Abstract
The formation of repetitive structures (such as stripes) in nature is often consistent with a reaction-diffusion mechanism, or Turing model, of self-organizing systems. We used mouse genetics to analyze how digit patterning (an iterative digit/nondigit pattern) is generated. We showed that the progressive reduction in Hoxa13 and Hoxd11-Hoxd13 genes (hereafter referred to as distal Hox genes) from the Gli3-null background results in progressively more severe polydactyly, displaying thinner and densely packed digits. Combined with computer modeling, our results argue for a Turing-type mechanism underlying digit patterning, in which the dose of distal Hox genes modulates the digit period or wavelength. The phenotypic similarity with fish-fin endoskeleton patterns suggests that the pentadactyl state has been achieved through modification of an ancestral Turing-type mechanism.
Digit patterning has commonly been interpreted in the context of a morphogen gradient model (1, 2). The proposed morphogen Sonic hedgehog (Shh) emanates from the zone of polarizing activity (a cluster of mesodermal cells in the posterior border of the limb bud) and establishes a gradient with maximum levels posteriorly. Gli3 is the major mediator of Shh signaling in limb development and a genetic cause of polydactyly (2). Because Shh prevents the processing of Gli3 to its repressor form (Gli3R), the Shh gradient is translated into an inverse gradient of Gli3R (3, 4). The surprising finding that mouse Gli3 and Shh;Gli3 null mutants display identical polydactylous limb phenotypes demonstrates that an iterative series of digits can form in the absence of Shh (4, 5). Rather than supporting a gradient model, this observation is consistent with a Turing-type model for digit patterning (6–11) in which dynamic interactions between activator and inhibitor molecules determine the wavelength of the specific pattern and produce periodic patterns of spots or stripes. This pattern has been hypothesized to act as a molecular prepattern for chondrogenesis. According to one of the specific predictions of the model, the digit period or wavelength, defined as the combined thickness of both digit and interdigital region, should be subject to modulation by perturbing the correct parameter of the gene network. This should lead to autopods with digits varying in thickness and number, which has never been clearly observed to date.
Although the core molecules of a self-organizing mechanism remain unknown, potential candidates for molecular modulators of the system include the Hox genes (10, 12). Distal Hoxa and Hoxd genes have a well-documented impact on digit number (13), though their specific role remains unclear, possibly due to their various interactions with the Shh-Gli3 pathway. These interactions include the mutual transcriptional regulation between Hox genes and Shh and the binding of Hoxd12 to Gli3R, resulting in a blockage of Gli3R repressor activity (14–16). In general, gain- and loss-of-function experiments suggest a positive relation between Hox genes and digit number (14, 17–22), which is also indicated by the ectopic anterior up-regulation of distal Hoxd genes in the Gli3-dependent polydactyly (4, 5). However, we showed that the combined deletion of Hoxd11–13 and Gli3 exacerbated the Gli3 polydactyly (23), suggesting instead a negative relation between distal Hoxd genes and digit number.
