Highlights
-
•
Signet ring cell carcinoma in the ampulla of vater is extremely uncommon.
-
•
Investigation to confirm the histological origin of signet ring cell carcinoma by immunohistochemical staining might inform the treatment strategy and identify patients with ampullary signet ring cell carcinoma who may have a good prognosis.
Abbreviations: SRCC, signet ring cell carcinoma; PD, pancreatoduodenectomy; PPPD, pylorus-preserving pancreatoduodenectomy
Keywords: Ampulla of vater, Signet ring cell carcinoma, Pancreatoduodenectomy
Abstract
Introduction
Signet ring cell carcinoma (SRCC) of the ampulla of vater is a very rare tumor that is reported infrequently in the literature.
Presentation of case
A 59-year-old woman visited our hospital for evaluation of elevated transaminase levels. On laboratory examination of tumor marker levels, carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19–9 levels were normal, and DUPAN-2 was elevated. Computed tomography (CT) confirmed a 2 cm, enhanced mass in the periampullary region, with marked common bile duct dilatation. Endoscopic retrograde cholangiopancreatography (ERCP) showed a swollen papilla of vater, with a reddish, erosive mucosa. Histological examination of biopsy samples from the ampulla of vater showed signet ring cell carcinoma (SRCC). The patient underwent radical pancreatoduodenectomy. Pathological examination showed that the SRCC had infiltrated into the duodenal muscularis propria and pancreatic parenchyma, and lymph node metastases were identified around the abdominal aorta and common hepatic artery. Based on the immunohistochemical staining patterns of the positive results for CDX2 and MUC2, the tumor cells in the present case appeared to have an intestinal type origin. The ampullary cancer was diagnosed as T3bN1M1, Stage IV according to the International Union Against Cancer TNM classification (UICC). After undergoing adjuvant chemotherapy with cisplatin–gemcitabine chemotherapy for 6 months, the patient has remained disease-free in the 7 months since surgery.
Discussion
SRCC of intestinal-type origin is associated with a favorable outcome.
Conclusion
Investigation to confirm the histological origin of SRCC by immunohistochemical staining might inform the treatment strategy and identify patients with ampullary SRCC who may have a good prognosis.
1. Introduction
Most tumors of the ampulla of vater are well-differentiated adenocarcinomas. Signet ring cell carcinoma (SRCC) at this site is uncommon [1], and only 26 resected cases have been previously reported in the English literature. A rare case of a 59-year-old woman with SRCC and paraaortic lymph node metastases is presented.
2. Case presentation
A 59-year-old woman visited our hospital for evaluation of elevated transaminase levels. She had no symptoms, and her past history was unremarkable. The physical examination findings were also unremarkable, with no abdominal tenderness or palpable masses. Routine laboratory test results were: aspartate aminotransferase (AST) 5 IU/L (normal 10–33 IU/L); alanine aminotransferase (ALT) 121 IU/L (normal 6–35 IU/L); alkaline phosphatase 884 IU/L (normal 120–340 IU/L); γ-glutamyltransferase 684 IU/L (normal 8–60 IU/L); total bilirubin (T-bil) 0.5 mg/dL (normal 0.4–1.4 mg/dL); amylase 87 IU/L (normal 31–106 IU/L); lipase 49 IU/L (normal 8–46 IU/L); and normal inflammatory