for class III obesity (all p < 0.05). Among the cost components, the largest incremental costs were from inpatient services associated with class III obesity ($1,084; p < 0.05). For subjects in the PreD stage, the incremental annual total costs associated with overweight or obesity relative to normal BMI ranged from $792 for overweight to $2,432 for class III obesity, and were only significant for class III obesity. This was mainly reflected by the incremental pharmacy costs ($1,165). For subjects in the T2D stage, the annual incremental total costs relative to normal BMI were significant and ranged from $1,139 for overweight to $4,649 for class III obesity (all p < 0.05). Inpatient costs associated with class III obesity ($2,629) had the largest incremental costs among the cost components.
Fig. 3.
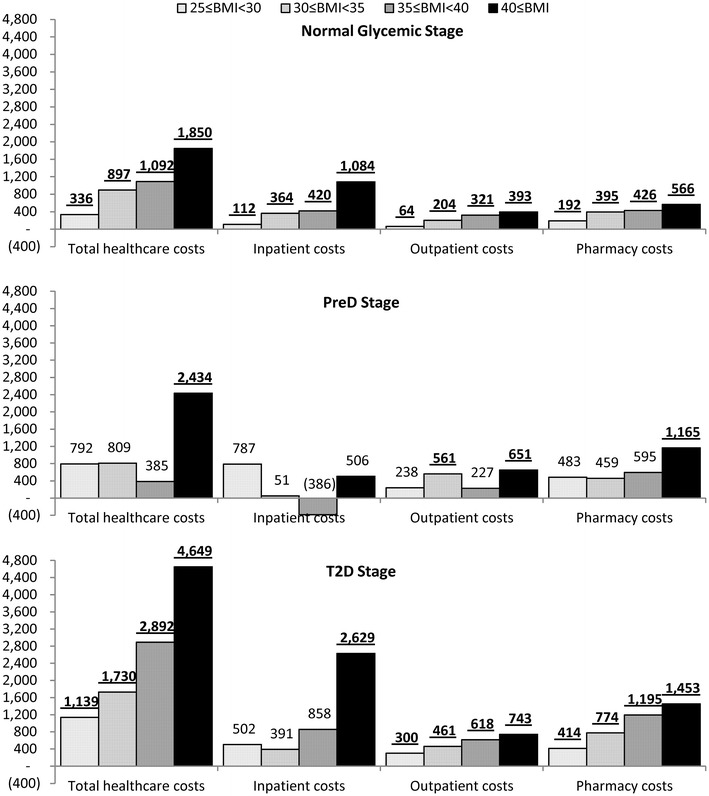
Adjusted incremental annualized costs, relative to 18.5 ≤ BMI < 25, in each glycemic stage. Incremental costs were adjusted for age, sex, race, smoking status, employment status, insurance type, and year starting the stage. Underscored incremental costs had p < 0.05 compared with normal BMI, based on generalized linear regression model with log-link and gamma distribution. BMI body mass index, PreD pre-diabetes, T2D type 2 diabetes
Discussion
Based on a regional US claims dataset, our study observed significantly increased healthcare costs with higher BMI level compared with normal BMI, after adjusting for sociodemographics. The positive associations varied by glycemic stage. The incremental costs of being overweight and obese were much higher among subjects with T2D than those with normal glycemic levels. Extreme obesity had a noticeable impact on healthcare costs regardless of the glycemic stages.
The economic burden of overweight and obesity has been well studied in the US. A systematic review on retrospective database studies published before 2009 estimated the direct medical costs of overweight and obesity were $266 and $1,723, respectively, per person per year (2008 US$) [25]. Recent assessments mostly utilized insurance claims data from large employers [26–28]. Data from 2003–2011 reported that the adjusted annual healthcare costs for obese employees were $2,055 higher than for those with BMI less than 27 kg/m2 [26]. Another study estimated that the incremental annual healthcare costs, compared with normal BMI, were $147, $712, and $1,977 (2005 US$) for overweight, obese, and class II–III obese (BMI ≥35 kg/m2), respectively, among employees from 2003–2005 [29]. Overall, the literature on the general overweight and obese population compares well with our study’s estimates of incremental BMI costs for the normal glycemic stage.
Despite the importance of weight control for subjects with PreD or T2D, there are limited studies on the cost of obesity for this specific population. Data from Germany found that obesity was associated with significant increments in the healthcare costs among adult patients with T2D, adjusted for age, sex, and diabetes complications [30]. No previous study has been found to evaluate the cost consequence of weight gain for subjects with PreD. In our study, the positive association between healthcare costs and BMI during the PreD stage was only significant for the extreme obesity level. This may be due to the small sample size and short duration of the identified PreD stage. Nevertheless, weight control among subjects with PreD is crucial. A clinical trial has shown that, compared with no intervention or use of metformin only, weight loss is associated with significantly slower progression from PreD to T2D [31].