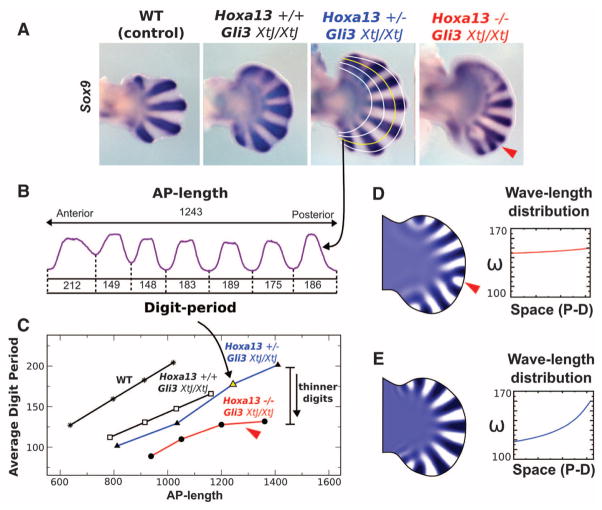
Hoxd11-13;Gli3 mutants displayed a gain of Hoxa13 expression, similar to Gli3 mutants (23). To address the relation between Hoxa13 and digit number, we generated double Hoxa13;Gli3 mutants (see supplementary materials and methods). At embryonic day 12.5 (E12.5), Sox9 expression marked the five digital chondrogenic condensations in control autopods and revealed the delay in differentiation in the anterior mesoderm and the polydactyly typical of the Gli3 deficiency (Fig. 1A) (24). Hoxa13−/−;Gli3XtJ/XtJ showed seven to eight digital condensations in the posterior mesoderm plus a diffuse Sox9 expression in the anterior mesoderm, which likely corresponded to presumptive digital condensations (Fig. 1A). Even though the number of digits could not be precisely determined, Sox9 expression suggested an increase in digit number in Hoxa13−/−;Gli3XtJ/XtJ compared with Gli3XtJ/XtJ mutants, supporting a negative effect of Hoxa13 on digit number, similar to distal Hoxd genes (23).
Fig. 1.
(A) Expression of Sox9 in E12.5 limbs of the Hoxa13;Gli3 allelic series. Note the delayed differentiation in the anterior mesoderm in the absence of Gli3. The curved white and yellow lines show the AP profiles used for the analysis of Sox9. The red arrowhead points to a digit bifurcation. WT, wild type. (B) Sox9 staining intensity along the yellow profile indicated by the curved arrow. AP length and the period of each digit (from minimum to minimum) are measured and shown for Hoxa13+/−;Gli3XtJ/XtJ. (C) Chart showing the average digit periods versus AP lengths for each profile and limb. A linear relation is observed in controls and in the Gli3XtJ/XtJ background for either the normal or heterozygous dose of Hoxa13, whereas a flatter relation that correlates with bifurcations (red arrowhead) is observed in the Hoxa13−/−;Gli3XtJ/XtJ limbs (red line). The curved arrow marks the yellow point corresponding to the profile in (B). (D and E) Two simulations of the reaction-diffusion model inside an E12.5 Gli3 mutant limb shape. (D) The activator concentration obtained in the simulation with a uniform modulation of wavelength ω (shown in the graph) shows digit bifurcation (red arrowhead) similar to the Hoxa13−/−Gli3XtJ/XtJ mutants. (E) The simulation result when wavelength is modulated according to a suitable PD gradient (in this case, a 2D gradient of simulated FGF signaling activity) avoids bifurcations, because the wavelength increases with increasing AP length. Limbs shown in all figures are forelimbs with distal to the right and anterior to the top.
Hoxa13−/−;Gli3XtJ/XtJ limbs also displayed reduced digit wavelength and digit bifurcations (Fig. 1A). To quantify both features, we analyzed curved anterior posterior (AP) profiles of Sox9 expression at four equidistant positions along the proximal distal (PD) axis of the digit region of the Hoxa13;Gli3 mutants shown in Fig. 1A (Fig. 1B and supplementary materials). We measured the average digit period and AP length of each profile (shown in Fig. 1B for Hoxa13+/−;Gli3XtJ/XtJ) and plotted results (Fig. 1C). In control, Hoxa13+/+;Gli3XtJ/XtJ, and Hoxa13+/−;Gli3XtJ/XtJ mutants, the average digit period increased along the PD axis, whereas the ratio between the average digit period and the AP length was constant, suggesting that the wavelength of a Turing-type mechanism was scaled along the PD axis to maintain a constant number of digits. However, this was not true for the Hoxa13−/−;Gli3XtJ/XtJ limbs, where the average digit period flattened off distally, and, in agreement with a Turing-type mechanism, digit bifurcations occurred as the AP length increased (Fig. 1A). Our quantification suggested that normal digit patterning involves an effective PD increase of the digit wavelength, which we hypothesized must be carefully controlled if bifurcations are to be avoided.
