Abstract
Introduction
Absolute CD4 T cell count and plasma viral load have been established as predictors of HIV disease progression, and CD4 T cell count is used as an indicator for initiation of antiretroviral therapy. Following long-term therapy, patients generally present with significant CD4 T cell recovery contrasting with persistently elevated CD8 T cell counts, which leads to a partial restoration of CD4:CD8 ratio. This review focuses on the relevance of the CD4:CD8 ratio on clinical outcomes, immune dysfunction and HIV reservoir size in long-term treated patients.
Method
We conducted a comprehensive literature review of publications in English language using major electronic databases. Our search was focused on factors contributing to CD4:CD8 T cell ratio and clinical outcome in adult HIV-positive patients in the context of treated infection.
Discussion
Low CD4:CD8 ratio has been linked to ageing and acts as a predictor of mortality in the general population. This ratio may represent the combined effects of inflammation and immunological changes called “inflammaging.” Although the mechanisms underlying partial correction of the CD4:CD8 ratio and persistently elevated CD8 T cell count in long-term treated patients remain poorly understood, it has been recently indicated that patients with optimal CD4 T cell recovery and low CD4:CD8 ratio still harbour increased immune activation, an immune senescent phenotype and have a higher risk of non-AIDS morbidity and mortality. This review reconsiders CD4:CD8 ratio in the light of advances in the understanding of immune dysfunction and examines its pathophysiological features and implications on clinical outcome and HIV reservoir size in long-term treated HIV-positive adults.
Conclusion
The CD4:CD8 ratio can contribute to the immunological evaluation of treated patients in a long-term follow-up and may be applied for monitoring both immune dysfunction and viral reservoir size in immune-based clinical trials.
Keywords: CD4:CD8 ratio, HIV infection, immune dysfunction, inflammation, viral reservoir
Introduction
HIV infection is characterized by profound CD4 T cell destruction, compromised mucosal barrier function and chronic immune activation. In addition, this infection is associated with a marked activation and expansion of HIV-specific and bystander CD8 T cells [1]. The impairment in CD4 T cell regeneration and the persistent elevation of CD8 T cell counts are considered to be a consequence of viral persistence and multiple inflammatory factors including gut microbial translocation, leading to major T cell dysfunction [2,3]. Antiretroviral therapy (ART) in a majority of patients suppresses HIV plasma viral load (VL) and stops the progression to AIDS, allowing progressive CD4 T cell recovery paired with a persistent elevation of CD8 T cells. Such changes on T cell populations over time result in a partial restoration of the CD4:CD8 ratio. Patients on suppressive ART who present with lower CD4:CD8 ratios have a higher risk for non-AIDS morbidity and mortality even with optimal CD4 T cell recovery [4]. However, the reason for the persistence of elevated CD8 T cell counts during HIV infection was reviewed but has not been elucidated [5].
This review focuses on the relationship between CD4:CD8 ratio and clinical outcomes, inflammation, as well as ageing and HIV reservoir size in the context of treated infection. We believe that the data reviewed here supports that the CD4:CD8 ratio represents a marker of immune dysfunction and may contribute to better patient management. Furthermore, this ratio may be helpful in allocating patients and assessing immune changes in immune-based clinical trials.
Methods
A comprehensive review of the English-language publications was implemented. We searched PubMed, JSTOR and Scopus electronic databases with keyword combinations including CD4:CD8 ratio, CD4 T cell, CD4 T cell reconstitution/restoration/recovery, CD8 T cell persistence/elevation and ageing. The same strategy was used with Google Scholar and ISI Proceedings to further include non-peer-reviewed literature and conference publications. We also considered the number of citations and study sample size of the relevant papers for their selection and description in the text and/or tables. Our search was limited to adult HIV-1-positive subjects using publications from year 2000 to 2015 at a time when advances in ART have allowed long-term control of viral replication.
Results and discussion
Historical perspective of the CD4:CD8 ratio as a prognostic factor for disease progression
In 1989, Taylor et al. compared the role of three immunological parameters in predicting disease progression to AIDS among 813 untreated HIV-seropositive men during a three-year longitudinal study, i.e. absolute CD4 T cell count, CD4 T cell percentage and the CD4:CD8 ratio. They found that these three parameters were strongly correlated to each other and showed comparable ability to predict the development of AIDS [6]. Older patient age can be used as a predictor of clinical events, so do the additional markers such as neopterin (a product of stimulated macrophages), β2-microglobulin, soluble interleukin-2 receptors (sIL-2R) and immunoglobulin A (IgA). However, among all these aforementioned markers, CD4 T cell count and the CD4:CD8 ratio stood out as the two best predictors for the progression to AIDS [7]. Level of CD8 T cell activation as measured by CD38 and HLA-DR expression is also an independent predictor of HIV disease progression [8,9]. Interestingly, elevated CD8 T cell counts and their level of activation represent independent factors associated with disease outcome.
Although the CD4:CD8 ratio has been used as a parameter to predict outcome [10–12], absolute CD4 T cell count is still considered the key criterion for ART initiation as it represents the best surrogate marker for the risks of opportunistic infections, AIDS-related cancers and death.