markers. Laboratory test results for tumor markers were: carcinoembryonic antigen (CEA) 2.9 ng/mL (normal 0–5.0 ng/mL); carbohydrate antigen (CA) 19–9 2 U/mL (normal 0–37 U/mL); DUPAN-2 940 U/mL (normal 0–150 U/mL); and SPAN-1 16 U/mL (normal 0–30 U/mL). Abdominal ultrasonography demonstrated gross dilatation of the common bile and pancreatic ducts. Subsequent computed tomography (CT) confirmed a 2 cm, enhanced mass in the periampullary region, marked common bile duct dilatation, and no evidence of lymphadenopathy or distant metastases (Fig. 1). Positron emission tomography with 2 [18 F]-fluoro-2-deoxy-D-glucose (FDG) showed a mass with an SUVmax of 4.2, consistent with the CT scan findings. Endoscopic retrograde cholangiopancreatography (ERCP) revealed a swollen papilla of vater with a reddish, erosive mucosa (Fig. 2). Cholangiography demonstrated an abrupt obstruction of the lower common bile duct. Histological examination of the biopsy samples from the ampulla of vater showed SRCC. To decompress the biliary system, an endoscopic retrospective biliary drainage tube (8.5 Fr) was inserted before surgery. The patient underwent surgery based on a diagnosis of SRCC of the ampulla of vater in September 2014. Surgery revealed no liver metastases or peritoneal dissemination, and a radical pancreatoduodenectomy was performed. Microscopic examination of the ampullary tumor revealed a poorly differentiated adenocarcinoma, of signet ring cell type that infiltrated the duodenal wall and adjacent pancreas (Fig. 3). There was lymphatic and vascular invasion. Lymph node metastases were identified around the abdominal aorta and common hepatic artery. Immunohistochemical staining was performed, and the signet ring cells were positive for CK7, CK19, CK20, CDX2, and MUC2, but negative for MUC5AC and E-cadherin (Fig. 4). The ampullary cancer was diagnosed as T3bN1M1, Stage IV according to the International Union Against Cancer TNM classification (UICC). After undergoing adjuvant chemotherapy with cisplatin-gemcitabine chemotherapy for 6 months, the patient has remained disease-free in the 7 months since surgery.
Fig. 1.
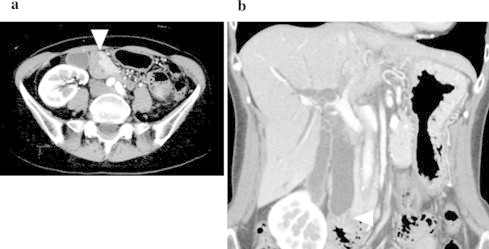
Computed tomography: CT scan confirms a 2 cm, enhanced mass in the periampullary region (arrowhead) and shows marked common bile duct dilatation.
Fig. 2.
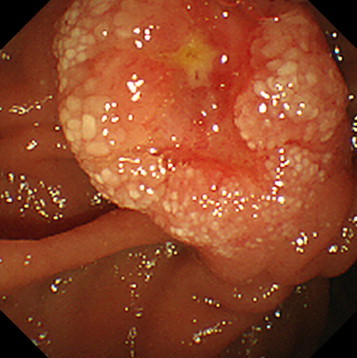
Endoscopic retrograde cholangiopancreatography: ERCP shows a swollen papilla of vater with a reddish, erosive mucosa.
Fig. 3.
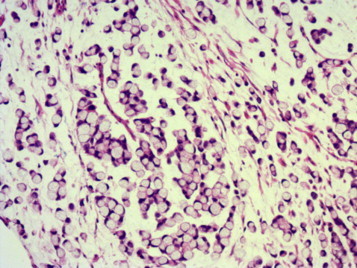
Microscopic examination: the ampullary tumor is a poorly differentiated adenocarcinoma of signet ring cell type.
Fig. 4.
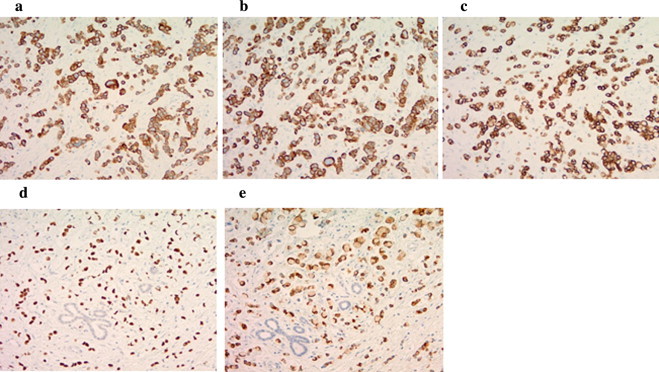
Immunohistochemical staining: the signet ring cells and adenocarcinoma cells are positive for CK7 (a), CK19 (b), CK20 (c), CDX2 (d), and MUC2 (e).
3. Discussion
Adenocarcinoma of the ampulla of vater is rare, with an incidence of less than 6 cases per million persons annually. It represents 0.2% of all gastrointestinal malignancies, and accounts for only 6% of all cancers developing in the periampullary region [1]. SRCC in the ampulla of vater is extremely uncommon, and in a PubMed search of the English literature using the key words SRCC and ampulla of vater; it was found that only 26 such well-documented; resected cases have been previously described (Table 1) [2–19].