To test this hypothesis, we built a two-dimensional (2D) finite-element model based on the shape of the Gli3XtJ/XtJ handplate at E12.5 and simulated a reaction-diffusion system by scaling the wavelength in either a uniform (remaining constant) or a graded manner along the PD axis of the digital region (Fig. 1, D and E, and supplementary text). We used a generic activator-inhibitor reaction-diffusion system, employing the minimal conditions to satisfy Turing instability (see supplementary text) (25). We determined whether a single reaction parameter of the model could be an effective target for wavelength modulation (figs. S4 and S5) and chose the effect of activator on inhibitor production (gu) as the most suitable (see supplementary text). Our simulations showed that digit bifurcations occurred in the uniform case (red arrowhead Fig. 1D), whereas no bifurcations were shown when we used graded wavelength scaling (Fig. 1E), reproducing the observed mutant patterns. Our model suggests that, in the absence of Gli3, the genetic reduction of Hoxa13 produces a global reduction in wavelength (ω), thereby increasing digit number, but also causes a shallower PD gradient of ω, which explains the observed digit bifurcations. Because there is no evidence for a PD-graded Hox expression, the simplest interpretation of the model is that ω is modulated by both the Hoxa13 gene (explaining the global reduction in wavelength) and fibroblast growth factor (FGF) signaling (explaining the PD gradient), possibly by co-regulating the same target genes (see supplementary text).
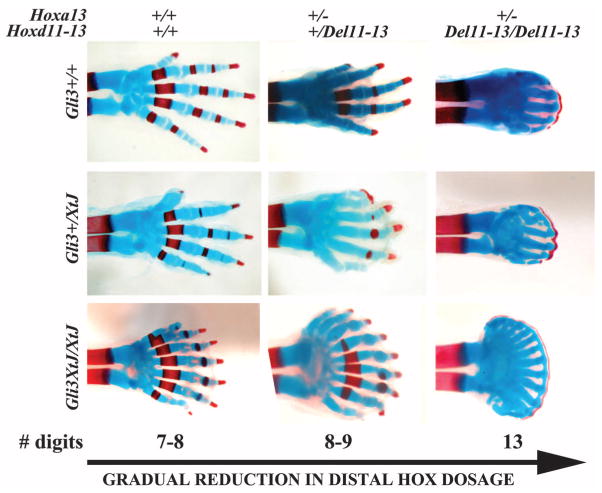
Because Hoxa13−/−;Gli3XtJ/XtJ double mutants display ectopic anterior expression of Hoxd12 and Hoxd13, similar to Gli3 mutants (fig. S1), it remained possible that the increased polydactyly was due to the gain of Hoxd12 and Hoxd13. To challenge such functional compensation and test whether the cumulative sum of distal functional Hox genes controls digit number by modulating the digit period, we generated triple mutants carrying the Hoxa13−, HoxdDel11-13, and Gli3XtJ alleles (Fig. 2). Skeletal preparations of neonates of the triple-mutant allelic series showed a variety of patterning defects, including syndactyly (fused digits), brachydactyly (shortened digits), absence of joints, ventral bending of digits, and delayed ossification. Most salient, however, was the clear trend toward increased digit number as progressively more alleles of distal Hox genes were removed in the absence of Gli3 (Fig. 2). The number of digits increased from 7 to 9 (typical of Gli3XtJ/XtJ) to 8 to 9 when one functional allele of both Hoxa13 and Hoxd11-13 was removed; further, to 9 to 11 when both Hoxd11-13 alleles were removed (23); and, finally, to at least 12 to 14 digits when only one copy of Hoxa13 remained (Hoxa13+/−;HoxdDel11-13/Del11-13;Gli3XtJ/XtJ) (Fig. 2). The midgestational lethality of Hoxa13 homozygous mutants precluded their analysis at this stage. The increase in digit number as distal Hox genes were removed did not rely on increased AP handplate size, as in Gli3 single mutants (26), but rather on a reduced wavelength as digits were thinner and had narrower gaps between them, while remaining regularly spaced (Figs. 1A and 2). These phenotypes cannot be explained by a model of positional information based on a morphogen gradient.