The CD4:CD8 ratio acts as a surrogate marker of T cell compartment balance: CD4 T cell recovery and CD8 T cell expansion
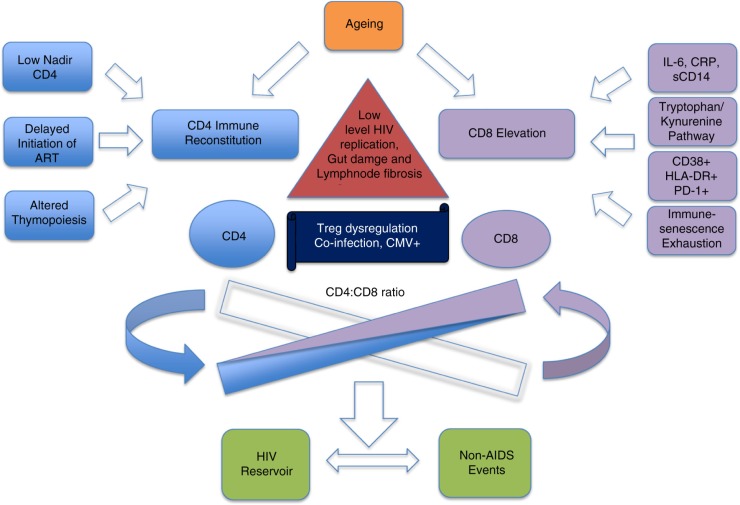
Long-term ART has successfully restored CD4 T cell counts in a large proportion of HIV-positive patients. However, the majority of these patients still demonstrate a persistent elevation of CD8 T cell count as well as dysfunction of CD8 T cell compartments [13]. The balance between immune reconstitution and immune activation/inflammation may be involved in the trend towards the normalization of the CD4:CD8 ratio with ART (Figure 1).
Figure 1.
Factors associated with low CD4:CD8 ratio in HIV treated infection.
The CD4:CD8 ratio foretells CD4 T cell recovery with long-term ART
CD4 T cell recovery is a sign of immune improvement following control of viral replication with ART [14]. The attainment of a CD4 T cell count over 500 cells/mm3 has been associated with a reduction in mortality [15]. Factors including gender, low pretreatment absolute CD4 T cell count (nadir T cell count), presence of CXCR4 (X4) tropic virus, altered thymopoiesis, overexpression of genes involved in immune activation and apoptosis and inflammation have been clearly identified as factors influencing CD4 T cell recovery [16–20]. Specifically, low CD4:CD8 ratio prior to initiation of ART was associated with a decreased probability of achieving CD4 T cell counts >500 cells/mm3 [4,21].
Serrano-Villar et al. have reported on the factors associated with a low CD4:CD8 ratio in HIV-positive adults from four distinct clinical cohorts and three clinical trials. They showed that a low CD4:CD8 ratio despite effective ART and CD4 T cell recovery above 500 cells/mm3 was associated with a skewed T cell phenotype from naïve towards terminally differentiated CD8+ T cells, with higher levels of CD8+ T cell activation (HLADR+CD38+), senescence (CD28- and CD57+CD28-) and higher kynurenine/tryptophan ratio [13]. Most significantly, by using multiparametric bioinformatics, Buggert et al. identified a correlation between the CD4:CD8 ratio and CD4 T cell populations that over-expressed activation and exhaustion markers such as programmed death-1 (PD-1) [22]. Equally, the CD4:CD8 ratio displayed a significant correlation with absolute CD4 T cell recovery during a two-year treatment period. Interestingly, compared with the absolute CD4 T cell count and HIV plasma VL, the CD4:CD8 ratio before ART initiation acted as a much better predictor for CD4 T cell recovery.
Ndumbi et al. monitored the longitudinal changes in CD4:CD8 ratio over a 10-year follow-up in ART treated patients [23]. They demonstrated that in patients with a CD4 T cell count<200 cells/mm3, the CD4:CD8 ratio failed to normalize. Importantly, they found that CD4:CD8 ratio normalization resulted from the combination of an optimal increase in CD4 T cell count with a concomitant decrease in CD8 T cell count. However, this only occurred in patients having a baseline CD4 T cell count>350 cells/mm3. Conversely, in patients with CD4 T cell counts<350 cells/mm3, CD8 T cells remained elevated, a possible result of persistent residual low-level HIV replication, gut damage associated with immune activation, lymph node fibrosis, coinfections, immunosenescence and Treg dysregulation [24]. The determinants of CD4 recovery in the context of treated infection have been recently reviewed [25] (Figure 1).
A recent prospective observational study by Mussini et al. [26] on 3236 participants with a median CD4:CD8 ratio of 0.39 before ART reported the estimated normalization probability of 4.4, 11.5 and 29.4% after 1, 2 and 5 years of treatment, respectively. Higher CD4 T cell counts and higher CD4:CD8 ratio at baseline as well as absence of cytomegalovirus (CMV) serology have been more likely associated with ratio normalization.