It would be interesting to explore the underlying drivers of the positive association between costs and BMI levels. Existing literature indicates that obesity-related co-morbidities, such as diabetes, hypertension, and coronary heart disease, contribute to the majority, if not all, of the economic burden of obesity [32]. Even after controlling for diabetes, one of the most common and costly co-morbidities of obesity, our study still observed higher healthcare costs for higher BMI levels.
In terms of healthcare settings, our study found that, during normal glycemic stage and T2D stage, incremental pharmacy costs for BMI between 25 and 40 kg/m2 were higher than incremental inpatient costs and incremental outpatient costs, while for extreme obesity (BMI ≥40 kg/m2), the largest incremental costs occurred in the inpatient setting. During the PreD stage, the economic burden of obesity was mainly from pharmacy costs associated with extreme obesity. The importance of pharmacy costs is, at least in part, due to the fact that obesity-related co-morbidities are chronic conditions which rely heavily on prescription medication treatment.
The demographics, measured at the start of the follow-up period, varied across BMI levels. During PreD and T2D stages, subjects with higher BMI level were much younger. This implies that subjects with high BMI progress to PreD or T2D at a younger age. Our study examined the mean BMI during the glycemic stage in order to summarize the overall BMI status and assess its association with the healthcare costs. In our study sample, approximately 30 % of subjects changed BMI level. Further research could be conducted to analyze how the variation of BMI affects the healthcare costs.
Our study used a cross-sectional study design to provide a snapshot assessment on the association between BMI and healthcare costs during the entire glycemic stage. The longitudinal feature of the data may complicate the assessment and thus requires a more advanced analysis approach. For example, a fixed-effect model with panel study design could account for individual heterogeneity effects. However, due to the chronic nature of obesity, the lack of variation of BMI makes it challenging to apply the comment panel data approaches. In addition, our study sample had, on average, 3–5 years of follow-up, and thus medical innovations in recent years may affect the cost and BMI association, especially for patients with T2D.
It is challenging to identify the glycemic stages in a retrospective database which usually has no requirement for periodic glycemic testing. This particularly affects our recognition of PreD as it can be determined only by laboratory values. The differences in patient demographics and economic outcomes between PreD and T2D were smaller than that between normal glycemic stage and PreD, which indicates that PreD in our study may not be identified until close to its progression to diabetes. For subjects with no FPG or HbA1c laboratory results, diagnosis of T2D, or use of antidiabetic medications in the dataset, their true glycemic staging is unknown to us but are assumed to be normal in our study. In the US, a substantial proportion (27.8 %, or 8.1 million of 29.1 million in 2012) of diabetic patients are undiagnosed [6]. The proportion of undiagnosed PreD might even be much higher. However, since a minimum of 2 years of continuous enrollment was required for all normal glycemic subjects, we at least know that they have no evidence of elevated glucose for at least 2 years. Costs may be assigned to a glycemic stage less severe than the genuine glycemic level (i.e. assign costs associated with PreD to the normal glycemic stage, and assign costs associated with T2D to the PreD or normal glycemic stage), which may lead to estimation bias. However, our glycemic staging algorithm utilized all the information available in the dataset, and has been used in a previously published study [33].
Our results should be interpreted in light of the study’s limitations. First and foremost, the use of a retrospective cohort design prevented us from understanding the causal effect of BMI on the increase in healthcare costs. Second, claim reimbursement data were not available in the dataset. We applied standard cost data derived from external sources to the utilization recorded in the claims in the MedMining dataset. The medical costs may be under-estimated since CMS fee schedules are usually lower than the commercial insurances, while the average wholesale price used in our study is likely to be higher than the acquisition cost of medication. However, our study aims to assess the relative differences in costs between BMI levels, thus the biasness in the absolute costs does not affect our findings. Third, verified enrollment in drug coverage is not available and thus use of medication may not be fully identified. Fourth, estimation bias may exist. For example, unobserved factors (e.g. health status) may affect both the availability of BMI and healthcare costs, and thus lead to sample selection bias. Also, specific physician disposition may also play a role in the BMI and cost association. Finally, the findings are based on data from a single integrated health system caring for subjects in northern Pennsylvania and may not be generalizable to larger populations or to other regions at county, state, or national level in the US.