Fig. 2.
Representative skeletal phenotypes of newborns of the Hoxa13;Hoxd11-13;Gli3 allelic series. Digit number (indicated for the Gli3XtJ/XtJ condition) increases as distal Hox dose is reduced. When only one functional copy of Hoxa13 remains (right column), the tip of the digits is connected by a continuous band of ossified (red) and cartilaginous (blue) tissue rimming the distal border of the limb and becoming more conspicuous as Gli3 copies are removed.
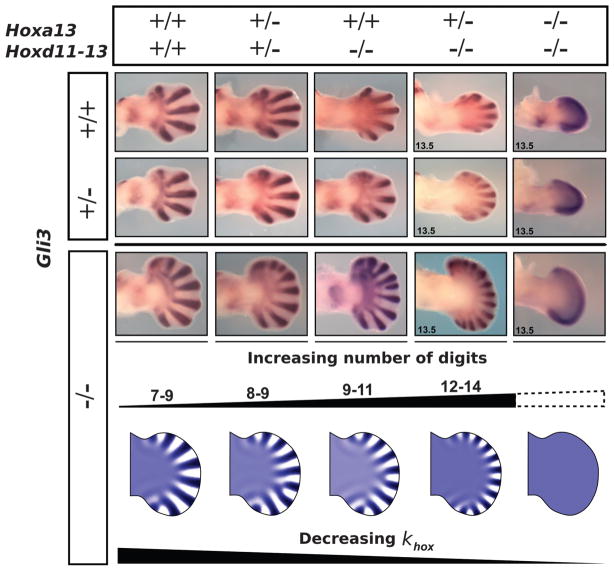
To compare the principal phenotypes of the triple allelic series with our computational model, we analyzed Sox9 expression at E12.5, when the condensations are being laid down and the Turing mechanism should be operative. Quantification of these mutants confirmed that there was no strict correlation between the progressive increase in digit number and handplate size, which instead coincided with thinner, more densely packed digits (Fig. 3). In our computer model, both Hox levels and FGF signaling contribute to wavelength modulation (Fig. 4A). Thus, by progressively reducing the global contribution from the Hox genes (khox), the resulting ω gradient became lower, but also shallower (fig. S6), reflecting the shallower wavelength gradients quantified from the mutants (Fig. 4B). In this way, the model was able to reproduce the observed smooth series of Sox9 phenotypes in the Gli3XtJ/XtJ background—both the increased number of digits and the greater tendency for bifurcations (Fig. 3 and supplementary text). Although the observed reduction in wavelength was strongest in the absence of Gli3, the same trend was clearly apparent in the Gli3+/+ and Gli3+/XtJ backgrounds (Fig. 4C and supplementary materials). In these cases, a reduced wavelength does not always produce a higher number of digits, because the AP width also decreases.
Fig. 3.
The phenotypes of triple mutants can be replicated by the Turing model. (Top) The first three rows show Sox9 expression at E12.5 and E13.5 for different combinations of the triple Hoxa13;Hoxd11-13;Gli3 allelic series. As more Hox are removed, the general trend shows an increase in digit number and a decrease in digit thickness. The trend is most strongly evident in the complete absence of Gli3 (third row). (Bottom) A similar behavior is shown by the reaction-diffusion simulations, where a decrease of the PD gradient used to modulate wavelength is correlated with reduced Hox dose (khox). Additionally, the model predicts a narrower digital region along the PD axis, which eventually shrinks to zero, and no pattern is formed.
Fig. 4.