Persistence of expansion of CD8 T cells with skewed differentiation phenotype
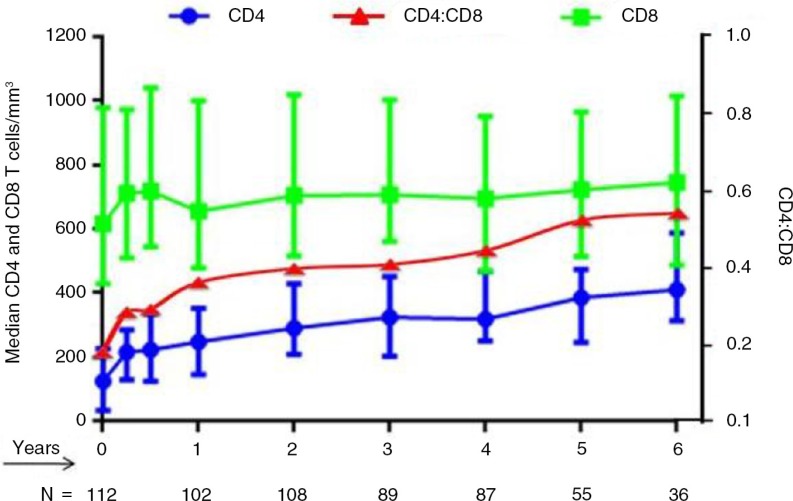
Following effective long-term ART, about 50 to 80% HIV-positive patients achieve CD4 T cell counts >500 cells/mm3 depending on their baseline values and time on ART [27]. However, there are continuous quantitative, qualitative and functional defects in CD8 T cell compartment, resulting in low CD4:CD8 ratios. Recently Helleberg et al. examined the trajectories of CD8 T cells before and after ART in 3882 HIV-positive patients [24]. Their results showed that CD8 T cells were elevated during untreated HIV infection and remained elevated through 10 years of ART. This finding is concordant with our cohort of 109 HIV-positive late presenters, who harboured a CD4:CD8 ratio of 0.66 (0.19–1.84) after a median follow-up of 4.5 years on ART, with a median baseline CD4:CD8 ratio of 0.15 (0.01–0.76) (Figure 2, unpublished data).
Figure 2.
Trajectories of CD4 and CD8 T cells and CD4:CD8 ratio in long-term treated patients. (Advanced patients initiating ART, enrolled in Beijing cohort.)
Emu et al. also found that the increased CD4:CD8 ratio in ART-suppressed subjects compared to non-controllers was driven by an increase in CD4 T cells, contrasting with continuously expanded CD8 T cells [28]. Expansion of differentiated CD8 T cell subpopulations (CD28-CD27-CD45RA+/CCR7-) persisted despite ART and minimal changes were noted in naïve T cell counts over time. Increased numbers of exhausted CD8+CD28- T cells were associated with a low CD4:CD8 ratio. Those persistently expanded CD8 T cells in treated HIV-positive patients exhibit characteristics of T cell immune-senescence and exhaustion. Whether those expanded CD8 T cells persist durably without continued cell division remains to be determined [29]. These factors might contribute to the failure of the majority of patients receiving ART to achieve normalized CD4:CD8 ratios.
Correlation of the CD4:CD8 ratio with immune activation and inflammation
The CD4:CD8 ratio is considered a marker for both immune-senescence and immune activation, even in patients with long-term suppressive viral control [30,31]. (Studies are depicted in Table 1.) Historically inflammation was regarded as a passive pathological consequence of injury and infection. Nowadays, it has been gradually considered also as a mechanism of immune defense and repair. Immune activation usually encompasses changes in cellular markers and soluble factors associated with inflammation [32], while inflammation refers more often to increases in soluble biomarkers such as interferon alpha (IFN-α), tumour necrosis factor alpha (TNF-α), IL-6 [33,34] and other cytokines and chemokines. Furthermore, the exhaustion marker PD-1 which regulates T cell activation and sCD14, a marker of monocyte/macrophage activation, has also been implicated in HIV pathogenesis [35–38].
Table 1.
Studies on association of CD4:CD8 ratio with immunological and clinical outcome in chronically treated HIV patients
| Correlations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Patients treated/untreated | Number of patients (N) | Duration of ART (years) | CD4: CD8 ratio criteria | Normalization of CD4:CD8 percentage | Factors associated with normalization of CD4:CD8 ratio | Activation HLA-DR+ CD38+ | Immuno- senescence CD57+CD28- | Inflammation IL-6, hs-CRP, sCD14 | Non-AIDS morbidity and mortality |
| [46] | Treated HIV- RNA<50 | 4206 | 2.7 | >1.2 | 7.2% | Baseline CD4>350, CD8<500,CD4:CD8>0.5 | NA | NA | NA | No |
| [13] | Treated with viral suppression and CD4≥500 cells/mm3 | C1>1500 C2>2200 C3=122 C4=2400 |
Different durations | ≤0.4 defined as low | NA | Positively correlated with TN, TCM and TTM and negatively correlated with TEM.; predictor of the K/T | Yes | Yes | Yes | Yes |
| [30] | Treated 76% undetectable |
112 | >15 | ≥0.9 | 37% | Older age, nadir CD4 and detectable HIV VL associated with inverted CD4:CD8 ratio | Yes | Yes | No | No |
| [23] | Treated HIV-RNA<50 |
288 | >10 | >1.2 | 16% (most in baseline CD4>350) | Failure to normalize the complete T cell phenotype when delaying cART with a CD4<200 | NA | NA | NA | NA |
| [4] | Treated | 407 | 4 | Low <0.4 | NA | NA | NA | Yes | ||
VL= viral load; C=Cohort; ART=antiretroviral therapy; K/T=Kynurenine/Tryptophan; NA=Not applicable. TN=Naïve T cells; TCM=Central; Memory T cells; TTM=Transitional Memory T cells; TEM=Effector Memory T cells.