Table 1.
Resected cases of signet ring cell carcinoma of the ampulla of vater reported in the English literature.
| First author | Ref. | Year | Age (y) | Sex | Size (mm) | UICC | Treatment | Follow-up (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Gardner | [2] | 1990 | 69 | F | 20 | T3N0M0 | PD | NA | NA |
| Hara | [3] | 2002 | 68 | M | 15 | T2N0M0 | PPPD | 10 | Alive |
| Tseng | [4] | 2002 | 47 | M | 20 | T3N0M0 | PD | 6 | Alive |
| Eriguchi | [5] | 2003 | 83 | M | 15 | T3N0M0 | PD | 18 | Alive |
| Li | [6] | 2004 | 56 | F | 15 | T2N1M0 | PD | 12 | Alive |
| Ramia | [7] | 2004 | 67 | F | 18 | T2N0M0 | PD | 12 | Alive |
| Fang | [8] | 2004 | 53 | M | 26 | T2N0M0 | PD | 25 | Alive |
| Bloomston | [9] | 2005 | 58 | F | 10 | T2N0M0 | PD | 134 | Alive |
| Akasu | [10] | 2007 | 43 | F | 20 | T2N0M0 | PD | 90 | Alive |
| Gao | [11] | 2009 | 38 | F | 20 | T3N0M0 | PD | 6 | Alive |
| Ishibashi | [12] | 2009 | 59 | M | 30 | T3N0M0 | PD | 18 | died |
| Gheza | [13] | 2011 | 66 | M | NA | NA | PD | 8 | Alive |
| Paplomata | [14] | 2011 | 45 | F | 30 | T4N1Mx | PPPD gemcitabine + oxaliplatin | 12 | Died |
| Maekawa | [15] | 2011 | 75 | M | 20 | T3N0M0 | PD | 6 | Died |
| Lesquereux-Martínez | [16] | 2012 | 78 | F | 11 | TxN1M0 | PD gemcitabine | 14 | Alive |
| Daoudi | [17] | 2012 | 55 | M | NA | T3N0M0 | PD gemcitabine + cisplatin | 8 | Alive |
| Acharya | [18] | 2013 | 78 | F | 30 | T3N0M0 | PD | 6 | Alive |
| Wen | [19] | 2014 | 40 | F | 30 | T3N0M0 | PD | 8 | Alive |
| Wen | [19] | 2014 | 64 | F | 65 | T4N x M0 | PD | 76 | Alive |
| Wen | [19] | 2014 | 75 | F | 35 | T4N x M0 | PD | 16 | Died |
| Wen | [19] | 2014 | 62 | M | 24 | T x N1M0 | PD | 27 | Died |
| Wen | [19] | 2014 | 62 | M | 30 | T x N1M0 | PD | 9 | Died |
| Wen | [19] | 2014 | 53 | M | 12 | T3N0M0 | PD | 45 | Alive |
| Wen | [19] | 2014 | 66 | F | 15 | T3N0M0 | PD | 54 | Alive |
| Wen | [19] | 2014 | 68 | M | 95 | T4N x M0 | PD | 72 | Alive |
| Our case | 2015 | 59 | F | 20 | T3N1M1 | PD gemcitabine + cisplatin | 7 | Alive |
PD: pancreatoduodenectomy.
PPPD: pylorus-preserving pancreatoduodenectomy.
NA: not available.
Including the present case, the 26 cases consisted of 12 men and 14 women. The median age at diagnosis was 61 years (range, 38–83 years). The median tumor diameter was 20 mm (mean, 26 mm; range, 10–95 mm). Six cases had UICC T2 (duodenal invasion) tumors, twelve cases had T3 (pancreatic invasion) tumors, and three cases had T4 tumors. Previous reports of ampullary SRCCs were limited to short-term follow-up with a median period of 12 months (mean, 28 months; range, 3–134 months). Long-term survival, more than 5 years, was documented in only four cases [9,10,19].