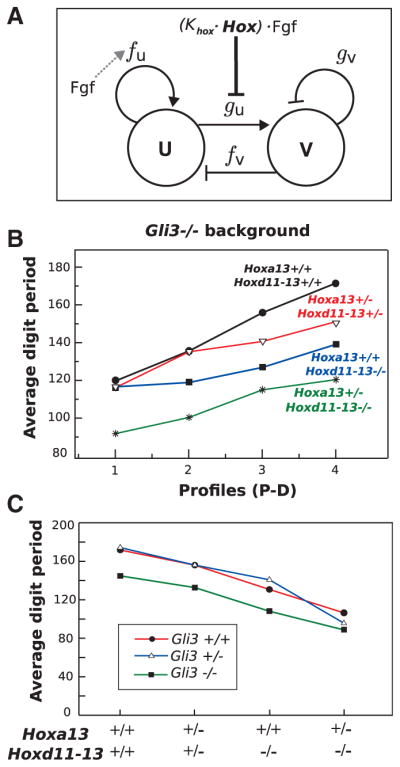
(A) Schematic representation of the network of a general activator-inhibitor Turing model. The four reaction kinetic parameters are shown: fu, fv, gu, and gv. Fgf promotes a PD-graded distribution of the parameter fu to drive stripe orientation (gray dashed arrow). Hox and Fgf inhibit the parameter gu to increase the wavelength in a PD-graded manner (bold line). U, activator; V, inhibitor. (B) Graphs of the average digit period of the triple mutants with Gli3−/− background at four equidistant positions along the PD axis of the digital region. With the exception of Hoxa13+/−;HoxdDel11-13/Del11-13;Gli3XtJ/XtJ, a clear trend is observed: The PD gradient of wavelength is generally shallower as distal Hox genes are removed. (C) Graphs of average digit period (wavelength) versus distal Hox gene dose in the three different Gli3 backgrounds. A smooth positive correlation between Hox gene dose and wavelength is observed in all three cases.
Our study highlighted a developmental delay in the appearance of the Sox9 pattern (fig. S2) and a reduction in the PD width of the digit-forming region (fig. S3). Theoretical analysis revealed that both features are naturally predicted by modulating gu in the model. The delayed patterning also supports the possible role of FGF signaling as a Turing modulator, as FGF4 treatment of micromass-cultured mesenchyme from limb buds sped up the appearance of the pattern (25). The simulated PD gradient of FGF signaling thus translates into a gradient of patterning speed, and further theoretical analysis revealed that this naturally predicts the progressive PD narrowing of the digital zone (fig. S6 and supplementary test). Below a certain Hox dose, the digital zone disappears entirely. Indeed, in triple mutants, no distinct digital condensations were scored, even at E13.5 (Fig. 3 and fig. S6).
In conclusion, our combination of genetics, quantitative analysis, and computer modeling reveals a missing piece of evidence for a Turing-type mechanism in digit patterning. Whereas numerous previous mutants have shown an abnormal digit number, evidence was lacking for a parameter that could smoothly tune digit wavelength. Our discovery and analysis of the smooth correlation between distal Hox gene number and digit wavelength provides this evidence. Additionally, the link between wavelength deregulation and the appearance of digit bifurcations also strongly supports the role of distal Hox genes as wavelength modulators of an intrinsic self-organizing Turing-type mechanism responsible for digit patterning. Our model predicts that overexpression of Hox genes should increase the digit wavelength, although this may require careful temporal examination at the time the Turing mechanism is operating and may have a subtle effect if Hox genes are normally expressed at saturating levels (22). The model makes no prediction about the temporal sequence of digit condensations along the AP axis. Also, the probability of bifurcations may be reduced in more extreme mutants (such as Hoxa13+/−;HoxdDel11-13/Del11-13;Gli3XtJ/XtJ) due to the narrower digital region. Additionally, our analysis suggests a role for Gli3 in tuning the wavelength, but this observation is not as notable as the smooth trend seen in our distal Hox allelic series (Figs. 3 and 4C and supplementary materials).