A recent cross-sectional study reported association of CD4:CD8 ratio with factors that include CD4 nadir, pre-ART plasma VL, duration of ART, level of expression of HLADR+CD38+ on CD4 T cells and CD57+ on CD8 T cells. CD4:CD8 ratio remained independently associated with T-cell activation [31].
Immune activation has been linked with CD4:CD8 ratio as reported by Sainz et al. in vertically infected children and adolescents where they correlated CD4:CD8 ratio and CD4 T cell percentage with immune activated (CD38+HLA-DR+) and senescent (CD28-CD57+) CD4 and CD8 T cells [39]. An inverted CD4:CD8 ratio was associated with higher frequencies of activated, senescent CD4 and CD8 T cells, and a skewed T-cell phenotype from naive towards effector memory. Furthermore, the CD4:CD8 ratio was strongly correlated with CD8 T cells expressing exhausted phenotype (HLA-DR+PD-1+).
The similar correlation between low CD4:CD8 ratios, altered T cell subsets and elevated CD8 T cell activation was reported by Serrano-Villar et al. in ART-treated patients demonstrating CD4 T cell counts>500 cells/mm3 [13]. In addition, they observed an inverse correlation between the CD4:CD8 ratio and hs-CRP, IL-6, and markers of myeloid cell activation such as sCD14 and indoleamine 2,3-dioxygenase (IDO). IDO is an immune-modulatory enzyme, which breaks down tryptophan into kynurenine and can be considered as a myeloid cell marker of immune suppression [40,41]. For patients with CD4 T cell counts >500/mm3, only the tryptophan/kynurenine (KT ratio), a marker of IDO activity, remained significantly correlated with the CD4:CD8 ratio and was able to predict non-AIDS events. These important findings sparked a renewed interest in the association of the CD4:CD8 ratio with the KT ratio, which has been recognized as a key factor contributing to HIV immune dysfunction [42,43]. Taken together, the correlation of CD4:CD8 ratio with IDO and KT ratio indicates that myeloid cells might play a more important role in immune activation than lymphoid cells in long-term ART treated patients [44,45].
The physiological CD4:CD8 ratio and ageing with its implications in HIV infection
In the general population a CD4:CD8 ratio of less than 1.0 is considered as a surrogate marker of immunosenescence and represents an independent predictor of overall mortality [47]. Convergent evidence indicates that a gradually declining CD4:CD8 ratio correlates with immune dysfunction leading to a poor response to immunization, as well as increased risks of severe infections and malignancies compared to younger individuals in the general population [48,49].
The characteristics of age-associated inflammation, termed “inflammaging,” are very similar to inflammatory changes that occur during HIV infection [50]. Our group recently reported that CD4:CD8 ratio was 2.11±0.99 in uninfected healthy adults and spanned from 0.70±0.47 in recently infected patients to 0.1±0.05 in very advanced patients [42,51]. This ratio gradually increased up to 0.94±0.37 after an average of nine years of ART. Interestingly, elite controllers, who are able to maintain an undetectable VL in absence of ART presented with a relatively low CD4:CD8 ratio when compared to the uninfected control subjects (1.13±0.59 vs. 2.1±0.99 respectively) [42]. We assessed ageing effect by a cross sectional analysis on 893 successfully treated HIV-positive patients with undetectable VL for at least one year, the CD4:CD8 ratio significantly lower in patients in their eighties when compared to those in their twenties 0.7 vs. 1.0, respectively (p=0.014*) (unpublished data). Of interest, we also note that male patients had lower CD4:CD8 ratio than females independent of age and duration of treatment. Inverted CD4:CD8 ratio together with loss of both naïve CD4 and CD8 T cells, expansion of activation and senescence markers on CD4 and CD8 T cells are presented both in HIV infection and ageing [52]. Differences in T cell compartments may echo changes observed in premature ageing associated with HIV infection [53]. The relationship of chronic inflammation, aberrant T cell function and phenotype as related to biologic ageing in HIV infection needs further investigation.
Low CD4:CD8 ratios predict non-AIDS related morbidity and mortality in treated HIV infection
Several studies reported a predictive value of CD4:CD8 ratio on clinical outcomes in treated patients. However, the cutoff values of CD4:CD8 ratio used in these studies are not consistent, ranging from 0.8 to 1.5. The ratio used in these studies may depend according to the sample size, nadir CD4 T cell count, timing and duration of ART. Despite this inconsistency in defining a cutoff of CD4:CD8 ratio, these studies still generate important information on its predictive value for clinical outcomes, as depicted in Table 1.
The relationship of the CD4:CD8 ratio and non-AIDS related mobility and mortality was conflicting. Leung et al. studied 4206 patients for a median follow-up of 2.7 years and identified 306 individuals (7.2%) who demonstrated normalized CD4:CD8 ratios (≥1.2) [46]. By using the Kaplan-Meier curves of the time to CD4:CD8 normalization, the probability of achieving a normal CD4:CD8 ratio was 6.1% for those with baseline CD4 T cells<200 cells/mm3, compared to 21% for those with CD4 T cells >350 cells/mm3. Interestingly, no plateau was reached; suggesting that the increase of CD4:CD8 ratio may continue. They found that low CD4 T cell counts, old age and intravenous drug use (IDU) were risk factors that were significantly associated with increasing risk of non-AIDS-events.