The origin of SRCCs remains controversial. Since they are predominantly found in gastric cancers, one theory is that these tumors originate from heterotopic gastric mucosa. Some authors have reported the presence of ectopic gastric mucosa near the ampullary tumor [2,7]. Another theory suggests that these carcinomas arise from areas of gastric-type metaplastic epithelia, which are considered to be a protective response to elevated acidity and are observable in the duodenal bulb of peptic ulcer patients [19]. In this theory, signet ring cells may originate from periampullary duodenal heterotopia of an ulcer etiology and expand secondarily to the ampulla of vater. In the present case, however, no ectopic gastric epithelium was found in the peritumoral region, and the patient also had no history of peptic ulcer disease. Immunohistochemical staining patterns allowing further classification of ampullary SRCC to an intestinal (I)- or pancreatobiliary (PB)-type have been described [15]. Expression of CK7, along with negativity for CK20, CDX2, and MUC2, signifies pancreatobiliary-type SRCC, and vice-versa. Hoedemaeker [20] attempted to classify ampullary SRCC into the following four types: I, PB, gastric, and mixed type. They concluded that the I-type ampullary SRC patients might have a more favorable prognosis than patients with PB-type ampullary SRCC differentiation. Based on the immunohistochemical staining patterns showing positive results for CDX2 and MUC2, the tumor cells in the present case appeared to have an I-type origin, which is associated with a favorable outcome.
The majority of patients in previous cases of ampullary SRCC underwent pancreatoduodenectomy (PD), occasionally with extended lymphadenectomy and/or partial gastrectomy. This radical approach facilitates lymph node dissection in advanced disease states, but a pylorus-preserving pancreatoduodenectomy (PPPD), which has been used in three previous cases of ampullary SRCC [3,14], may be more applicable in early disease, where curability is balanced by a more moderate resection. In the present case, a radical PD with an extensive lymphadenectomy including paraaortic lymph nodes dissection was performed. PPPD was not chosen in this case because this procedure could result in an insufficient dissection for the infrapyloric nodes.
Chemoradiotherapy based on 5-fluorouracil has been used as an adjunctive treatment modality following curative resection of ampullary adenocarcinomas. However, there is debate as to whether this actually affords a significant survival benefit, since many patients develop metastatic disease. Presently, no established adjuvant chemotherapeutic regimen exists specifically for ampullary SRCC, although documented anti-neoplastic agents have been used in 4 cases, including the present case [14,16,17]. In the report of Daoudi et al., a 55-year-old male patient with pT3N0M0 disease received six cycles of adjuvant gemcitabine/cisplatin, without disease recurrence at eight months [17].
4. Conclusions
Confirmation of the histological origin of SRCC by immunohistochemical staining may inform the treatment strategy and identify patients with ampullary SRCC who may have a good prognosis.
Conflict of interest
Masaki Wakasugi and the other co-authors have no conflict of interest to declare.
Funding
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan to M.T. (No. 25462129).
Ethical approval
Written, informed consent was obtained from the patient for her information to be included in our manuscript. Her information has been de-identified to the best of our ability to protect her privacy.
Authors contribution
Each author participated in writing the manuscript, and all agreed to accept equal responsibility for the accuracy of the content of the paper.