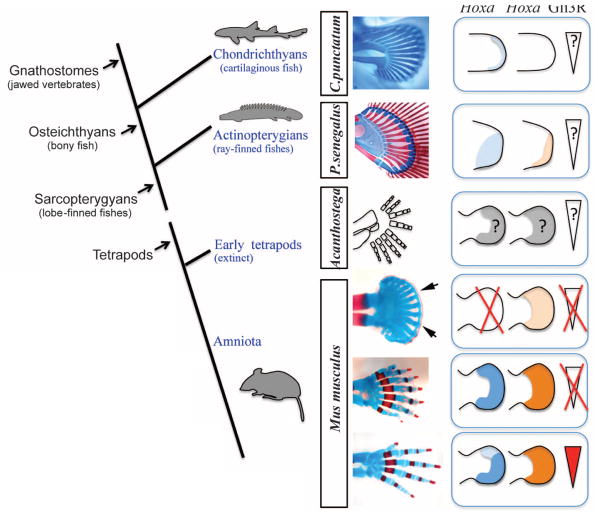
Our results also permit a reassessment of distal Hox gene function in one of the most important vertebrate innovations: the fin-to-limb transition. The emerging consensus suggests that the genetic toolkit patterning fins and limbs is largely conserved, and the evolution of digits was driven by accumulated regulatory changes controlling the spatial and temporal deployment of that common toolkit (27–32). The reduction of the distal Hox gene number in the absence of Gli3, which renders Shh signaling irrelevant, resulted in mouse digits losing defining characteristics (pentadactyl constraint and segmented morphologies) and exhibiting patterns reminiscent of the endoskeleton patterns in chondrichthyan and basal actinopterygian fins (numerous, iterative, densely packed, infrequently segmented elements) (Fig. 5). Thus, our data provide evidence that an ancestral Turing-like mechanism patterning fins has been conserved in tetrapods and modified by the implementation of regulatory changes in the evolution of digits. In particular, our data suggest that the equilibrium resulting from the cross-regulation between Shh-Gli3 and distal Hox genes may have led to the stabilization of the pentadactyl state more than 360 million years ago.
Fig. 5.
Vertebrate limb evolution and distal Hox gene function. A phylogenetic tree of representative taxa and appendage skeletal patterns is shown, as well as corresponding distal Hox expression [in actinopterygians shown for Polyodon spathula (28)] and Gli3R gradient (when known). Genetically abrogating Shh signaling and reducing distal Hox function in mouse autopods (Hoxa13+/−;HoxdDel11-13/Del11-13;Gli3XtJ/XtJ) reveals ancestral skeletal characteristics shared with the pectoral fins of sharks (Chiloscyllium punctatum) and primitive ray-finned fishes (Polypterus senegalus): numerous, densely packed, and iterative elements, with a distal cartilaginous band corresponding to the distal radials of fish fins (arrows). The periodic pattern of skeletal elements evident in fins and mutant limbs strongly suggests that a self-organizing Turing-type mechanism of chondrogenesis is deeply conserved in vertebrate phylogeny. Our results further indicate that distal Hox gene dose regulates the number and spacing of skeletal elements formed, implicating distal Hox gene regulatory networks as critical drivers of the evolution of the pentadactyl limb.
Supplementary Material
Acknowledgments
We thank D. Duboule and P. Chambon for the mutant mice, A. Munteanu for comments on the mathematical analysis, and M. Torres and M. Towers for critical reading of the manuscript. This work was supported by grant BFU2011-24972 from the Spanish Ministry of Science and Innovation to M.A.R. and by grant MOP-82880 from the Canadian Institutes of Health Research to M.K. R.S. was supported by a Formación Profesorado Universitario fellowship from the Spanish Ministry of Science and Innovation; L.M. was supported by a fellowship from the EMBL-CRG Systems Biology Program.