The relationship between CD4:CD8 ratio and non-AIDS events, was investigated in a case-control study performed by Serrano-Villar et al. [4]. Multivariate analyses adjusted for age, sex, nadir CD4, proximal CD4 T cell count, year of ART initiation and ART duration were performed on 407 patients for the prediction of non-AIDS events, including malignancies, cardiovascular and kidney diseases. A low CD4:CD8 ratio was an independent factor for both non-AIDS morbidity and mortality in long-term treated HIV-positive patients and was independent of nadir CD4 T cell count. Encouragingly, Saracino et al. reported that patients with more than 15 years of ART had a progressive increase in the CD4:CD8 ratio without reaching a plateau. Factors associated with a persistently low CD4:CD8 ratio included older age, low nadir CD4 T cell count and detectable HIV plasma VL. No association was found between low CD4:CD8 ratio, HIV clade, co-receptor tropism, or co-infections with CMV, hepatitis B or hepatitis C viruses [30]. Interestingly, the metabolic status of treated patients having diabetes and/or hypertriglyceridemia was also linked with low CD4:CD8 ratio [30].
Cumulatively, these studies indicate that CD4 T cell count loses its predictive value, whereas the CD4:CD8 ratio remains a predictor of non-AIDS-associated morbidity and mortality even after long-term ART.
Recently, two groups reported results concerning the contribution of the CD4:CD8 ratio in prediction of cardiovascular events, the most frequent non-AIDS condition. Menozzi et al. performed a study on 914 patients receiving ART for over two years, and a threshold CD4:CD8 ratio value of 0.8 was chosen as a median value of the cohort [54]. In multivariable analyses, CD4:CD8 <0.8 was a predictor for risk of cardiovascular disease, and this effect was not evident for multimorbidity. Bernal et al. analyzed the associations between the CD4:CD8 ratio (≥1.0), cardiovascular risk factors and classes of ART drugs [55]. The inversion of the CD4:CD8 ratio in treated patients was independently associated with intimate media thickness, a marker of subclinical atherosclerosis. So far, there are no large-scale studies with sufficient statistical power to clearly assess the association between the CD4:CD8 ratio with other conditions, such as non-AIDS-associated malignancies, liver and kidney diseases.
The CD4:CD8 ratio association with the size of the HIV reservoir
Viral rebound after cessation of long-term ART clearly indicates the existence of an HIV reservoir in long-lived infected cells [56,57]. Great efforts have been made to understand the mechanisms of viral latency, the size of the HIV reservoir and to design potential therapeutic approaches to target the latency [58,59].
Chun et al. explored the correlation between the CD4:CD8 ratio and HIV reservoir size [60]. They found an inverse correlation between the frequency of CD4 T cells carrying HIV-1 proviral DNA and the CD4:CD8 ratio in treated patients. Our group extended this study and confirmed that CD4 T cell nadir, the CD4:CD8 ratio and CD4 T cell counts were also inversely associated with HIV-1 proviral DNA levels. However, in multivariate analysis, only CD4 T cell nadir significantly predicted levels of HIV-1 proviral DNA independently of other factors [61].
The viral reservoir in peripheral blood exists predominantly in memory CD4 T cells endowed with reproductive potential, including central memory T cells (TCM) and transitional memory T cells (TTM). TCM are characterized by a high clonogenic potential and a relatively long half-life, whereas TTM represent an intermediate differentiation stage between TCM and effector memory T cells (TEM) [62,63]. Cockerham et al. found that the CD4 and CD8 T cell activation as measured by the expression of CD38, HLA-DR, CCR5 and PD-1, was associated with HIV DNA in resting CD4+ T cells in long-term ART-treated patients [64]. Based on these findings, CD4:CD8 ratio can be considered to reduce bias in patient allocation for clinical trials aiming at reducing the size of HIV reservoir [65].
The impact of ART initiation on the normalization of CD4:CD8 ratio
The CD4:CD8 ratio normalization depends on several factors, such as level of immune activation, imbalance of the gut mucosa and the timing of ART initiation. From recent work, it appears that ART initiation in the early phases of infection allows for greater recovery of the CD4:CD8 ratio. Hocqueloux et al. reported on CD4:CD8 ratio recovery in a cohort where 35 patients began treatment during the first four months of infection, while 272 began later on in the chronic phase [66]. The early-treated group had a more rapid and sustained immune reconstitution and a CD4:CD8 ratio of 1.31 versus 0.77 in chronic patients (p<0.0001). A recent report by Thornhill et al. confirmed these findings in a prospective observational study on 353 patients, 253 of whom began early treatment while the remainder deferred ART initiation [67]. In early treated patients 45% normalized their CD4:CD8 ratio compared to only 11% in the deferred group. We further reported that ART initiated in the first year of infection normalized KT ratio and immune activation but failed to improve markers of gut mucosal dysfunction [42]. As of today only early ART initiation has been reported to be able to normalize CD4:CD8 ratio indicating the importance of further investigation.