References
- 1.Howe J.R., Klimstra D.S., Moccia R.D., Conlon K.C., Brennan M.F. Factors predictive of survival in ampullary carcinoma. Ann. Surg. 1998;228:87–94. doi: 10.1097/00000658-199807000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner H.A., Matthews J., Ciano P.S. A signet-ring cell carcinoma of the ampulla of vater. Arch. Pathol. Lab. Med. 1990;114:1071–1072. [PubMed] [Google Scholar]
- 3.Hara T., Kawashima H., Ishigooka M., Kashiyama M., Takanahi S., Hosokawa Y. Signet-ring-cell carcinoma of the ampulla of vater: a case report. Hepatogastroenterology. 2002;49:561–563. [PubMed] [Google Scholar]
- 4.Tseng L.J., Jao Y.T., Mo L.R. Signet ring cell carcinoma of major papilla. Gastrointest. Endosc. 2002;56:733. doi: 10.1067/mge.2002.128698. [DOI] [PubMed] [Google Scholar]
- 5.Eriguchi N., Aoyagi S., Jimi A. Signet-ring cell carcinoma of the ampulla of vater: report of a case. Surg. Today. 2003;33:467–469. doi: 10.1007/s10595-002-2509-9. [DOI] [PubMed] [Google Scholar]
- 6.Li L., Chen Q.H., Sullivan J.D., Breuer F.U. Signet-ring cell carcinoma of the ampulla of vater. Ann. Clin. Lab. Sci. 2004;34:471–475. [PubMed] [Google Scholar]
- 7.Ramia J.M., Mansilla A., Villar J., Muffak K., Garrote D., Ferron J.A. Signet-ring cell carcinoma of the Vater’s ampulla. JOP. 2004;5:495–497. [PubMed] [Google Scholar]
- 8.Fang C.L., Chu J.S., Hsieh M.C., Wu M.S. Signet-ring cell carcinoma of the ampulla of vater. J. Formos. Med. Assoc. 2004;103:793–796. [PubMed] [Google Scholar]
- 9.Bloomston M., Walker M., Frankel W.L. Radical resection in signet ring carcinoma of the ampulla of vater: report of an 11-year survivor. Am. Surg. 2006;72:193–195. [PubMed] [Google Scholar]
- 10.Akatsu T., Aiura K., Takahashi S., Kameyama K., Kitajima M., Kitagawa Y. Signet-ring cell carcinoma of the ampulla of vater: report of a case. Surg. Today. 2007;37:1110–1114. doi: 10.1007/s00595-007-3534-4. [DOI] [PubMed] [Google Scholar]
- 11.Gao J.M., Tang S.S., Fu W., Fan R. Signet-ring cell carcinoma of the ampulla of vater: contrast-enhanced ultrasound findings. World J. Gastroenterol. 2009;15:888–891. doi: 10.3748/wjg.15.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishibashi Y., Ito Y., Omori K., Wakabayashi K. Signet ring cell carcinoma of the ampulla of vater: a case report. JOP. 2009;10:690–693. [PubMed] [Google Scholar]
- 13.Gheza F., Ceryi E., Pulcini G., Villanacci V., Giulini S.M., Schiavo- Lena M. Signet ring cell carcinoma of the ampulla of vater: demonstration of pancreatobiliary origin. Pancreas. 2011;40:791–793. doi: 10.1097/MPA.0b013e318216db94. [DOI] [PubMed] [Google Scholar]
- 14.Paplomata E., Wilfong L. Signet ring cell carcinoma of the ampulla of vater with leptomeningeal metastases: a case report. J. Clin. Oncol. 2011;29:e627–e629. doi: 10.1200/JCO.2011.35.2385. [DOI] [PubMed] [Google Scholar]
- 15.Maekawa H., Sakurada M., Orita H., Sato K. Signet-ring cell carcinoma co-existing with adenocarcinoma of the ampulla of vater a case report. JOP. 2011;12:162–166. [PubMed] [Google Scholar]
- 16.Lesquereux-Martínez L., Fernández-Pérez A., Bustamante- Montalvo M. Signet ring cell adenocarcinoma of the ampulla of vater: a rare pathology. Rev. Esp. Enferm. Dig. 2012;104:501–502. doi: 10.4321/s1130-01082012000900013. [DOI] [PubMed] [Google Scholar]
- 17.Daoudi K., El Haoudi K., Bouyahia N., Benlemlih A., Arifi S., Mellas N. Signet ring cell carcinoma of the vater's ampulla: a very rare malignancy. Case Rep. Oncol. Med. 2012;2012:1–2. doi: 10.1155/2012/402798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acharya M.N., Panagiotopoulos N., Cohen P., Ahmad R., Jiao L.R. Poorly-differentiated signet-ring cell carcinoma of the ampulla of vater: report of a rare malignancy. JOP. 2013;14:190–194. doi: 10.6092/1590-8577/1267. [DOI] [PubMed] [Google Scholar]
- 19.Wen X., Wu W., Wang B., Yao H., Teng X. Signet ring cell carcinoma of the ampulla of vater: immunophenotype and differentiation. Oncol. Lett. 2014;8:1687–1692. doi: 10.3892/ol.2014.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoedemaeker P.J. Heterotopic gastric mucosa in the duodenum. Digestion. 1970;3:165–173. doi: 10.1159/000197027. [DOI] [PubMed] [Google Scholar]