Regardless of these limitations, our study is the first analysis to assess the economic burden of being overweight and obese at different levels of severity, stratified by glycemic stage. Our study has other strengths. First, we followed subjects for at least 1 year until the end of a glycemic stage (within the continuous enrollment in the health plan), instead of only a 1-year snapshot of data, as examined in previous studies [25]. Findings from longer follow-up can provide a more comprehensive evaluation on the economic burden of obesity, since the healthcare costs were found to vary by duration of time in the glycemic stage, particularly for subjects with elevated glucose [13, 34]. Moreover, BMI values were clinically measured in the current study, while a majority of the published studies relied on self-reported weight and height. Self-reported weight and height considerably underestimate the subjects’ measured BMI [35, 36], which leads to systematic bias in the results. In addition, our study contained detailed demographic information, such as race, smoking status, and employment status, which are important confounders in cost-of-illness studies, but have not been widely captured and adjusted for in published studies.
Conclusions
Higher BMI level is associated with higher annual healthcare costs within all glycemic stages, after adjusting for demographic characteristics. The economic burden of overweight and obesity is higher among subjects with PreD or T2D. Targeted weight-control programs aimed at patients with pre-diabetes, or at-risk normal glycemic subjects, as well as those with frank T2D, should be able to generate a significant return on investment by effectively reducing the economic burden of overweight and obesity in the US. The impact on the health of the individual and the population is likely even more dramatic.
Acknowledgments
Qian Li and Steven W. Blume are employees of Evidera, which provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. In their salaried positions, they work with a variety of companies and organizations and are precluded from receiving payment or honoraria directly from these organizations for services rendered. They analyzed the data and drafted the manuscript with review and revision by all authors. Joanna C. Huang and Mette Hammer are employees and stockholders of Novo Nordisk Inc. Thomas Graf is an employee of Geisinger Health System, the company that produced the data that was purchased for use in the study, and provided consultation with regards to study design and review of the research. He has no conflicts of interest to declare.
Appendix
See Table 4.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cawley J, Meyerhoefer C, Biener A, Hammer M, Wintfeld N. Savings in medical expenditures associated with reductions in body mass index among US adults with obesity, by diabetes status. Pharmacoeconomics. 2014 doi: 10.1007/s40273-014-0230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity. 2008;16(10):2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Statistics about diabetes: data from the National Diabetes Statistics Report, released June 10, 2014. Alexandria (VA); 2014. http://www.diabetes.org/diabetes-basics/statistics/. Accessed 19 Jun 2014.
- 6.Centers for Disease Control and Prevention (CDC). National diabetes statistics report, 2014. Atlanta (GA); 2014. http://www.cdc.gov/diabetes/library/reports/surveillance.html. Accessed 19 Jun 2014.
- 7.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamseddeen H, Getty JZ, Hamdallah IN, Ali MR. Epidemiology and economic impact of obesity and type 2 diabetes. Surg Clin North Am. 2011;91(6):1163–72, vii. doi:10.1016/j.suc.2011.08.001. [DOI] [PubMed]
- 9.American Diabetes Association Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes. 2013;6:327–338. doi: 10.2147/DMSO.S51325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf AM, Colditz GA. The cost of obesity: the US perspective. Pharmacoeconomics. 1994;5(Suppl 1):34–37. doi: 10.2165/00019053-199400051-00007. [DOI] [PubMed] [Google Scholar]
- 12.Nichols GA, Hillier TA, Brown JB. Progression from newly acquired impaired fasting glusose to type 2 diabetes. Diabetes Care. 2007;30(2):228–233. doi: 10.2337/dc06-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols GA, Glauber HS, Brown JB. Type 2 diabetes: incremental medical care costs during the 8 years preceding diagnosis. Diabetes Care. 2000;23(11):1654–1659. doi: 10.2337/diacare.23.11.1654. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO). BMI classification, 1995, 2000, 2004. Global database on body mass index: an interactive surveillance tool for monitoring nutrition transition. Geneva. http://apps.who.int/bmi/index.jsp?intro. Accessed 19 Jun 2014.
- 15.RED BOOK™ from Micromedex Solutions®. Truven Health Analytics, Ann Arbor (MI). http://micromedex.com/redbook. Accessed 19 Jun 2014.
- 16.United States Centers for Medicare and Medicaid Services (CMS). Fee schedules. Baltimore (MD). http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/FeeScheduleGenInfo/index.html/. Accessed 19 Jun 2014.