Future directions of research on the CD4:CD8 ratio
Many questions have yet to be answered. Relationship of the CD4:CD8 ratio and non-AIDS events may not be identified due to the lack of statistical power in some studies and deserves further evaluation. Therefore, virologically suppressed patients with low CD4:CD8 ratio should be monitored through long-term cohorts. Meanwhile, additional investigations on a large scale of patients with non-AIDS-associated cancer and neurocognitive impairment, as well as the impact of ART drug classes like integrase inhibitor are necessary. Association between the CD4:CD8 ratio and levels of microbial translocation, viral co-infections particularly CMV, needs to be further explored. Continued investigation on monocytes, innate immunity and immune-metabolism may also be helpful in elucidating the CD4:CD8 trajectory in treated HIV infection. Research should also focus on mechanisms associated with persistent CD8 T cell expansion and its impact on clinical outcome.
Conclusions
Collectively, a persistently low CD4:CD8 ratio during long-term effective ART represents a marker of continuing immune dysfunction, “inflammaging” and high risk of non-AIDS morbidity and mortality. In long-term treated patients, the progressive correction of the CD4:CD8 ratio is solely a result of CD4 recovery, as CD8 T cell counts remains constant. Encouragingly, earlier ART initiation contributes to a more rapid CD4:CD8 ratio normalization when compared to late treatment initiation. However when ART is initiated in chronic phase, a moderate increase in the CD4:CD8 ratio is observed. Based on patient clinical outcome CD8 T cell count normalization should become a focus of research.
Overall, recent developments highlight the importance of CD4:CD8 ratio as a new tool for assessing patient clinical outcomes and response to immune-based therapies in the context of treated HIV infection.
Acknowledgements
The authors acknowledge Angie Massicotte and Alexandra Averback for coordination and assistance. The authors also acknowledge Jacquie Sas and Jim Pankovich for coordinating the research collaboration between McGill University Health Centre and Peking Union Medical College Hospital with the unwavering support of Canadian Institutes of Health Research (CIHR)/Canadian HIV Trials Network (CTN).
Competing interests
The authors have no competing interests to declare.
Authors' contributions
WL, VM and JPR designed the study, contributed significantly to the review of literature and drafted and revised the manuscript. KV and WC contributed in reviewing the literature, constructing the figures and table and editing the manuscript. TL critically reviewed the manuscript and contributed by releasing data from his Beijing cohort used in this review. All authors have read and approved the final manuscript.
Funding
This study was supported by the Canadian Institutes of Health Research (grant MOP #103230 and CTN #257), Fonds de la Recherche Québec-Santé (FRQ-S): Thérapiecellulaire, Réseau SIDA/Maladies infectieuses, Québec, Canada, and Chinese National Key Technologies R&D Program for the 12th Five-year Plan [grant 2012ZX10001003-001 and 2013ZX10001002-001-001] and by The Canadian HIV Cure Enterprise Team Grant HIG-133050 (JPR) from the CIHR in partnership with CANFAR and IAS.
Dr. Wei Lu is supported by China Scholarship Council (201308110367) and is currently a visiting professor at McGill University Health Centre. Dr. Vikram Mehraj is supported by FRQ-S postdoctoral fellowship award and Dr. Wei Cao is supported by CTN postdoctoral fellowship award. Dr. Jean-Pierre Routy is the holder of Louis Lowenstein Chair in Hematology & Oncology, McGill University.
References
- 1.Frazer IH, Mackay IR, Crapper RM, Jones B, Gust ID, Sarngadharan MG, et al. Immunological abnormalities in asymptomatic homosexual men: correlation with antibody to HTLV-III and sequential changes over two years. Q J Med. 1986;61(234):921–33. [PubMed] [Google Scholar]
- 2.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–33. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vyboh K, Jenabian M-A, Mehraj V, Routy J-P. HIV and the gut microbiota, partners in crime: breaking the vicious cycle to unearth new therapeutic targets. J Immunol Res. 2015;2015:314127. doi: 10.1155/2015/614127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serrano-Villar S, Perez-Elias MJ, Dronda F, Casado JL, Moreno A, Royuela A, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One. 2014;9(1):85798. doi: 10.1371/journal.pone.0085798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mudd JC, Lederman MM. CD8 T cell persistence in treated HIV infection. Curr Opin HIV AIDS. 2014;9(5):500–5. doi: 10.1097/COH.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor JM, Fahey JL, Detels R, Giorgi JV. CD4 percentage, CD4 number, and CD4:CD8 ratio in HIV infection: which to choose and how to use. J Acquir Immune Defic Syndr. 1989;2(2):114–24. [PubMed] [Google Scholar]
- 7.Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322(3):166–72. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(2):83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179(4):859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 10.Clifford GM, Rickenbach M, Lise M, Dal Maso L, Battegay M, Bohlius J, et al. Hodgkin lymphoma in the Swiss HIV Cohort Study. Blood. 2009;113(23):5737–42. doi: 10.1182/blood-2009-02-204172. [DOI] [PubMed] [Google Scholar]
- 11.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med. 2010;170(14):1228–38. doi: 10.1001/archinternmed.2010.197. [DOI] [PubMed] [Google Scholar]