Footnotes
References and Notes
- 1.Bastida MF, Ros MA. Curr Opin Genet Dev. 2008;18:374. doi: 10.1016/j.gde.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Zeller R, López-Ríos J, Zuniga A. Nat Rev Genet. 2009;10:845. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Fallon JF, Beachy PA. Cell. 2000;100:423. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 4.Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Nature. 2002;418:979. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- 5.te Welscher P, et al. Science. 2002;298:827. doi: 10.1126/science.1075620. [DOI] [PubMed] [Google Scholar]
- 6.Kondo S, Miura T. Science. 2010;329:1616. doi: 10.1126/science.1179047. [DOI] [PubMed] [Google Scholar]
- 7.Turing AM. Philos Trans R Soc London Ser B. 1952;237:37. [Google Scholar]
- 8.Newman SA, Frisch HL. Science. 1979;205:662. doi: 10.1126/science.462174. [DOI] [PubMed] [Google Scholar]
- 9.Gierer A, Meinhardt H. Kybernetik. 1972;12:30. doi: 10.1007/BF00289234. [DOI] [PubMed] [Google Scholar]
- 10.Miura T, Shiota K, Morriss-Kay G, Maini PK. J Theor Biol. 2006;240:562. doi: 10.1016/j.jtbi.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Maini PK, Solursh M. Int Rev Cytol. 1991;129:91. doi: 10.1016/s0074-7696(08)60510-0. [DOI] [PubMed] [Google Scholar]
- 12.Newman SA. Bioessays. 1996;18:171. doi: 10.1002/bies.950180302. [DOI] [PubMed] [Google Scholar]
- 13.Zákány J, Duboule D. Cell Tissue Res. 1999;296:19. doi: 10.1007/s004410051262. [DOI] [PubMed] [Google Scholar]
- 14.Zákány J, Kmita M, Duboule D. Science. 2004;304:1669. doi: 10.1126/science.1096049. [DOI] [PubMed] [Google Scholar]
- 15.Kmita M, et al. Nature. 2005;435:1113. doi: 10.1038/nature03648. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, et al. Development. 2004;131:2339. doi: 10.1242/dev.01115. [DOI] [PubMed] [Google Scholar]
- 17.Kmita M, Fraudeau N, Hérault Y, Duboule D. Nature. 2002;420:145. doi: 10.1038/nature01189. [DOI] [PubMed] [Google Scholar]
- 18.Fromental-Ramain C, et al. Development. 1996;122:2997. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- 19.Zákány J, Fromental-Ramain C, Warot X, Duboule D. Proc Natl Acad Sci USA. 1997;94:13695. doi: 10.1073/pnas.94.25.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis AP, Capecchi MR. Development. 1996;122:1175. doi: 10.1242/dev.122.4.1175. [DOI] [PubMed] [Google Scholar]
- 21.Goff DJ, Tabin CJ. Development. 1997;124:627. doi: 10.1242/dev.124.3.627. [DOI] [PubMed] [Google Scholar]
- 22.Knezevic V, et al. Development. 1997;124:4523. doi: 10.1242/dev.124.22.4523. [DOI] [PubMed] [Google Scholar]
- 23.Sheth R, Bastida MF, Ros M. Dev Biol. 2007;310:430. doi: 10.1016/j.ydbio.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 24.McGlinn E, et al. Mech Dev. 2005;122:1218. doi: 10.1016/j.mod.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Miura T, Maini PK. Bull Math Biol. 2004;66:627. doi: 10.1016/j.bulm.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Rios J, et al. Dev Cell. 2012;22:837. doi: 10.1016/j.devcel.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahn RD, Davis MC, Pappano WN, Shubin NH. Nature. 2007;445:311. doi: 10.1038/nature05436. [DOI] [PubMed] [Google Scholar]
- 28.Davis MC, Dahn RD, Shubin NH. Nature. 2007;447:473. doi: 10.1038/nature05838. [DOI] [PubMed] [Google Scholar]
- 29.Freitas R, Zhang G, Cohn MJ. PLoS ONE. 2007;2:e754. doi: 10.1371/journal.pone.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woltering JM, Duboule D. Dev Cell. 2010;18:526. doi: 10.1016/j.devcel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Schneider I, et al. Proc Natl Acad Sci USA. 2011;108:12782. doi: 10.1073/pnas.1109993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shubin N, Tabin C, Carroll S. Nature. 2009;457:818. doi: 10.1038/nature07891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.