- 17.United States Department of Health and Human Services. Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP). Rockville (MD). http://www.hcup-us.ahrq.gov/. Accessed 19 Jun 2014.
- 18.United States Centers for Medicare and Medicaid Services. Physician fee schedule. RVU12D file. Baltimore (MD); 2012. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/. Accessed 19 Jun 2014.
- 19.United States Centers for Medicare and Medicaid Services. Clinical lab fee schedule. 12CLAB file. Baltimore (MD); 2012. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html. Accessed 19 Jun 2014.
- 20.United States Centers for Medicare and Medicaid Services. Ambulance fee schedule. CY 2012 file. Baltimore (MD); 2012. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AmbulanceFeeSchedule/afspuf.html. Accessed 19 Jun 2014.
- 21.United States Centers for Medicare and Medicaid Services. DMEPOS fee schedule (durable medical equipment, prosthetics/orthotics, and supplies). DME12_C file. Baltimore (MD); 2012. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/DMEPOSFeeSched/DMEPOS-Fee-Schedule.html. Accessed 19 Jun 2014.
- 22.United States Department of Labor, Bureau of Labor Statistics. Consumer price index. Washington, DC; 2014. http://www.bls.gov/cpi/. Accessed 19 Jun 2014.
- 23.SAS/STAT® 9.2 User’s guide, second edition. The GENMOD Procedure SAS Institute Inc., Cary (NC). http://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#statug_genmod_sect004.htm. Accessed 19 Jun 2014.
- 24.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652–659. doi: 10.1111/j.0006-341X.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obes Rev. 2011;12(1):50–61. doi: 10.1111/j.1467-789X.2009.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinman N, Abouzaid S, Andersen L, Wang Z, Powers A. Cohort analysis assessing medical and nonmedical cost associated with obesity in the workplace. J Occup Environ Med. 2014;56(2):161–170. doi: 10.1097/JOM.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 27.Van Nuys K, Globe D, Ng-Mak D, Cheung H, Sullivan J, Goldman D. The association between employee obesity and employer costs: evidence from a panel of U.S. employers. Am J Health Promot. 2014;28(5):277–285. doi: 10.4278/ajhp.120905-QUAN-428. [DOI] [PubMed] [Google Scholar]
- 28.Ostbye T, Stroo M, Eisenstein EL, Peterson B, Dement J. Is overweight and class I obesity associated with increased health claims costs? Obesity. 2014;22(4):1179–1186. doi: 10.1002/oby.20669. [DOI] [PubMed] [Google Scholar]
- 29.Durden ED, Huse D, Ben-Joseph R, Chu BC. Economic costs of obesity to self-insured employers. J Occup Environ Med. 2008;50(9):991–997. doi: 10.1097/JOM.0b013e318182f730. [DOI] [PubMed] [Google Scholar]
- 30.von Lengerke T, Hagenmeyer EG, Gothe H, Schiffhorst G, Happich M, Haussler B. Excess health care costs of obesity in adults with diabetes mellitus: a claims data analysis. Exp Clin Endocrinol Diabetes. 2010;118(8):496–504. doi: 10.1055/s-0030-1253400. [DOI] [PubMed] [Google Scholar]
- 31.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quesenberry CP, Jr, Caan B, Jacobson A. Obesity, health services use, and health care costs among members of a health maintenance organization. Arch Intern Med. 1998;158(5):466–472. doi: 10.1001/archinte.158.5.466. [DOI] [PubMed] [Google Scholar]
- 33.Blume SW, Li Q, Huang JC, Hammer M, Graf TR. Variation in the risk of progression between glycemic stages across different levels of body mass index: evidence from a United States electronic health records system. Curr Med Res Opin. 2014:1–10. doi:10.1185/03007995.2014.971356. [DOI] [PubMed]
- 34.Brown JB, Nichols GA, Glauber HS, Bakst AW. Type 2 diabetes: incremental medical care costs during the first 8 years after diagnosis. Diabetes Care. 1999;22(7):1116–1124. doi: 10.2337/diacare.22.7.1116. [DOI] [PubMed] [Google Scholar]
- 35.Burkhauser RV, Cawley J. Beyond BMI: the value of more accurate measures of fatness and obesity in social science research. J Health Econ. 2008;27(2):519–529. doi: 10.1016/j.jhealeco.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Plankey MW, Stevens J, Flegal KM, Rust PF. Prediction equations do not eliminate systematic error in self-reported body mass index. Obes Res. 1997;5(4):308–314. doi: 10.1002/j.1550-8528.1997.tb00556.x. [DOI] [PubMed] [Google Scholar]