- 12.Syrjala H, Surcel HM, Ilonen J. Low CD4/CD8 T lymphocyte ratio in acute myocardial infarction. Clin Exp Immunol. 1991;83(2):326–8. doi: 10.1111/j.1365-2249.1991.tb05636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10(5):e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann GR, Perrin L, Pantaleo G, Opravil M, Furrer H, Telenti A, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163(18):2187–95. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 15.Lewden C, Chene G, Morlat P, Raffi F, Dupon M, Dellamonica P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46(1):72–7. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- 16.Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol. 2012;2012:670957. doi: 10.1155/2012/670957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimenez E, Vicente A, Sacedon R, Munoz JJ, Weinmaster G, Zapata AG, et al. Distinct mechanisms contribute to generate and change the CD4:CD8 cell ratio during thymus development: a role for the Notch ligand, Jagged1. J Immunol. 2001;166(10):5898–908. doi: 10.4049/jimmunol.166.10.5898. [DOI] [PubMed] [Google Scholar]
- 18.Kosmrlj A, Read EL, Qi Y, Allen TM, Altfeld M, Deeks SG, et al. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature. 2010;465(7296):350–4. doi: 10.1038/nature08997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Routy JP, Angel J, Patel M, Kanagaratham C, Radzioch D, Kema I, et al. Assessment of chloroquine as a modulator of immune activation to improve CD4 recovery in immune nonresponding HIV-infected patients receiving antiretroviral therapy. HIV Med. 2015;16(1):48–56. doi: 10.1111/hiv.12171. [DOI] [PubMed] [Google Scholar]
- 20.Tinago W, Coghlan E, Macken A, McAndrews J, Doak B, Prior-Fuller C, et al. Clinical, immunological and treatment-related factors associated with normalised CD4+/CD8+ T-cell ratio: effect of naive and memory T-cell subsets. PLoS One. 2014;9(5):97011. doi: 10.1371/journal.pone.0097011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torti C, Prosperi M, Motta D, Digiambenedetto S, Maggiolo F, Paraninfo G, et al. Factors influencing the normalization of CD4+ T-cell count, percentage and CD4+/CD8+ T-cell ratio in HIV-infected patients on long-term suppressive antiretroviral therapy. Clin Microbiol Infect. 2012;18(5):449–58. doi: 10.1111/j.1469-0691.2011.03650.x. [DOI] [PubMed] [Google Scholar]
- 22.Buggert M, Frederiksen J, Noyan K, Svard J, Barqasho B, Sonnerborg A, et al. Multiparametric bioinformatics distinguish the CD4/CD8 ratio as a suitable laboratory predictor of combined T cell pathogenesis in HIV infection. J Immunol. 2014;192(5):2099–108. doi: 10.4049/jimmunol.1302596. [DOI] [PubMed] [Google Scholar]
- 23.Ndumbi P, Falutz J, Pant Pai N, Tsoukas CM. Delay in cART initiation results in persistent immune dysregulation and poor recovery of T-cell phenotype despite a decade of successful HIV suppression. PLoS One. 2014;9(4):e94018. doi: 10.1371/journal.pone.0094018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helleberg M, Kronborg G, Ullum H, Ryder LP, Obel N, Gerstoft J. Course and clinical significance of CD8+ T-cell counts in a large cohort of HIV-infected individuals. 2015;211(11):1726–34. doi: 10.1093/infdis/jiu669. [DOI] [PubMed] [Google Scholar]
- 25.Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011;117(21):5582–90. doi: 10.1182/blood-2010-12-322453. [DOI] [PubMed] [Google Scholar]
- 26.Mussini C, Lorenzini P, Cozzi-Lepri A, Lapadula G, Marchetti G, Nicastri E, et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV. 2015;2(3):e98–e106. doi: 10.1016/S2352-3018(15)00006-5. [DOI] [PubMed] [Google Scholar]
- 27.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emu B, Moretto WJ, Hoh R, Krone M, Martin JN, Nixon DF, et al. Composition and function of T cell subpopulations are slow to change despite effective antiretroviral treatment of HIV disease. PLoS One. 2014;9(1):e85613. doi: 10.1371/journal.pone.0085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204(8):1217–26. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saracino A, Bruno G, Scudeller L, Volpe A, Caricato P, Ladisa N, et al. Chronic inflammation in a long-term cohort of HIV-infected patients according to the normalization of the CD4:CD8 ratio. AIDS Res Hum Retroviruses. 2014;30(12):1178–84. doi: 10.1089/aid.2014.0080. [DOI] [PubMed] [Google Scholar]
- 31.Serrano-Villar S, Gutierrez C, Vallejo A, Hernandez-Novoa B, Diaz L, Abad Fernandez M, et al. The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect. 2013;66(1):57–66. doi: 10.1016/j.jinf.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Wallach D, Kang TB, Kovalenko A. Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol. 2014;14(1):51–9. doi: 10.1038/nri3561. [DOI] [PubMed] [Google Scholar]
- 33.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214(2):231–41. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 34.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 36.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16(4):452–9. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 38.Sereti I, Estes JD, Thompson WL, Morcock DR, Fischl MA, Croughs T, et al. Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. PLoS Pathog. 2014;10(1):e1003890. doi: 10.1371/journal.ppat.1003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sainz T, Serrano-Villar S, Diaz L, Gonzalez Tome MI, Gurbindo MD, de Jose MI, et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS. 2013;27(9):1513–6. doi: 10.1097/QAD.0b013e32835faa72. [DOI] [PubMed] [Google Scholar]
- 40.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2(32):32ra6. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenabian MA, Patel M, Kema I, Kanagaratham C, Radzioch D, Thebault P, et al. Distinct tryptophan catabolism and Th17/Treg balance in HIV progressors and elite controllers. PLoS One. 2013;8(10):78146. doi: 10.1371/journal.pone.0078146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenabian MA, El-Far M, Vyboh K, Kema I, Costiniuk CT, Thomas R, et al. Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis. 2015 doi: 10.1093/infdis/jiv037. [DOI] [PubMed] [Google Scholar]
- 43.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–59. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hileman CO, Kinley B, Scharen-Guivel V, Melbourne K, Szwarcberg J, Robinson J, et al. Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor-based initial antiretroviral therapy. J Infect Dis. 2015 doi: 10.1093/infdis/jiv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehraj V, Jenabian MA, Vyboh K, Routy JP. Immune suppression by myeloid cells in HIV infection: new targets for immunotherapy. Open AIDS J. 2014;8:66–78. doi: 10.2174/1874613601408010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leung V, Gillis J, Raboud J, Cooper C, Hogg RS, Loutfy MR, et al. Predictors of CD4:CD8 ratio normalization and its effect on health outcomes in the era of combination antiretroviral therapy. PLoS One. 2013;8(10):77665. doi: 10.1371/journal.pone.0077665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176(4):2645–53. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 48.Strindhall J, Nilsson BO, Lofgren S, Ernerudh J, Pawelec G, Johansson B, et al. No Immune risk profile among individuals who reach 100 years of age: findings from the Swedish NONA immune longitudinal study. Exp Gerontol. 2007;42(8):753–61. doi: 10.1016/j.exger.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998;102(2–3):187–98. doi: 10.1016/s0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 50.Kroger L, Vahasalo P, Tynjala P, Aalto K, Saila H, Malin M, et al. [Medical treatment of juvenile idiopathic arthritis] Duodecim. 2012;128(5):477–86. [PubMed] [Google Scholar]
- 51.Smith GH, Boulassel MR, Klien M, Gilmore N, MacLeod J, LeBlanc R, et al. Virologic and immunologic response to a boosted double-protease inhibitor-based therapy in highly pretreated HIV-1-infected patients. HIV Clin Trials. 2005;6(2):63–72. doi: 10.1310/HAG3-8YA5-UDQC-36NX. [DOI] [PubMed] [Google Scholar]
- 52.Rickabaugh TM, Jamieson BD. A challenge for the future: aging and HIV infection. Immunol Res. 2010;48(1–3):59–71. doi: 10.1007/s12026-010-8167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zapata HJ, Shaw AC. Aging of the human innate immune system in HIV infection. Curr Opin Immunol. 2014;29:127–36. doi: 10.1016/j.coi.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menozzi M, Zona S, Santoro A, Carli F, Stentarelli C, Mussini C, et al. CD4/CD8 ratio is not predictive of multi-morbidity prevalence in HIV-infected patients but identify patients with higher CVD risk. J Int AIDS Soc. 2014;17(4 Suppl 3):19709. doi: 10.7448/IAS.17.4.19709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernal E, Serrano J, Perez A, Valero S, Garcia E, Marin I, et al. The CD4:CD8 ratio is associated with IMT progression in HIV-infected patients on antiretroviral treatment. J Int AIDS Soc. 2014;17(4 Suppl 3):19723. doi: 10.7448/IAS.17.4.19723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94(24):13193–7. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davey RT, Jr., Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96(26):15109–14. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Autran B, Descours B, Bacchus C. Immune control of HIV-1 reservoirs. Curr Opin HIV AIDS. 2013;8(3):204–10. doi: 10.1097/COH.0b013e32835fe6d2. [DOI] [PubMed] [Google Scholar]
- 59.International ASSWGoHIVC, Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12(8):607–14. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chun TW, Justement JS, Pandya P, Hallahan CW, McLaughlin M, Liu S, et al. Relationship between the size of the human immunodeficiency virus type 1 (HIV-1) reservoir in peripheral blood CD4+ T cells and CD4+:CD8+ T cell ratios in aviremic HIV-1-infected individuals receiving long-term highly active antiretroviral therapy. J Infect Dis. 2002;185(11):1672–6. doi: 10.1086/340521. [DOI] [PubMed] [Google Scholar]
- 61.Boulassel MR, Chomont N, Pai NP, Gilmore N, Sekaly RP, Routy JP. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J Clin Virol. 2012;53(1):29–32. doi: 10.1016/j.jcv.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 62.Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014;20(2):139–42. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cockerham LR, Siliciano JD, Sinclair E, O'Doherty U, Palmer S, Yukl SA, et al. CD4+ and CD8+ T cell activation are associated with HIV DNA in resting CD4+ T cells. PLoS One. 2014;9(10):e110731. doi: 10.1371/journal.pone.0110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barouch DH, Deeks SG. Immunologic strategies for HIV-1 remission and eradication. Science. 2014;345(6193):169–74. doi: 10.1126/science.1255512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hocqueloux L, Avettand-Fenoel V, Jacquot S, Prazuck T, Legac E, Melard A, et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother. 2013;68(5):1169–78. doi: 10.1093/jac/dks533. [DOI] [PubMed] [Google Scholar]
- 67.Thornhill J, Inshaw J, Oomeer S, Kaleebu P, Cooper D, Ramjee G, et al. Enhanced normalisation of CD4/CD8 ratio with early antiretroviral therapy in primary HIV infection. J Int AIDS Soc. 2014;17(4 Suppl 3):19480. doi: 10.7448/IAS.17.4.19480. [DOI] [PMC free article] [PubMed] [Google Scholar]